Regularities of Manganese Charge State Formation and Luminescent Properties of Mn-Doped Al2O3, YAlO3, and Y3Al5O12 Single Crystalline Films
Abstract
:1. Introduction
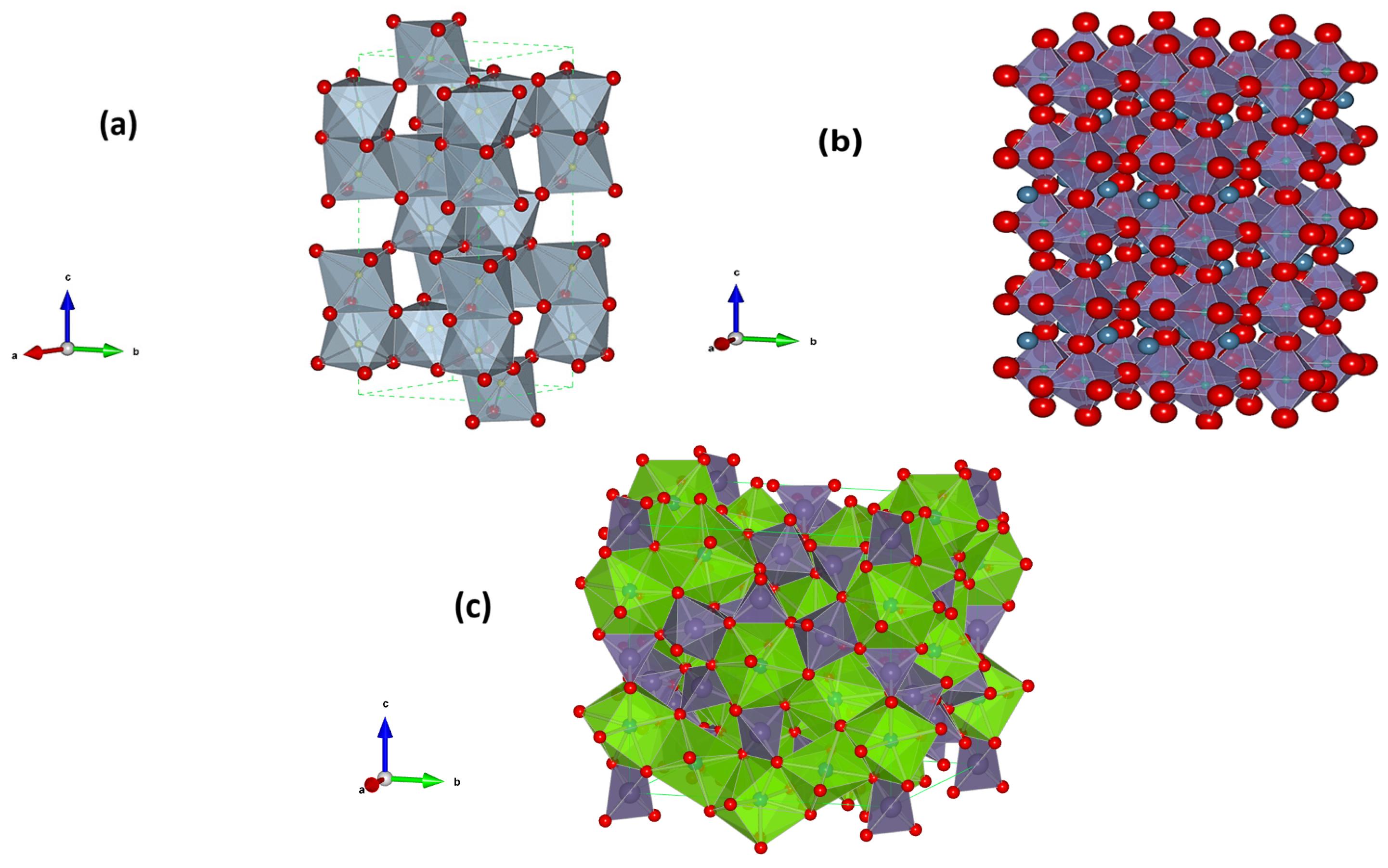
Crystal Structure Oxides under Study
2. Materials and Methods
3. Results
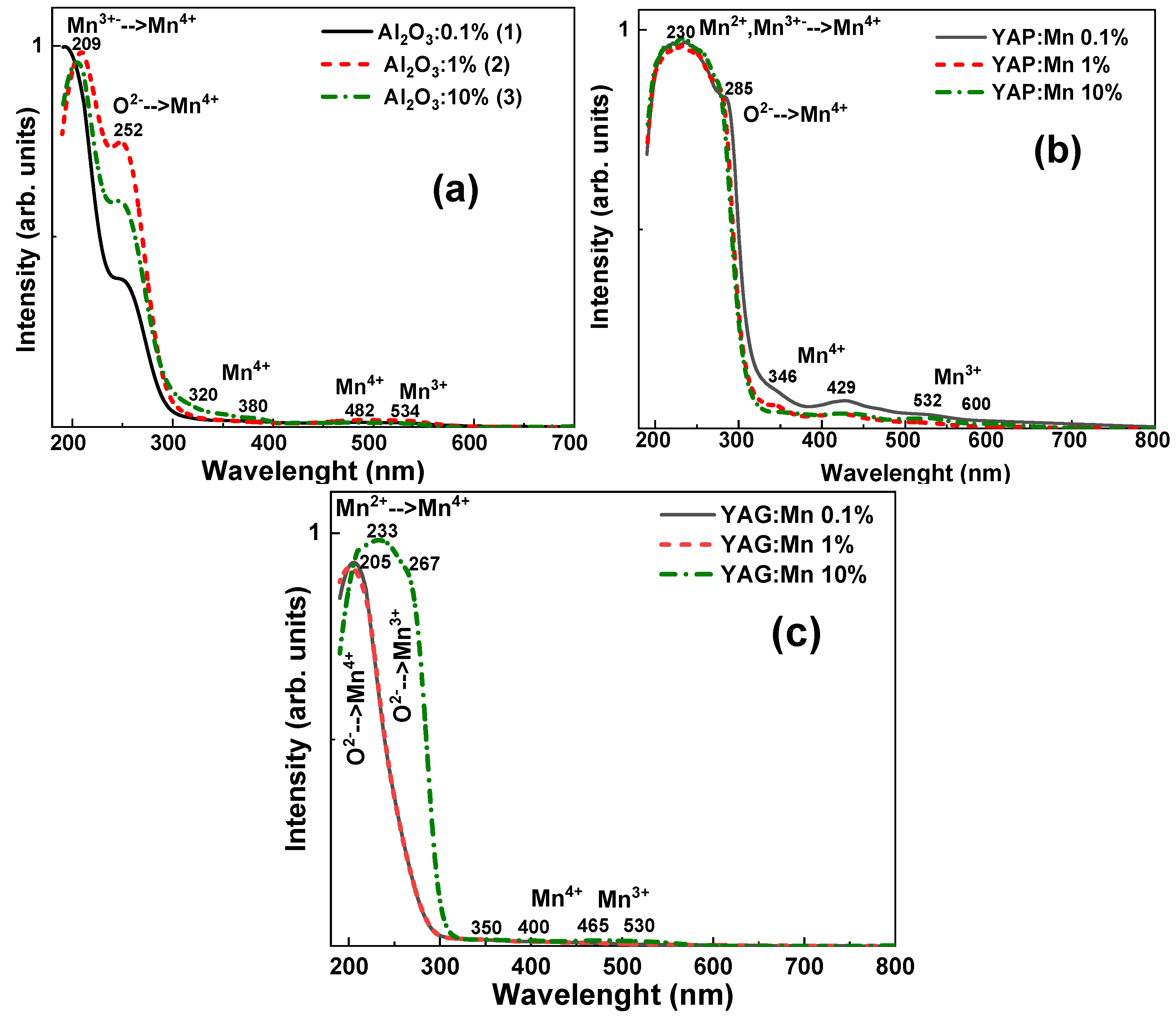
3.1. Absorption Spectra
3.2. CL Spectra
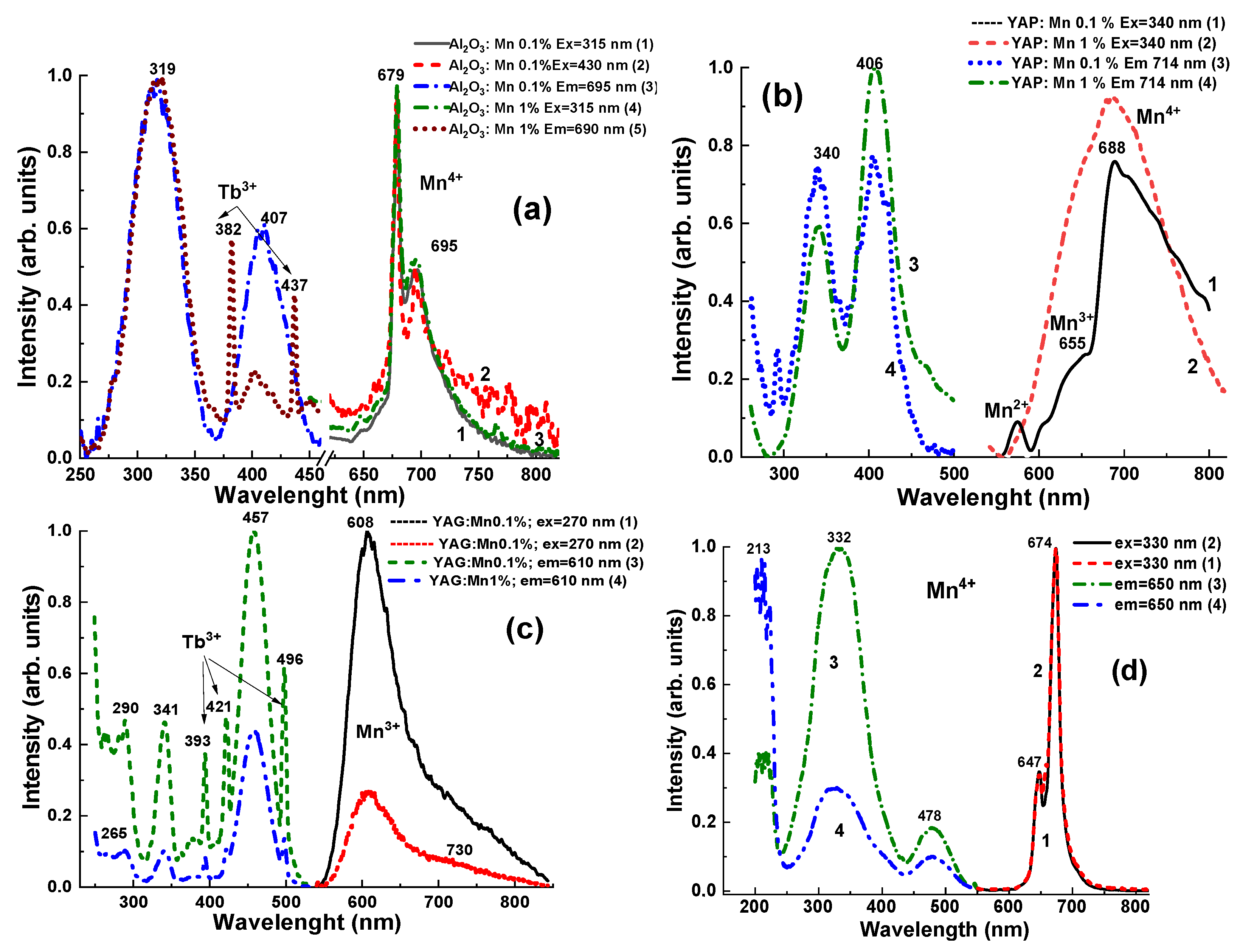
3.3. Photoluminescence Emission and Excitation Spectra
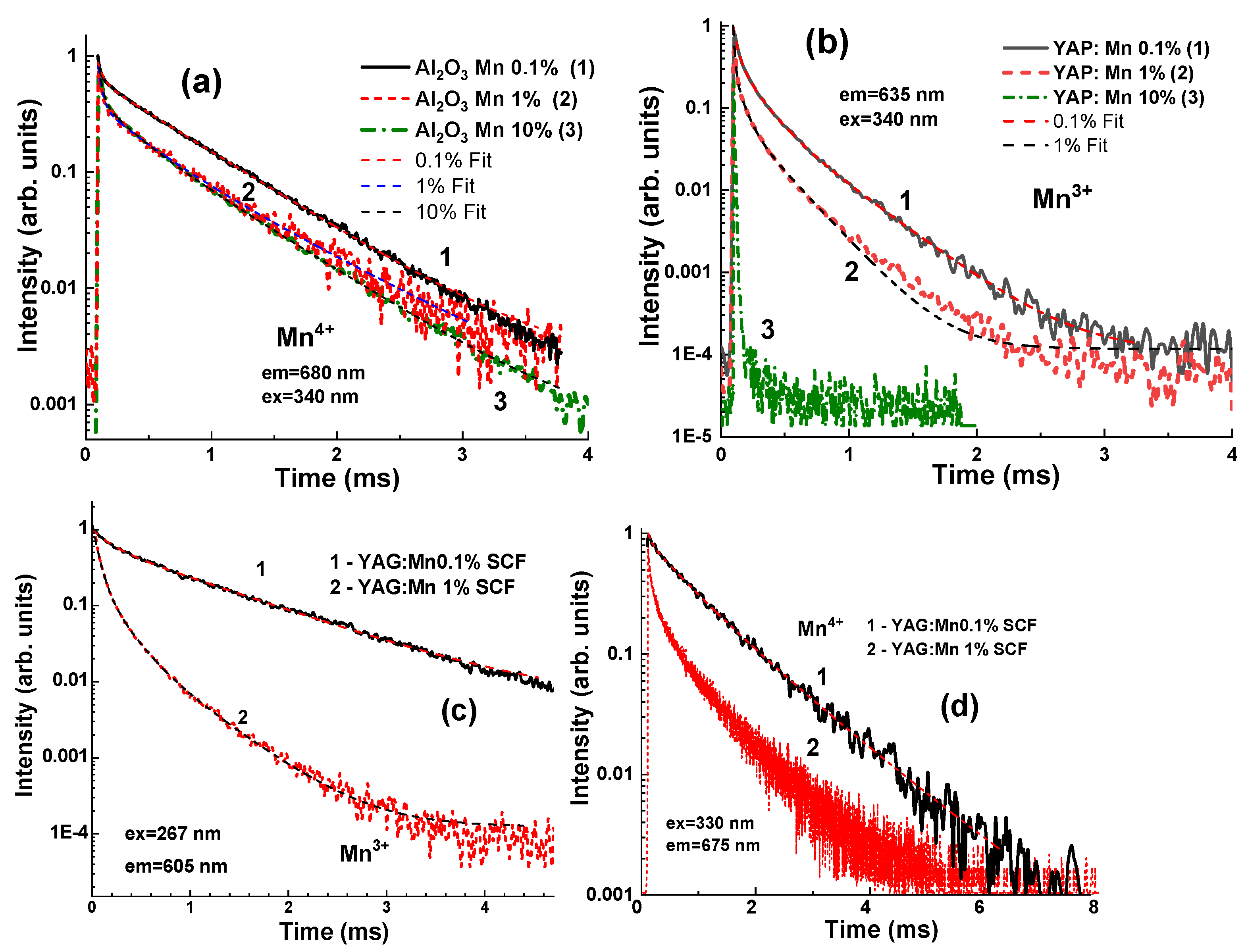
3.4. Photoluminescence Decay Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khomenko, O.V.; Berezovskaya, I.V.; Stoyanova, I.V.; Efryushina, N.P.; Dotsenko, V.P.; Poletaev, N.I.; Khlebnikova, M.E. Synthesis and Luminescence Study of Mn-doped Nanosized Al2O3. In Proceedings of the 2018 IEEE 8th International Conference Nanomaterials: Application & Properties (NAP), Zatoka, Ukraine, 9–14 September 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Zhydachevskii, Y.; Galanciak, D.; Kobyakov, S.; Berkowski, M.; Kamińska, A.; Suchocki, A.; Zakharko, Y.; Durygin, A. Photoluminescence studies of Mn4+ ions in YAlO3 crystals at ambient and high pressure. J. Phys. Condens. Matter 2006, 18, 11385. [Google Scholar] [CrossRef]
- Singh, S.; Gavrilovic, P. US Patent No. 22483. 1994. [Google Scholar]
- Zhydachevskii, Y.; Suchocki, A.; Berkowski, M.; Zakharko, Y. Optically stimulated luminescence of YAlO3: Mn2+ for radiation dosimetry. Radiat. Meas. 2007, 42, 625–627. [Google Scholar] [CrossRef]
- Yang, S.H.; Lu, C.Y. Influence of doping and coating on the photoluminescence properties of yttrium aluminum garnet phosphors. J. Electrochem. Soc. 2007, 154, J397. [Google Scholar] [CrossRef]
- Zorenko, Y.; Gorbenko, V.; Zorenko, T.; Kuklinski, B.; Grinberg, M.; Wiśniewski, K.; Bilski, P. Luminescent properties of Mn-doped Y3Al5O12 single crystalline films. Opt. Mater. 2014, 36, 1680–1684. [Google Scholar] [CrossRef]
- Tian, C.; Lin, H.; Zhang, D.; Zhang, P.; Hong, R.; Han, Z.; Qian, X.; Zou, J. Mn4+ activated red-emitting ceramic phosphor with excellent thermal conductivity. Opt Express 2019, 27, 32666–32678. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.; Mansoor, M.; Mansoor, M.; Er, Z.; Çinar Şahin, F. Ab-initio study of paramagnetic defects in Mn and Cr doped transparent polycrystalline Al2O3 ceramics. Synth. Sinter. 2021, 1, 135–142. [Google Scholar] [CrossRef]
- Piskunov, S.; Isakoviča, I.; Popov, A.I. Electronic structure of MnAl3+ and MnAl2+ doped YAlO3: Prediction from the first principles . Opt. Mater. 2018, 85, 162–166. [Google Scholar] [CrossRef]
- Zorenko, Y.; Zorenko, T.; Voznyak, T.; Mandowski, A.; Xia, Q.; Batentschuk, M.; Friedrich, J. Luminescence of F+ and F centers in AI2O3-Y2O3 oxide compounds. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2010; Volume 15, p. 012060. [Google Scholar]
- Zhang, R.; Lin, H.; Yu, Y.L.; Chen, D.Q.; Xu, J.; Wang, Y.S. A new-generation color converter for high-power white LED: Transparent Ce3+:YAG phosphor-in-glass. Laser Photonics Rev. 2014, 8, 158–164. [Google Scholar] [CrossRef]
- Liao, C.X.; Cao, R.P.; Ma, Z.J.; Li, Y.; Dong, G.P.; Sharafudeen, K.N.; Qiu, J.R. Synthesis of K2SiF6: Mn4+, phosphor from SiO2, powders via redox reaction in HF/KMnO4, solution and their application in warm-white LED. J. Am. Chem. Soc. 2013, 96, 3552–3556. [Google Scholar] [CrossRef]
- Qiu, Z.X.; Luo, T.T.; Zhang, J.L.; Zhou, W.L.; Yu, L.P.; Lian, S.X. Effectively enhancing blue excitation of red phosphor Mg2TiO4: Mn4+ by Bi3+ sensitization. J. Lumin. 2015, 158, 130–135. [Google Scholar] [CrossRef]
- Makhov, V.N.; Lushchik, A.; Lushchik, C.B.; Kirm, M.; Vasil’chenko, E.; Vielhauer, S.; Harutunyan, V.V.; Aleksanyan, E. Luminescence and radiation defects in electron-irradiated Al2O3 and Al2O3:Cr. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 2949–2952. [Google Scholar] [CrossRef]
- Stanek, C.R.; Levy, M.R.; McClellan, K.J.; Uberuaga, B.P.; Grimes, R.W. Defect identification and compensation in rare earth oxide scintillators. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 2657–2664. [Google Scholar] [CrossRef]
- Babin, V.; Gorbenko, V.; Makhov, A.; Mares, J.A.; Nikl, M.; Zazubovich, S.; Zorenko, Y. Luminescence characteristics of Pb2+ centres in undoped and Ce3+-doped Lu3Al5O12 single-crystalline films and Pb2+→Ce3+ energy transfer processes. J. Lumin. 2007, 127, 384–390. [Google Scholar] [CrossRef]
- Ntwaeaborwa, O.M.; Mhlongo, G.H.; Pitale, S.S.; Dhlamini, M.S.; Kroon, R.E.; Swart, H.C. Cathodoluminescence Properties of SiO2: Ce3+, Tb3+, SiO2: Ce3+, Pr3+ and SiO2: PbS. In Cathodoluminescence; IntechOpen: London, UK, 2012. [Google Scholar]
- Yacobi, B.G.; Holt, D.B. Cathodoluminescence. In Cathodoluminescence Microscopy of Inorganic Solids; Springer: Boston, MA, USA, 1990. [Google Scholar] [CrossRef]
- Vedda, A.; Fasoli, M.; Nikl, M.; Laguta, V.V.; Mihokova, E.; Pejchal, J.; Yoshikawa, A.; Zhuravleva, M. Trap-center recombination processes by rare earth activators in YAlO3 single crystal host. Phys. Rev. B 2009, 80, 045113. [Google Scholar] [CrossRef]
- Donegan, J.F.; Glynn, T.J.; Imbusch, G.F.; Remeika, J.P. Luminescence and fluorescence line narrowing studies of Y3Al5O12:Mn4+. J. Lumin. 1986, 36, 93–100. [Google Scholar] [CrossRef]

| Type of SCF | Mn Content, Mole% | Mn Segregation Coefficient | Substrate | Flux | h, µm | T, °C | f, μm/min |
|---|---|---|---|---|---|---|---|
| Al2O3:Mn | 10% | Al2O3 SC | PbO + B2O3 + Bi2O3 | 11 | 950 | 0.8 | |
| 1% | 0.08 | Al2O3 SC | PbO + B2O3 + Bi2O3 | 5 | 930 | 1.6 | |
| 0.1% | Al2O3 SC | PbO + B2O3 + Bi2O3 | 14 | 945 | 2.14 | ||
| YAO3:Mn | 10% | YAO3 SC | PbO | 14.7 | 1020 | 0.18 | |
| 1% | 0.14 | YAO3 SC | PbO | 29 | 999 | 1.23 | |
| 0.1% | YAO3 SC | PbO | 19 | 1012 | 2.2 | ||
| Y3A5O12:Mn | 10% | Y3A5O12 SC | PbO | 3.7 | 980 | 0.19 | |
| 1% | 0.2 | Y3A5O12 SC | PbO | 24.8 | 985 | 0.22 | |
| 0.1% | Y3A5O12 SC | PbO | 60 | 983 | 0.21 |
| Type of SCF | Mn Content in MELT Solution, Mole% | Registered Mn State in Absorption Spectra | Registered Mn State in CL Spectra | Other Emission Centers in CL Spectra |
|---|---|---|---|---|
| Al2O3:Mn | 0.1 | Mn4+ | Mn4+ | Pb2+, ex(Pb) |
| 1 | Mn3+, Mn4+ | Mn4+ | Pb2+, ex(Pb) | |
| 10 | Mn3+, Mn4+ | Mn3+, Mn4+ | Pb2+, ex(Pb) | |
| YAO3:Mn | 0.1 | Mn3+, Mn4+ | Mn3+ | |
| 1 | Mn2+, Mn3+, Mn4+ | Mn2+, Mn3+ | Pb2+ | |
| 10 | Mn2+, Mn3+, Mn4+ | Mn2+, Mn3+, Mn4+ | ||
| Y3A5O12:Mn | 0.1 | Mn4+ | Mn2+, Mn3+, Mn4+ | |
| 1 | Mn4+ | Mn2+, Mn3+, Mn4+ | Pb2+ | |
| 10 | Mn4+, Mn3+, Mn2+ | Mn2+, Mn3+, Mn4+ |
| SCF Type | Mn Content,% | Mn State | A1/t1, ms | A2/t2, ms | A3/t3, ms |
|---|---|---|---|---|---|
| Al2O3:Mn | 0.1 | Mn4+ | 0.04 (5.9%) | 0.64 (94.1%) | - |
| 1 | Mn4+ | 0.03 (2.8%) | 0.33 (29.4%) | 0.76 (67.8%) | |
| 10 | Mn4+ | 0.026 (3%) | 0.22 (24.8%) | 0.64 (72.2%) | |
| YAO3:Mn | 0.1 | Mn3+ | 0.14 (27.4) | 0.03 (6%) | 0.34 (66.6%) |
| 1 | Mn3+ | 0.11 (25.6%) | 0.06 (14%) | 0.26 (60.4%) | |
| Y3A5O12:Mn | 0.1 | Mn3+ | 0.17 (14.8%) | 0.98 (85.2%) | - |
| 1 | Mn3+ | 0.17 (24.6%) | 0.05 (3.3%) | 0.47 (68.1%) | |
| 0.1 | Mn4+ | 0.066 (3.6%) | 0.65 (34.6%) | 1.16 (61.8%) | |
| 1 | Mn3+ | 0.06 (40.5%) | 0.265 (33.1%) | 0.875 (26.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewski-Napierkowski, A.; Gorbenko, V.; Zorenko, T.; Witkiewicz-Łukaszek, S.; Zorenko, Y. Regularities of Manganese Charge State Formation and Luminescent Properties of Mn-Doped Al2O3, YAlO3, and Y3Al5O12 Single Crystalline Films. Crystals 2023, 13, 1481. https://doi.org/10.3390/cryst13101481
Majewski-Napierkowski A, Gorbenko V, Zorenko T, Witkiewicz-Łukaszek S, Zorenko Y. Regularities of Manganese Charge State Formation and Luminescent Properties of Mn-Doped Al2O3, YAlO3, and Y3Al5O12 Single Crystalline Films. Crystals. 2023; 13(10):1481. https://doi.org/10.3390/cryst13101481
Chicago/Turabian StyleMajewski-Napierkowski, Artur, Vitaliy Gorbenko, Tatiana Zorenko, Sandra Witkiewicz-Łukaszek, and Yuriy Zorenko. 2023. "Regularities of Manganese Charge State Formation and Luminescent Properties of Mn-Doped Al2O3, YAlO3, and Y3Al5O12 Single Crystalline Films" Crystals 13, no. 10: 1481. https://doi.org/10.3390/cryst13101481
APA StyleMajewski-Napierkowski, A., Gorbenko, V., Zorenko, T., Witkiewicz-Łukaszek, S., & Zorenko, Y. (2023). Regularities of Manganese Charge State Formation and Luminescent Properties of Mn-Doped Al2O3, YAlO3, and Y3Al5O12 Single Crystalline Films. Crystals, 13(10), 1481. https://doi.org/10.3390/cryst13101481








