Solid Phase and Stability Investigation of a Co-Crystal in the l-Valine/l-Leucine System
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Experimental Procedures
2.3. Analytical Techniques
2.4. Computational Details
3. Results and Discussion
3.1. Solution Behavior of the V3L Compound in Water and Water–Ethanol Mixtures: The Ternary l-Val/l-Leu/Solvent System
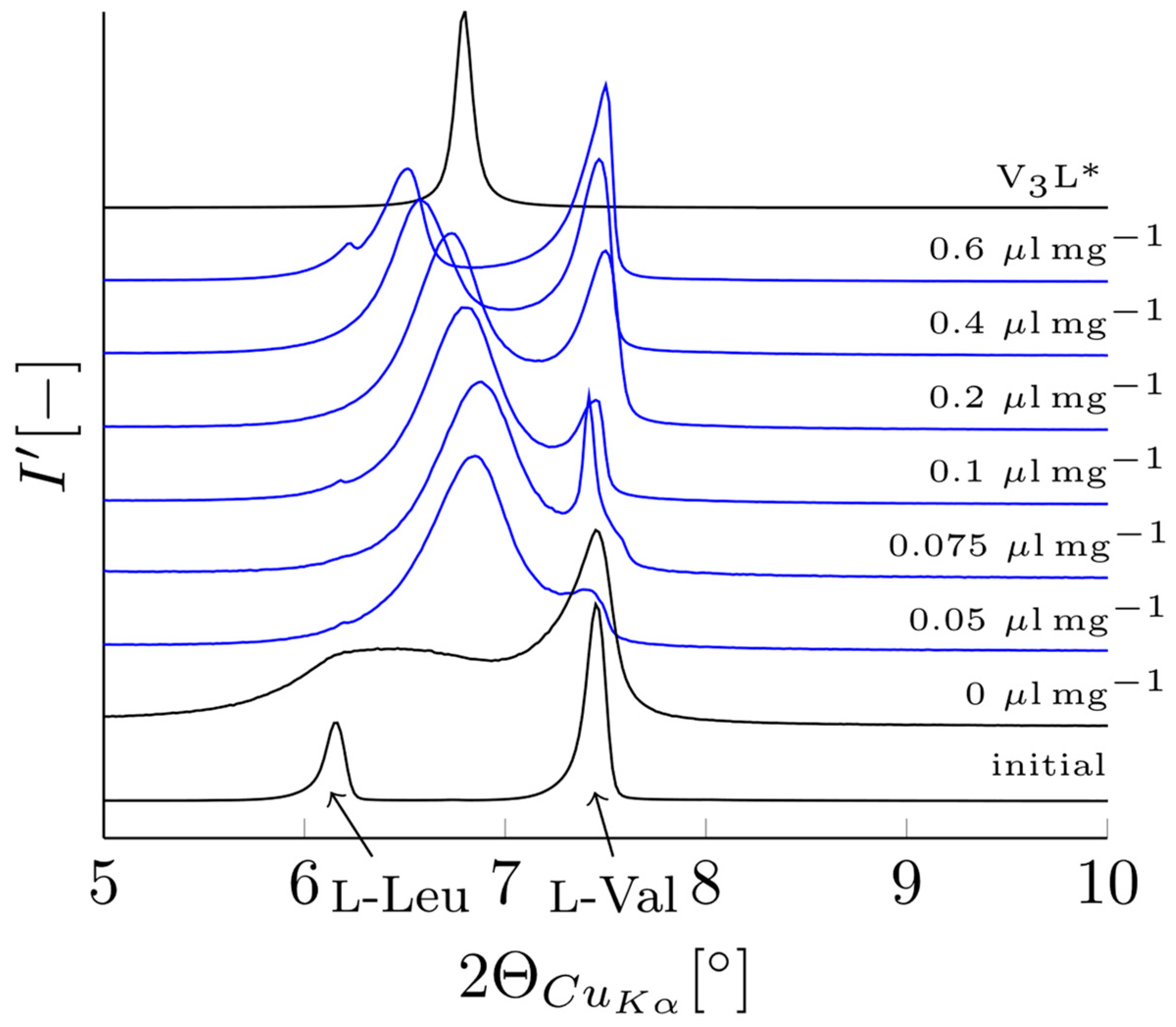
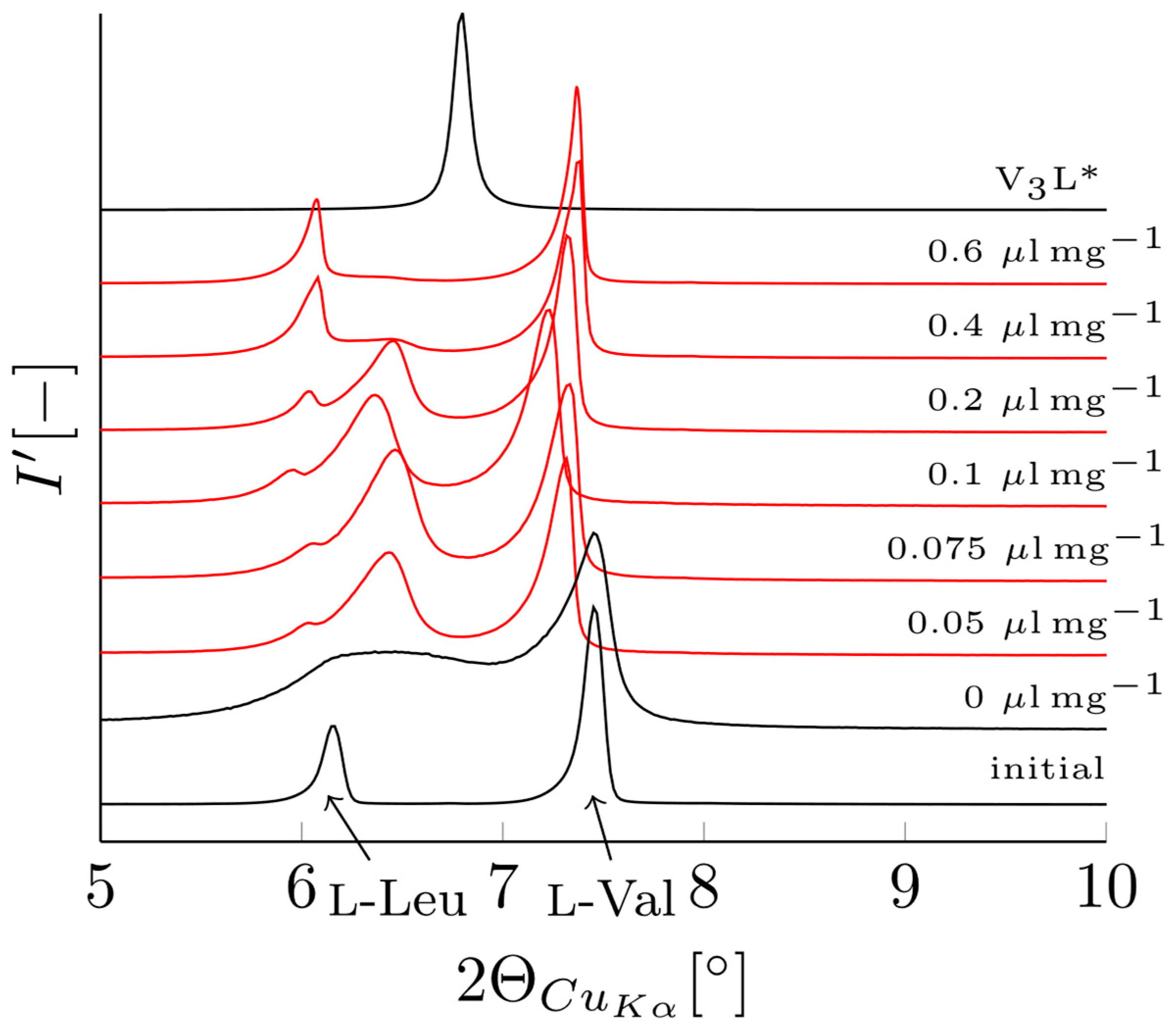
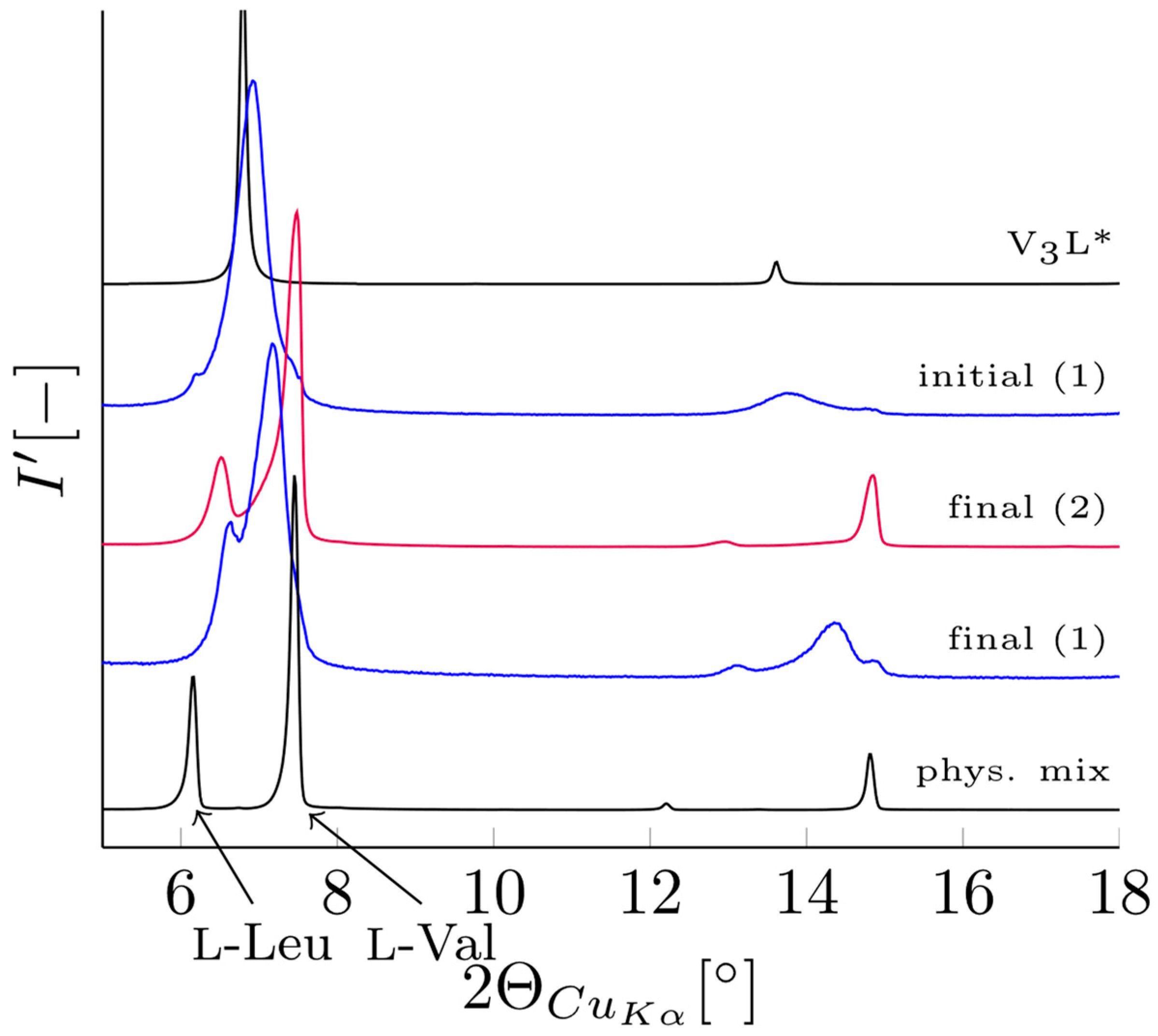
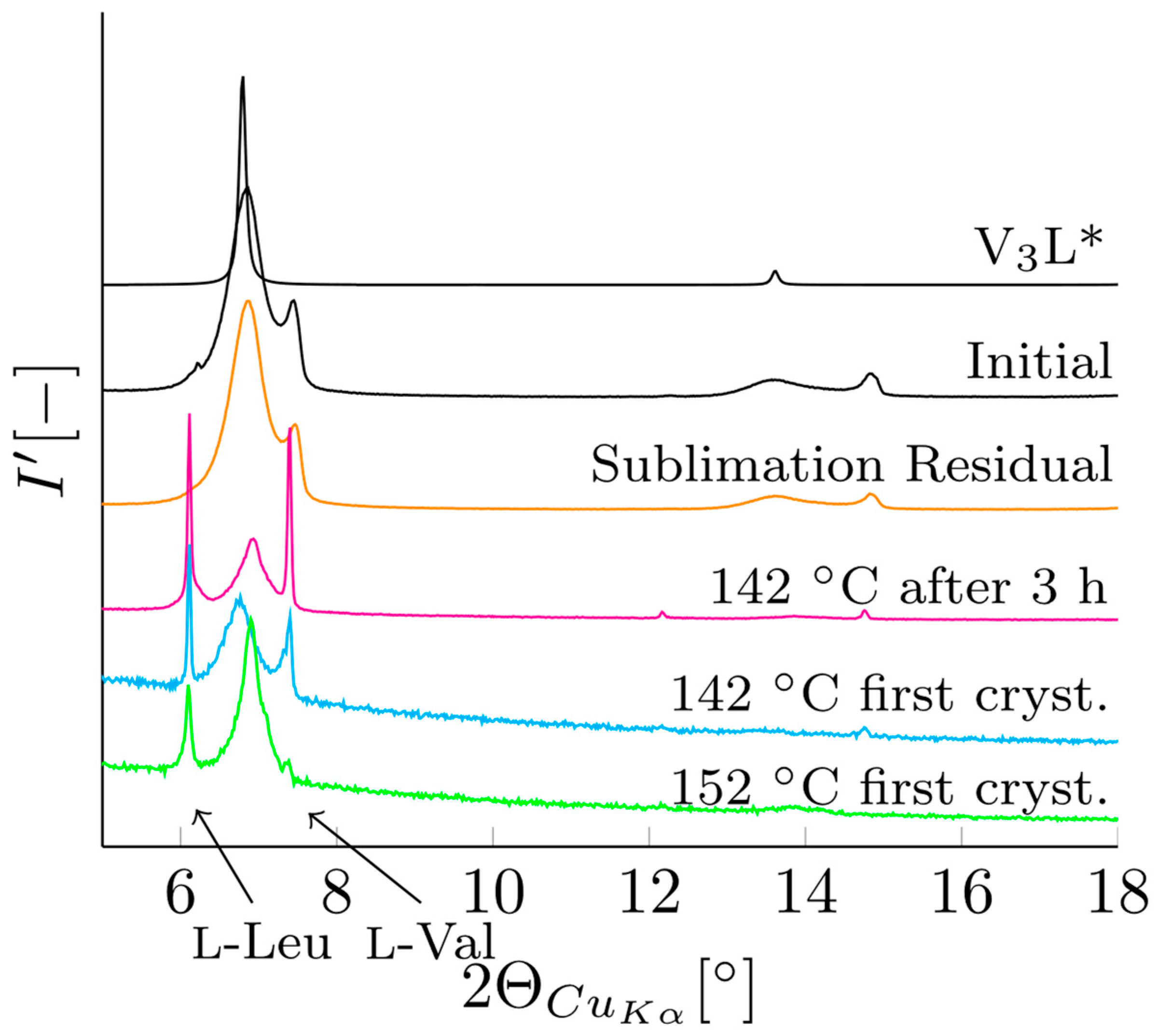
3.2. Verification of V3L Phase Behavior via Mechanochemistry, Slurry, and Sublimation Studies
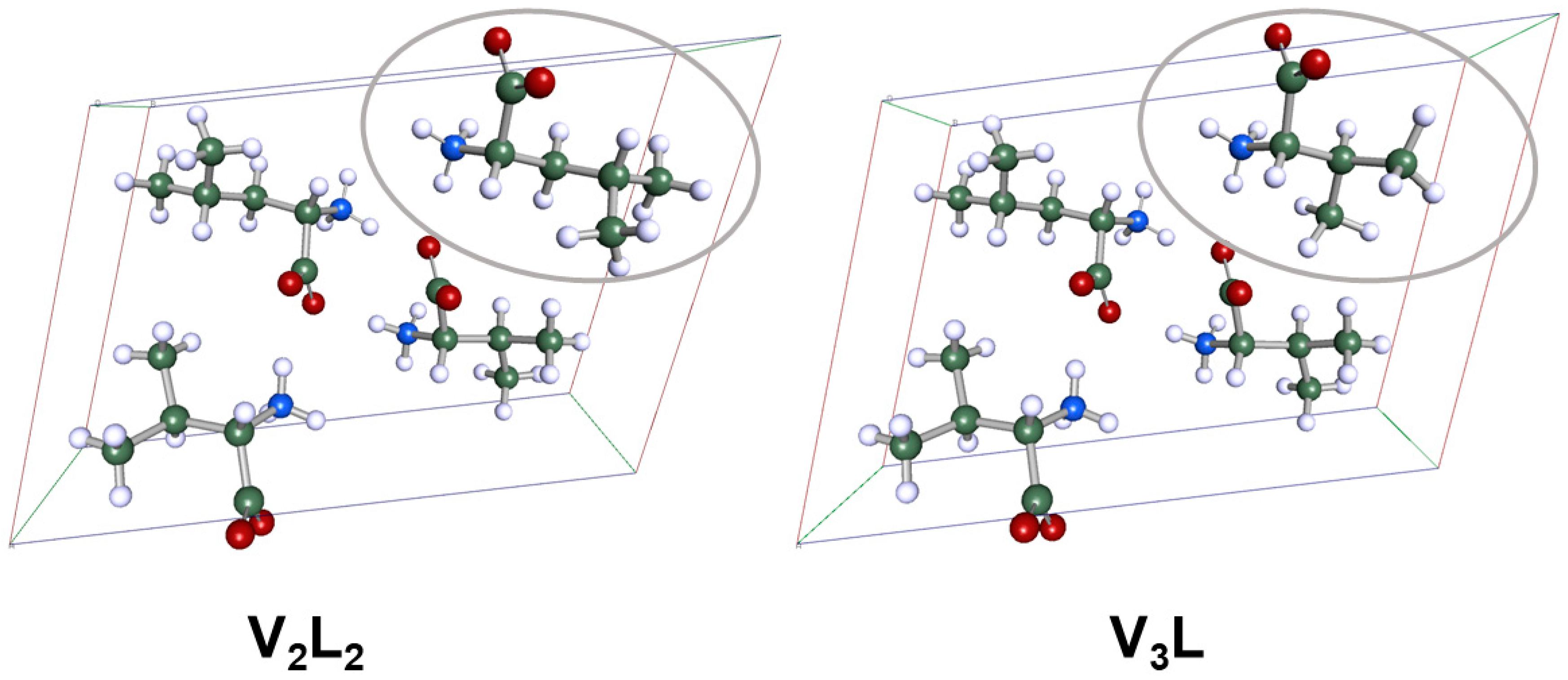
3.3. Intermolecular Interactions in the Crystals of the l-Val/l-Leu System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myerson, S. Handbook of Industrial Crystallization; Butterworth Heinemann: Oxford, UK, 2002. [Google Scholar]
- Kotelnikova, E.N.; Isakov, A.I.; Lorenz, H. Non-equimolar discrete compounds in binary chiral systems of organic substances. CrystEngComm 2017, 19, 1851–1869. [Google Scholar] [CrossRef]
- Hilfiker, R. Polymorphism of Crystalline, Systems. In Crystallization: Basic Concepts and Industrial Applications; Beckmann, W., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 85–103. [Google Scholar]
- Lusi, M. A rough guide to molecular solid solutions: Design, synthesis and characterization of mixed crystals. CrystEngComm 2018, 20, 7042–7052. [Google Scholar] [CrossRef]
- Kitamura, M. Polymorphism in the crystallization of L-glutamic acid. J. Cryst. Growth 1989, 96, 541–546. [Google Scholar] [CrossRef]
- Gónzalez, L.J.; Shimizu, T.; Satomi, Y.; Betancourt, L.; Besada, V.; Padrón, G.; Orlando, R.; Shirasawa, T.; Shimonishi, Y.; Takao, T. Differentiating α- and β-aspartic acids by electrospray ionization and low-energy tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2000, 14, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Sadovnichii, R.; Kotelnikova, E.; Lorenz, H. Thermal Deformations of Crystal Structures in the L-Aspartic/L-Glutamic Acid System and DL-Aspartic Acid. Crystals 2021, 11, 1102. [Google Scholar] [CrossRef]
- Isakov, I.; Lorenz, H.; Zolotarev, A.A., Jr.; Kotelnikova, E.N. Heteromolecular compounds in binary systems of amino acids with opposite and same chiralities. CrystEngComm 2020, 22, 986–997. [Google Scholar] [CrossRef]
- Sadeghi, M.; Tenberg, V.; Münzberg, S.; Lorenz, H.; Seidel-Morgenstern, A. Phase Equilibria of L-Valine/L-Leucine Solid Solutions. J. Mol. Liq. 2021, 340, 117315. [Google Scholar] [CrossRef]
- Tenberg, V.; Sadeghi, M.; Seidel-Morgenstern, A.; Lorenz, H. Bypassing thermodynamic limitations in the crystallization-based separation of solid solutions. Sep. Purif. Technol. 2022, 283, 120169. [Google Scholar] [CrossRef]
- Tenberg, V.; Hokmabadi, M.; Seidel-Morgenstern, A.; Lorenz, H.; Sadeghi, M. Investigation of the Antisolvent Effect on the Phase Behavior of Amino Acid Solid Solutions. Ind. Eng. Chem. Res. 2023, 62, 753–761. [Google Scholar] [CrossRef]
- Daltrup, J.-B.G.; Held, C.; Ruether, F.; Schembecker, G.; Sadowski, G. Measurement and Modeling Solubility of Aqueous Multisolute Amino-Acid Solutions. Ind. Eng. Chem. Res. 2010, 49, 1395–1401. [Google Scholar] [CrossRef]
- Kurosawa, I.; Teja, A.S.; Rousseau, R.W. Solid-liquid equilibria in L-leucine + L-valine + water. Fluid Phase Equilibr. 2005, 228–229, 83–87. [Google Scholar] [CrossRef]
- Viedma, C.; Ortiz, J.E.; de Torres, T.; Cintas, P. Enantioenrichment in sublimed amino acid mixtures. Chem. Commun. 2012, 48, 3623–3625. [Google Scholar] [CrossRef] [PubMed]
- Reilly, A.M.; Tkatchenko, A. Understanding the role of vibrations, exact exchange, and many-body van der Waals interactions in the cohesive properties of molecular crystals. J. Chem. Phys. 2013, 139, 024705. [Google Scholar] [CrossRef]
- Dolgonos, G.A.; Hoja, J.; Boese, A.D. Revised values for the X23 benchmark set of molecular crystals. Phys. Chem. Chem. Phys. 2019, 44, 24333–24344. [Google Scholar] [CrossRef] [PubMed]
- Otero-de-la-Roza, A.; Johnson, E.R. A benchmark for non-covalent interactions in solids. J. Chem. Phys. 2012, 137, 054103. [Google Scholar] [CrossRef]
- Buchholz, H.K.; Stein, M. Accurate lattice energies of organic molecular crystals from periodic turbomole calculations. J. Comput. Chem. 2018, 39, 1335–1343. [Google Scholar] [CrossRef]
- Stein, M.; Heimsaat, M. Intermolecular Interactions in Molecular Organic Crystals upon Relaxation of Lattice Parameters. Crystals 2019, 9, 665. [Google Scholar] [CrossRef]
- Otero-de-la-Roza, A.; Cao, B.H.; Price, I.K.; Hein, J.E.; Johnson, E.R. Predicting the Relative Solubilities of Racemic and Enantiopure Crystals by Density-Functional Theory. Angew. Chem. Int. Ed. 2014, 53, 7879–7882. [Google Scholar] [CrossRef] [PubMed]
- Geatches, D.; Rosbottom, I.; Robinson, R.L.M.; Byrne, P.; Hasnip, P.; Probert, M.I.J.; Jochym, D.; Maloney, A.; Roberts, K.J. Off-the-shelf DFT-DISPersion methods: Are they now “on-trend” for organic molecular crystals. J. Chem. Phys. 2019, 151, 044106. [Google Scholar] [CrossRef]
- Isakov, A.I.; Kotelnikova, E.N.; Münzberg, S.; Bocharov, S.N.; Lorenz, H. Solid Phases in the System L-Valine—L-Isoleucine. Cryst. Growth Des. 2016, 16, 2653–2661. [Google Scholar] [CrossRef]
- Raza, S.A.; Schacht, U.; Svoboda, V.; Edwards, D.P.; Florence, A.J.; Pulham, C.R.; Sefcik, J.; Oswald, I.D.H. Rapid Continuous Antisolvent Crystallization of Multicomponent Systems. Cryst. Growth Des. 2018, 18, 210–218. [Google Scholar]
- Viedma, C.; Noorduin, W.L.; Oritz, J.E.; de Torres, T.; Cintas, P. Asymmetric amplification in amino acid sublimation involving racemic compound to conglomerate conversion. Chem. Commun. 2010, 47, 671–673. [Google Scholar]
- Lähde, A.; Raula, J.; Malm, J.; Kauppinen, E.I.; Karpinen, M. Sublimation and vapour pressure estimation of L-leucine using thermogravimetric analysis. Thermochim. Acta 2009, 482, 17–20. [Google Scholar]
- Glavin, P.; Bada, J.L. Isolation of Amino Acids from Natural Samples Using Sublimation. Anal. Chem. 1998, 70, 3119–3122. [Google Scholar] [PubMed]
- TURBOMOLE V7.5 2019, University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, since 2007. Available online: https://www.turbomole.com (accessed on 24 October 2023).
- Furche, F.; Ahlrichs, R.; Hattig, C.; Klopper, W.; Sierka, M.; Weigend, F. Turbomole. Interdiscip. Rev. Comput. Mol. Sci. 2014, 4, 91–100. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction DFT-D for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar]
- Tao, J.M.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the density functional ladder: Nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar]
- Wigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for h to rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate coulomb potentials. Theor. Chem. Acc. 1997, 97, 119–124. [Google Scholar]
- Eichkorn, K.; Treutler, O.; Ohm, H.; Haser, M.; Ahlrichs, R. Auxiliary basis-sets to approximate coulomb potentials. Chem. Phys. Lett. 1995, 240, 283–290, Erratum in Chem. Phys. Lett. 1995, 242, 652–660. [Google Scholar] [CrossRef]
- Łarzarski, R.; Burow, A.M.; Grajciar, L.; Sierka, M. Density functional therory for molecular and periodic systems using density fitting and continuous fast multipole method: Analytical gradients. J. Comput. Chem. 2016, 37, 2518–2526. [Google Scholar] [CrossRef]
- Łazarski, R.; Burow, A.M.; Sierka, M. Density functional theory for molecular and periodic systems using density fitting and continuous fast multipole methods. J. Chem. Theory Comput. 2015, 11, 3029–3041. [Google Scholar]
- Grajciar, L. Low-memory iterative density fitting. J. Comput. Chem. 2015, 36, 1521–1535. [Google Scholar] [PubMed]
- Kotelnikova, E.; Isakov, A.; Lorenz, H. Thermal deformations of crystal structures formed in the systems of malic acid enantiomers and L-valine-L-isoleucine enantiomers. CrystEngComm 2018, 20, 2562–2572. [Google Scholar]
- Prieto, M. Thermodynamics of Solid Solution-Aqueous Solution Systems. Rev. Mineral. Geochem. 2009, 70, 47–85. [Google Scholar]
- Dorofeeva, O.V.; Ryzhova, O.N. Gas-phase enthalpies of formation and enthalpies of sublimation of amino acids based on isodesmic reaction calculations. J. Phys. Chem. A 2014, 118, 3490–3502. [Google Scholar] [PubMed]




| B97-D3/def2-TZVPP | TPSS-D3/def2TZVPP | Exp. | |
|---|---|---|---|
| l-Leucine | −176 | −158 | −145 |
| l-Valine | −155 | −154 | −144 |
| V2L2 | −160 | −151 | |
| V3L | −155 | −150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenberg, V.; Stein, M.; Lorenz, H. Solid Phase and Stability Investigation of a Co-Crystal in the l-Valine/l-Leucine System. Crystals 2023, 13, 1542. https://doi.org/10.3390/cryst13111542
Tenberg V, Stein M, Lorenz H. Solid Phase and Stability Investigation of a Co-Crystal in the l-Valine/l-Leucine System. Crystals. 2023; 13(11):1542. https://doi.org/10.3390/cryst13111542
Chicago/Turabian StyleTenberg, Vico, Matthias Stein, and Heike Lorenz. 2023. "Solid Phase and Stability Investigation of a Co-Crystal in the l-Valine/l-Leucine System" Crystals 13, no. 11: 1542. https://doi.org/10.3390/cryst13111542
APA StyleTenberg, V., Stein, M., & Lorenz, H. (2023). Solid Phase and Stability Investigation of a Co-Crystal in the l-Valine/l-Leucine System. Crystals, 13(11), 1542. https://doi.org/10.3390/cryst13111542








