Density Testing Method for Undercooling Solidification of High-Temperature Metal Melts
Abstract
1. Introduction
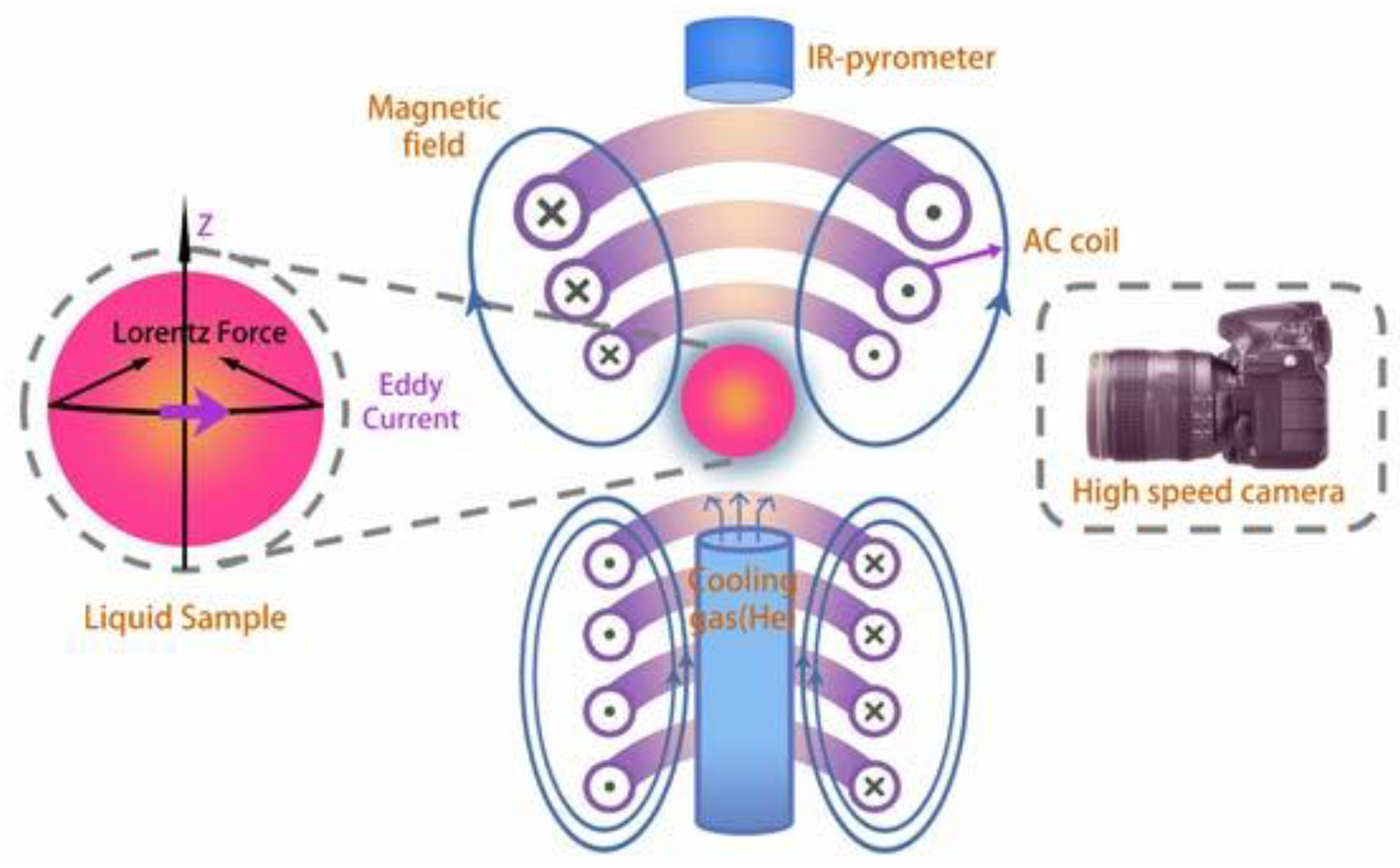
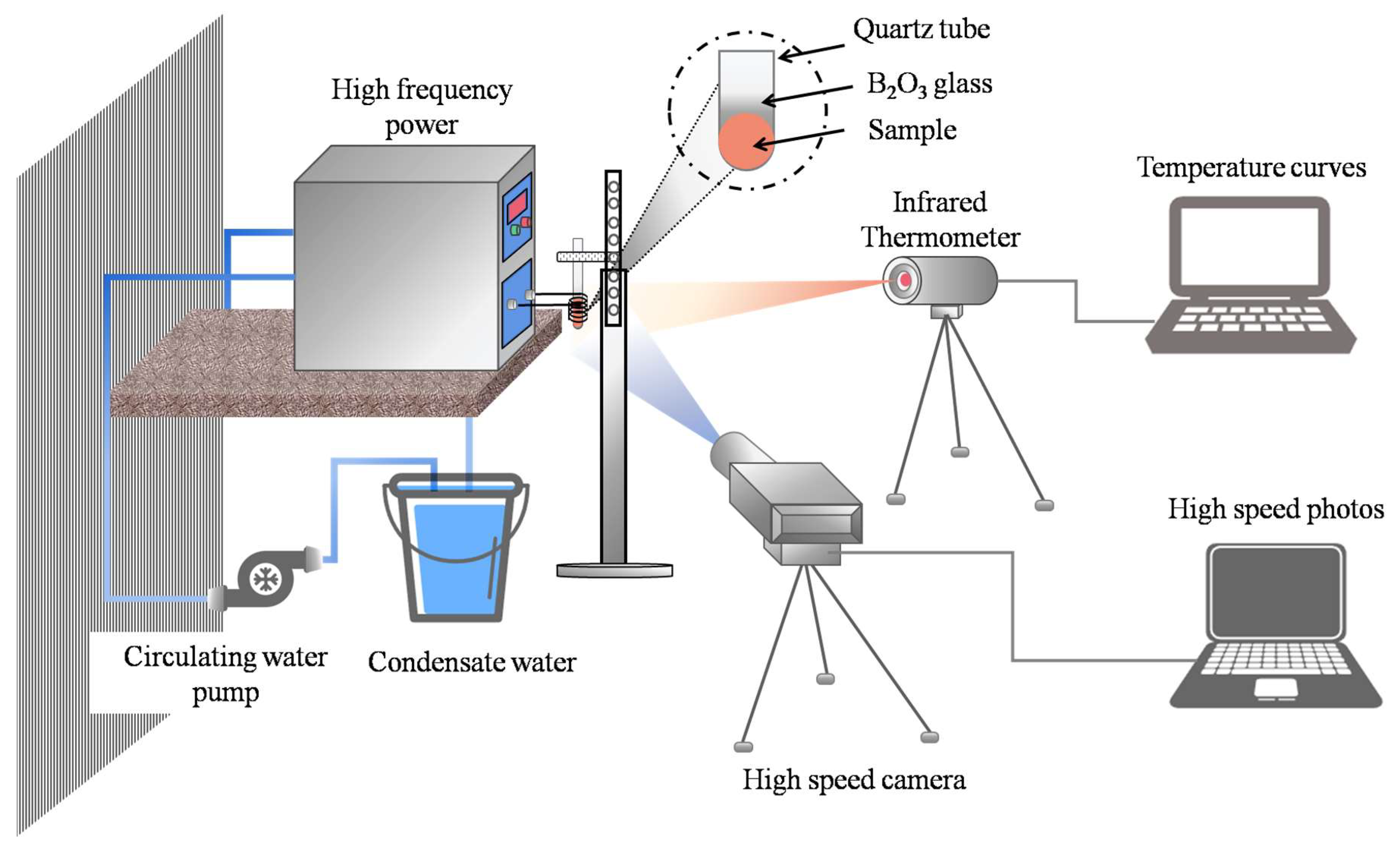
2. Experiment
3. Results
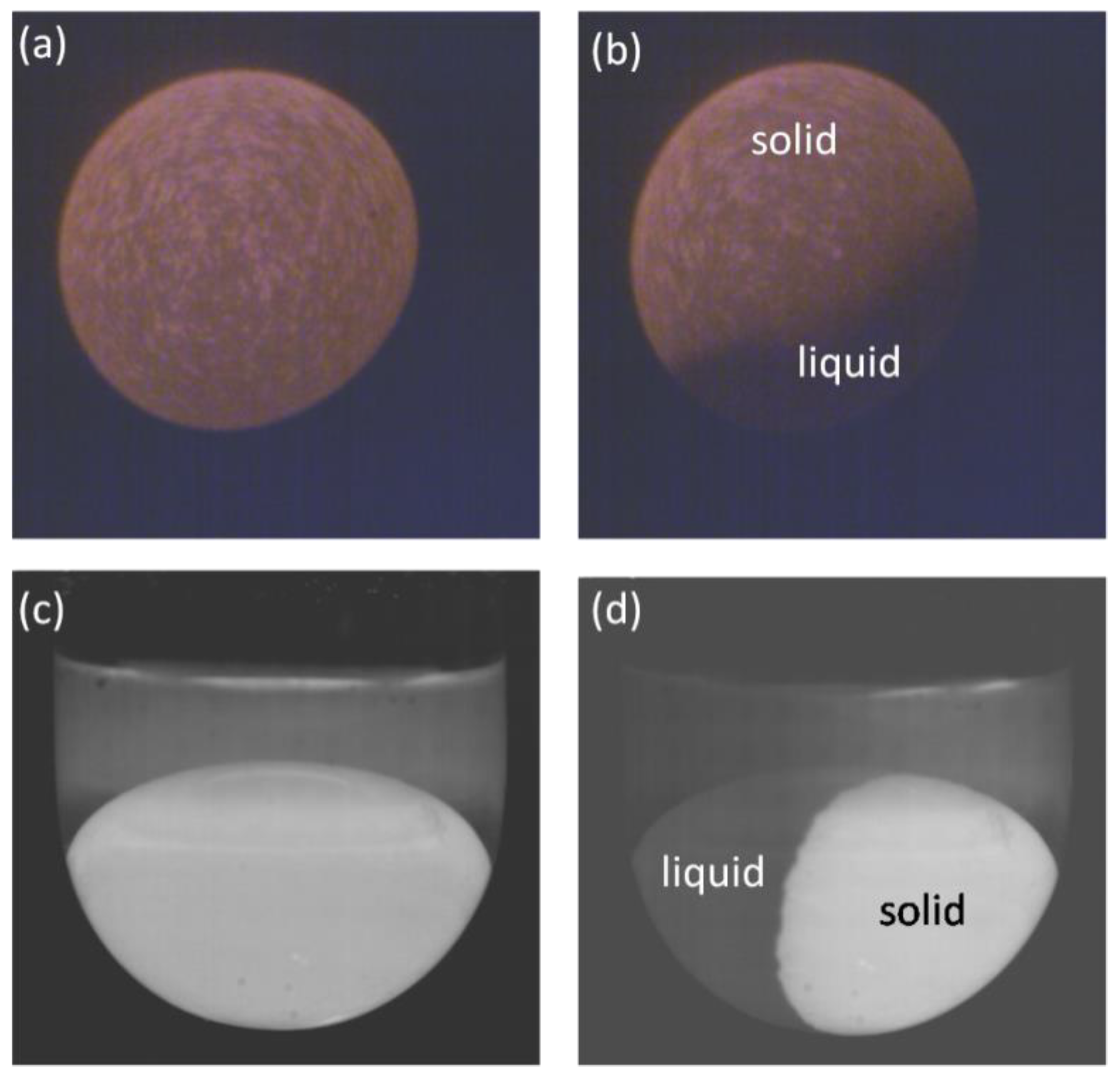
3.1. Sample Images
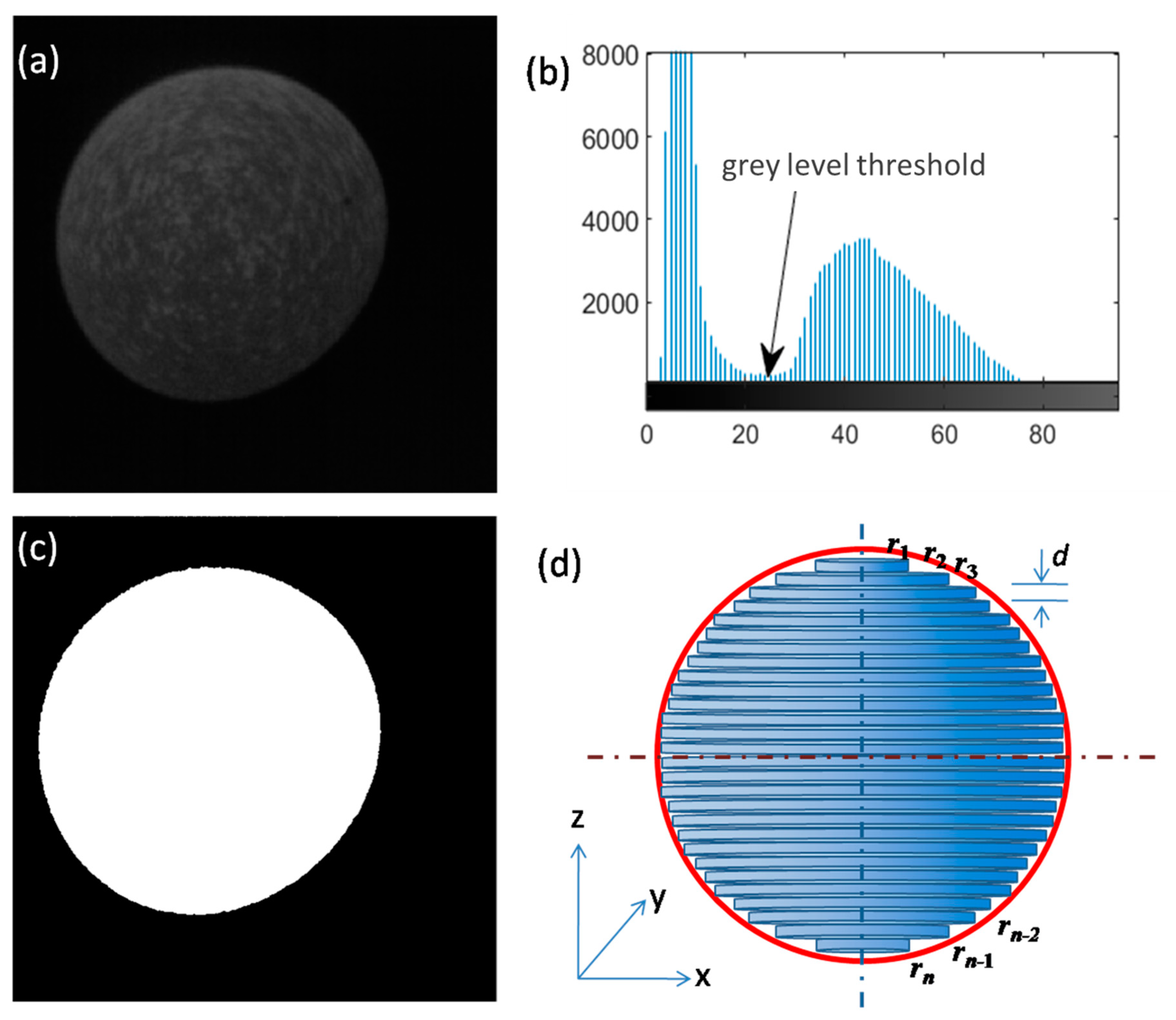
3.2. Ordinary Image Threshold Segmentation Method
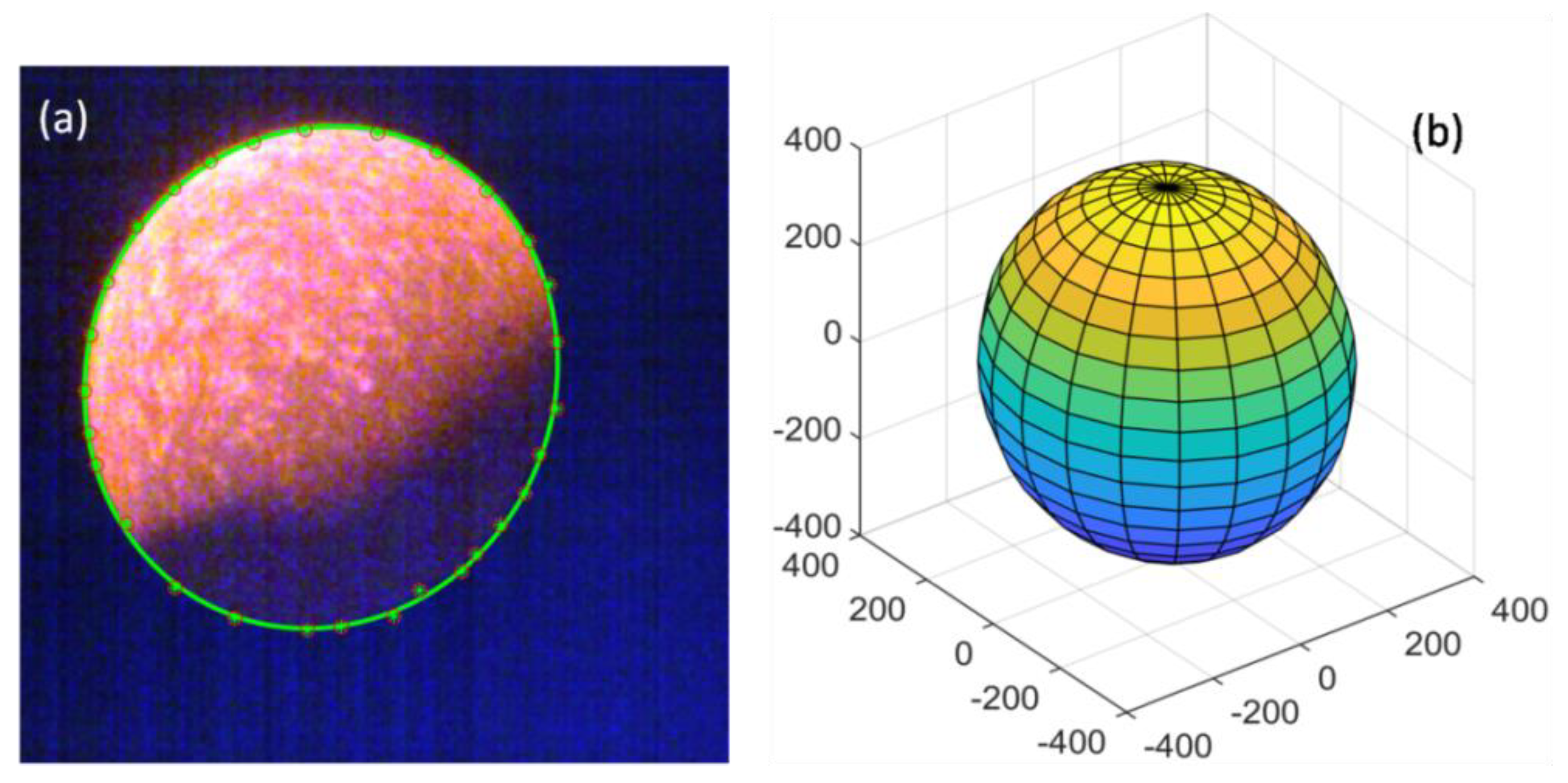
3.3. Ellipse Fitting Method
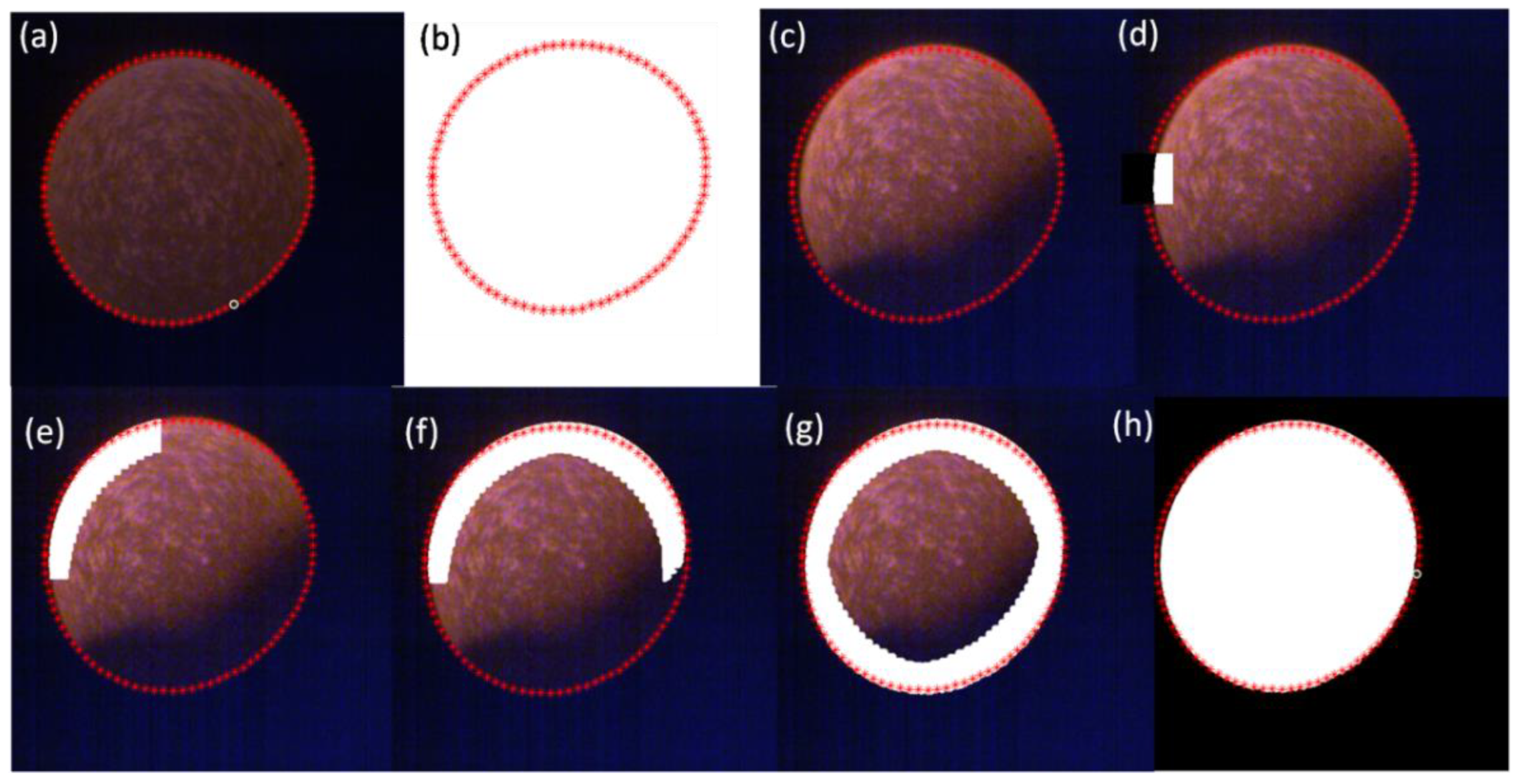
3.4. Local Dynamic Threshold Segmentation Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ushakov, S.V.; Niessen, J.; Quirinale, D.G.; Prieler, R.; Navrotsky, A.; Telle, R. Measurements of Density of Liquid Oxides with an Aero-Acoustic Levitator. Materials 2021, 14, 822. [Google Scholar] [CrossRef]
- Watanabe, M.; Adachi, M.; Fukuyama, H. Densities of Au-X (X = Cu, Ni and Pd) binary melts and thermodynamic correlations. J. Mol. Liq. 2022, 348, 118050. [Google Scholar] [CrossRef]
- Naeem, M.; He, H.; Harjo, S.; Kawasaki, T.; Lin, W.; Kai, J.J.; Wu, Z.; Lan, S.; Wang, X.L. Temperature-dependent hardening contributions in CrFeCoNi high-entropy alloy. Acta Mater. 2021, 221, 117371. [Google Scholar] [CrossRef]
- Dogan, A.; Arslan, H. Thermophysical properties of Cu-In-Sn liquid Pb-free alloys: Viscosity and surface tension. Philos. Mag. 2018, 98, 37–53. [Google Scholar] [CrossRef]
- Xu, J.F.; Sun, G.Z.; Liu, Z.T.; Wang, Y.L.; Jian, Z.Y. Preparation and properties of Ge4Se96 glass. Infrared Phys. Technol. 2018, 89, 59–63. [Google Scholar] [CrossRef]
- Korobeinikov, A.I.; Endo, R.; Seetharaman, S.; Volkova, O. Density of Liquid Manganese Measured Using the Maximum Bubble Pressure Method. Metall. Mater. Trans. B 2021, 52, 571–575. [Google Scholar] [CrossRef]
- Fukuta, M.; Sumiyama, J.; Motozawa, M.; Yanagisawa, T. Surface tension measurement of oil/refrigerant mixture by maximum bubble pressure method. Int. J. Refrig. 2017, 73, 125–133. [Google Scholar] [CrossRef]
- Leitner, T.; Werkovits, A.; Kleber, S.; Pottlacher, G. Surface Tension and Density of Liquid Hot Work Tool Steel W360 by voestalpine BÖHLER Edelstahl GmbH & Co KG Measured with an Electromagnetic Levitation Apparatus. Int. J. Thermophys. 2021, 42, 16. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, G.; Qi, X.; McLean, A. Modeling and Characterization of Surface Deformation and Current Distribution of Molten Droplets Under Electromagnetic Levitation. Metall. Mater. Trans. B 2023, 54, 1798–1806. [Google Scholar] [CrossRef]
- Watanabe, S.; Amatatu, M.; Saito, T. Densities of Fe-Ni, Co-Ni, Co-Mo and Co-W alloys in liquid State. Trans. Jpn. Inst. Met. 1971, 12, 337–342. [Google Scholar] [CrossRef][Green Version]
- Pstruś, J.; Moser, Z.; Gąsior, W.; Dębski, A. Surface tension and density measurements of liquid Sn-Zn alloys. Experiment vs. SURDAT database of Pb-free solders. Arch. Metall. Mater. 2006, 51, 335–343. [Google Scholar] [CrossRef]
- Shiraishi, S.Y.; Ward, R.G. The density of nickel in the superheated and supercooled liquid states. Can. Metall. Q. 1964, 3, 117–122. [Google Scholar] [CrossRef]
- Saito, T.; Sakuma, Y. Densities of Pure Iron, Cobalt and Nickel in the molten state. Sci. Rep. Res. Ins. 1970, 22, 57–65. [Google Scholar]
- Reiplinger, B.; Brillo, J. Density and excess volume of the liquid Ti–V system measured in electromagnetic levitation. J. Mater. Sci. 2022, 57, 7954–7964. [Google Scholar] [CrossRef]
- Lohöfer, G. High-resolution inductive measurement of electrical resistivity and density of electromagnetically levitated liquid metal droplets. Rev. Sci. Instrum. 2018, 89, 124709. [Google Scholar] [CrossRef]
- Racz, L.M.; Egry, I. Advances in the measurement of density and thermal expansion of undercooled liquid metals. Rev. Sci. Instrum. 1995, 66, 4254–4258. [Google Scholar] [CrossRef]
- Brillo, J.; Egry, I. Density determination of liquid copper, nickel, and their alloys. Int. J. Thermophys. 2003, 24, 1155–1170. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, G.; Yi, B.; Barati, M. Electromagnetic Levitation and Solidification of Ferrosilicon Alloy and Resulting Microstructural Evolution Mechanisms. J. Mater. Res. Technol. 2023, 24, 8296–8306. [Google Scholar] [CrossRef]
- Saito, T.; Shiraishi, Y.; Sakuma, Y. Density measurement of molten metals by levitation technique at temperatures between 1800 °C and 2200 °C. Trans. Iron. Steel. Inst. Jpn. 1969, 9, 118–126. [Google Scholar] [CrossRef]
- Brillo, J.; Egry, I.; Matsushita, T. Density and surface tension of liquid ternary Ni-Cu-Fe alloys. Int. J. Mater. Res. 2006, 97, 28–34. [Google Scholar] [CrossRef]
- Schmon, A.; Azizand, K.; Pottlacher, G. Density determination of liquid copper and liquid nickel by means of fast resistive pulse heating and electromagnetic levitation. Metall. Mater. Trans. A 2015, 46, 2674–2679. [Google Scholar] [CrossRef]
- Bradshaw, R.C.; Schmidt, D.P.; Rogers, J.R.; Kelton, K.F.; Hyers, R.W. Machine vision for high-precision volume measurement applied to levitated containerless material processing. Rev. Sci. Instrum. 2005, 76, 125108. [Google Scholar] [CrossRef]
- Chung, S.K.; Thiessen, D.B.; Rhim, W.K. A noncontact measurement technique for the density and thermal expansion coefficient of solid and liquid materials. Rev. Sci. Instrum. 1996, 67, 3175–3181. [Google Scholar] [CrossRef]
- Watanabe, M.; Takahashi, Y.; Imaizumi, S.; Zhao, Y.; Adachi, M.; Ohtsuka, M.; Chiba, A.; Koizumi, Y.; Fukuyama, H. Thermophysical properties of liquid Co–Cr–Mo alloys measured by electromagnetic levitation in a static magnetic field. Thermochim. Acta 2022, 708, 179119. [Google Scholar] [CrossRef]
- Matson, D.M.; Battezzati, L.; Galenko, P.K.; Gandin, C.-A.; Gangopadhyay, A.K.; Henein, H.; Kelton, K.F.; Kolbe, M.; Valloton, J.; Vogel, S.C.; et al. Electromagnetic levitation containerless processing of metallic materials in microgravity: Rapid solidification. Npj Microgravity 2023, 9, 65. [Google Scholar] [CrossRef]
- Toropova, L.V.; Alexandrov, D.V.; Kao, A.; Rettenmayr, M.; Galenko, P.K. Electromagnetic levitation method as a containerless experimental technique. Phys. Usp. 2023, 66, 722–733. [Google Scholar] [CrossRef]
- Rodriguez, J.E.; Kreischer, C.; Volkmann, T.; Matson, D.M. Solidification velocity of undercooled Fe–Co alloys. Acta Mater. 2017, 122, 431–437. [Google Scholar] [CrossRef]
- Liu, L.; Yang, L.; Li, J. Solidification Pathways in Highly Undercooled Co79.3B20.7 Alloy. Metall. Mater. Trans. A 2021, 52, 4324–4330. [Google Scholar] [CrossRef]
- An, H.; Xu, X. Study of non-equilibrium dendrite growth in an undercooled alloy. Mater. Sci. Technol. 2020, 36, 1720–1727. [Google Scholar] [CrossRef]
- Rahul, M.R.; Phanikumar, G. Solidification behaviour of undercooled equiatomic FeCuNi alloy. J. Alloys Compd. 2020, 815, 152334. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, T.; Xu, J.; Yao, Z.; Jian, Z.; Galenko, P.K. Density Testing Method for Undercooling Solidification of High-Temperature Metal Melts. Crystals 2023, 13, 1502. https://doi.org/10.3390/cryst13101502
Niu T, Xu J, Yao Z, Jian Z, Galenko PK. Density Testing Method for Undercooling Solidification of High-Temperature Metal Melts. Crystals. 2023; 13(10):1502. https://doi.org/10.3390/cryst13101502
Chicago/Turabian StyleNiu, Tongzhuang, Junfeng Xu, Zhirui Yao, Zengyun Jian, and Peter K. Galenko. 2023. "Density Testing Method for Undercooling Solidification of High-Temperature Metal Melts" Crystals 13, no. 10: 1502. https://doi.org/10.3390/cryst13101502
APA StyleNiu, T., Xu, J., Yao, Z., Jian, Z., & Galenko, P. K. (2023). Density Testing Method for Undercooling Solidification of High-Temperature Metal Melts. Crystals, 13(10), 1502. https://doi.org/10.3390/cryst13101502






