The Ultrasound-Assisted Preparation of Crystal Seeds for the Hydrolysis of TiOSO4 to H2TiO3
Abstract
:1. Introduction
2. Experiment
2.1. Materials
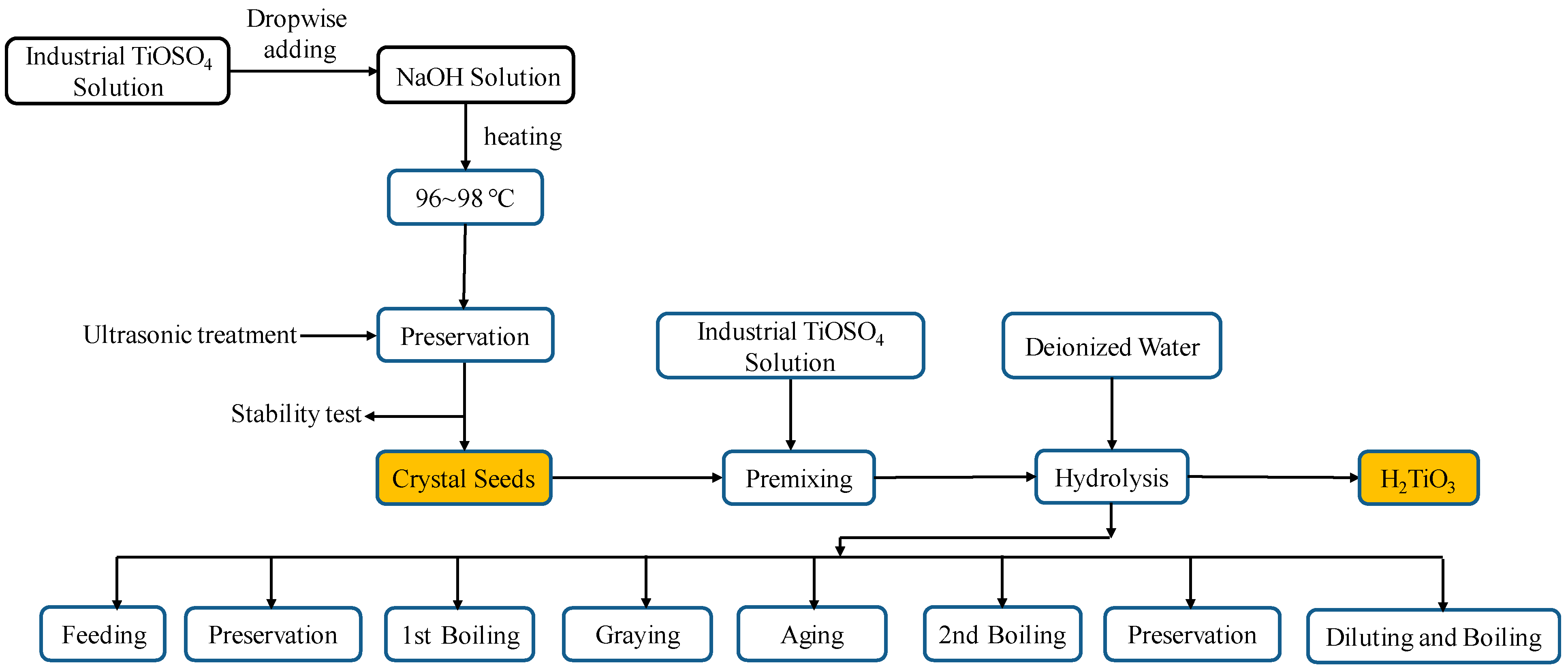
2.2. Preparation of Hydrolytic Crystal Seeds
2.3. Hydrolysis of TiOSO4 to H2TiO3
2.4. Characterization
3. Results and Discussion
3.1. Optimum Process Conditions for Preparing Hydrolytic Crystal Seeds
3.1.1. NaOH/TiO2 Ratio
3.1.2. F Value
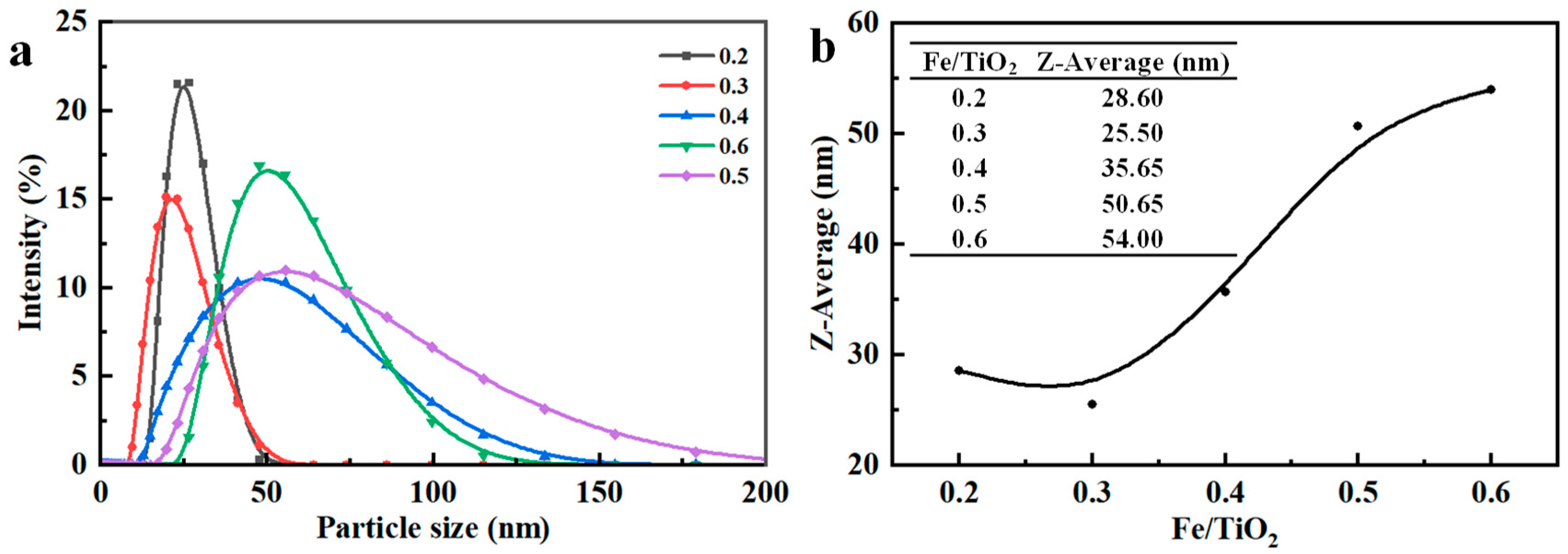
3.1.3. Fe/TiO2 Value
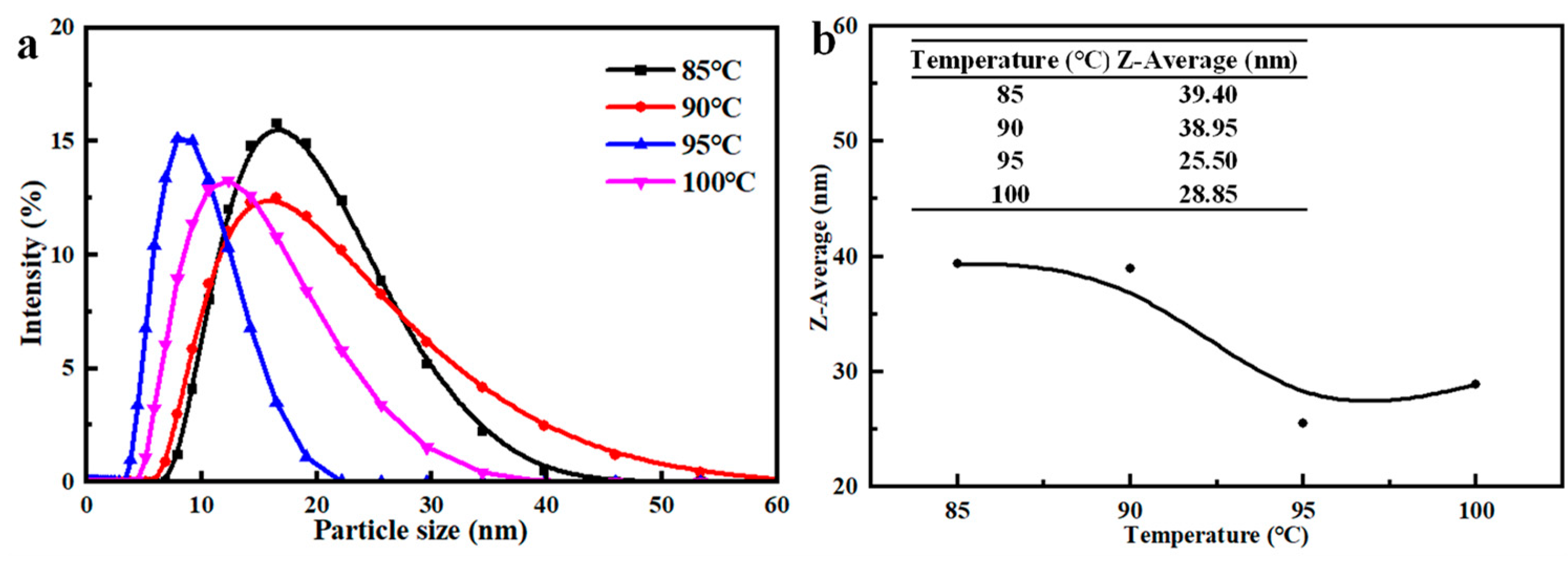
3.1.4. Reaction Temperature
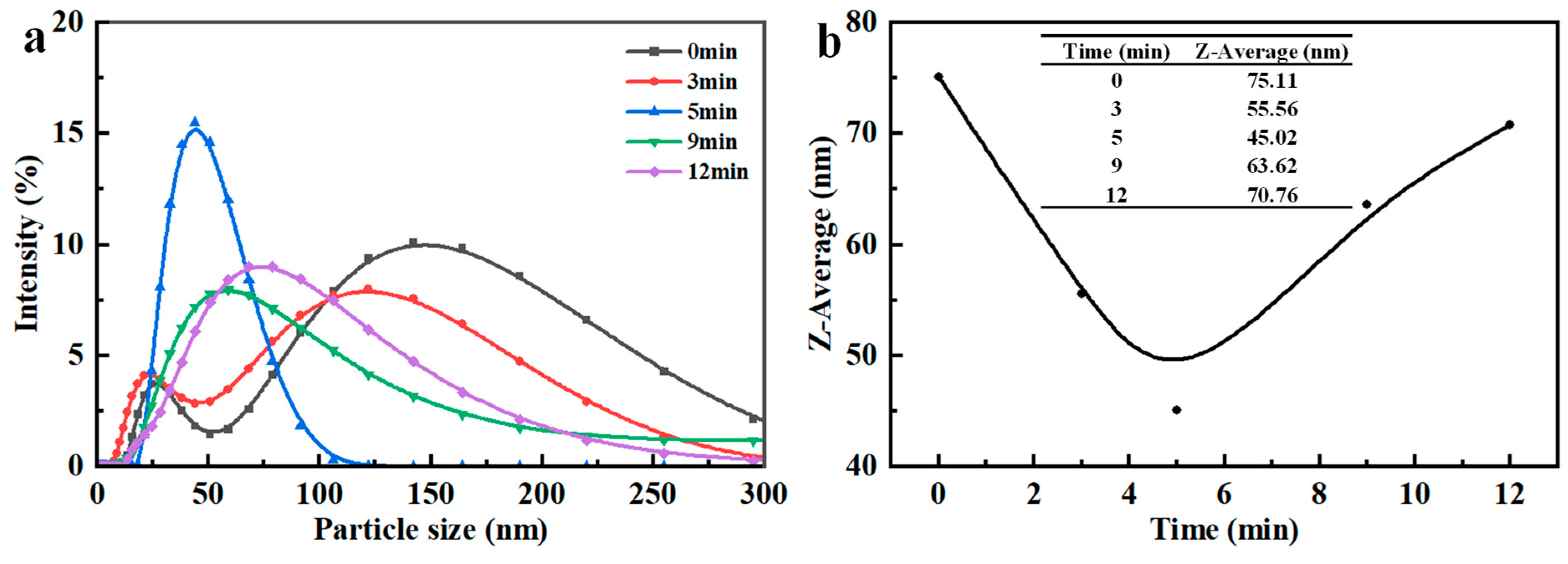
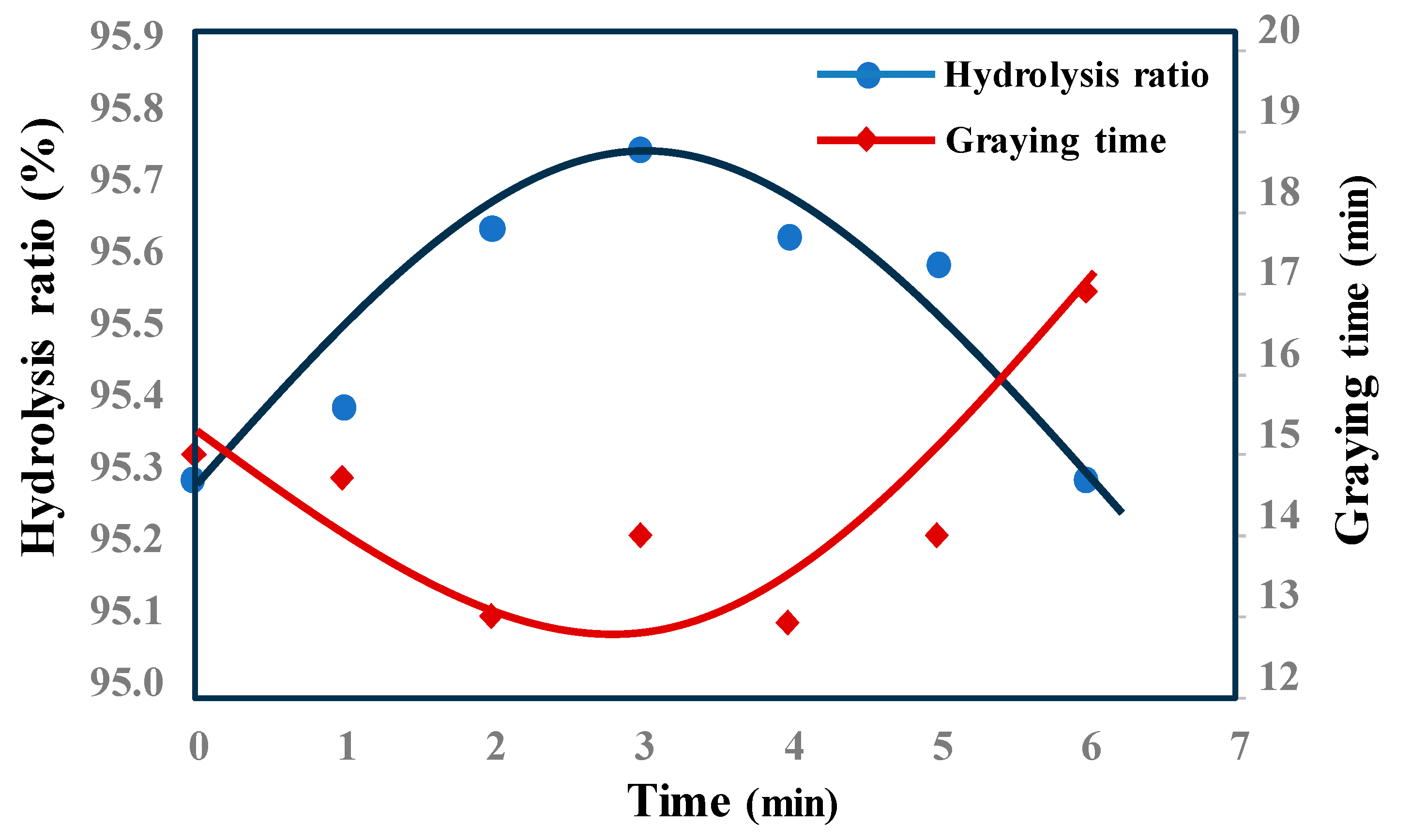
3.1.5. Hydrolysis Time
3.2. Ultrasonic-Assisted Preparation of Hydrolytic Seeds
3.2.1. Effect of Ultrasonic Time
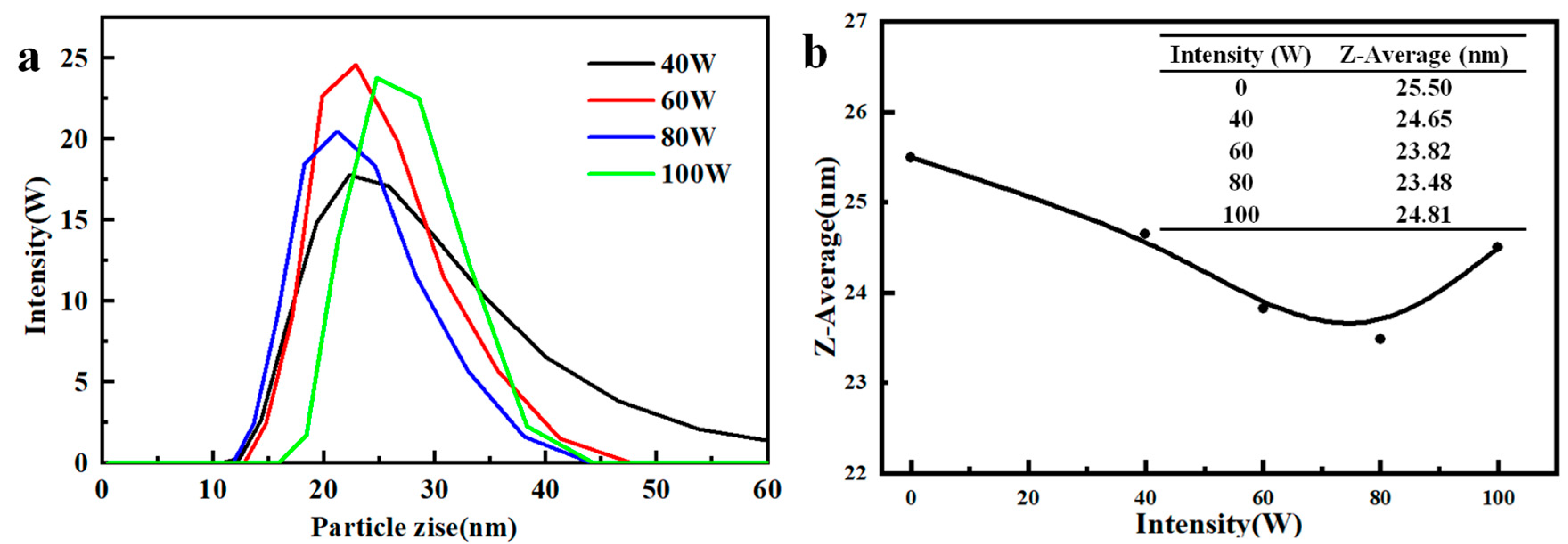
3.2.2. Effect of Ultrasonic Intensity
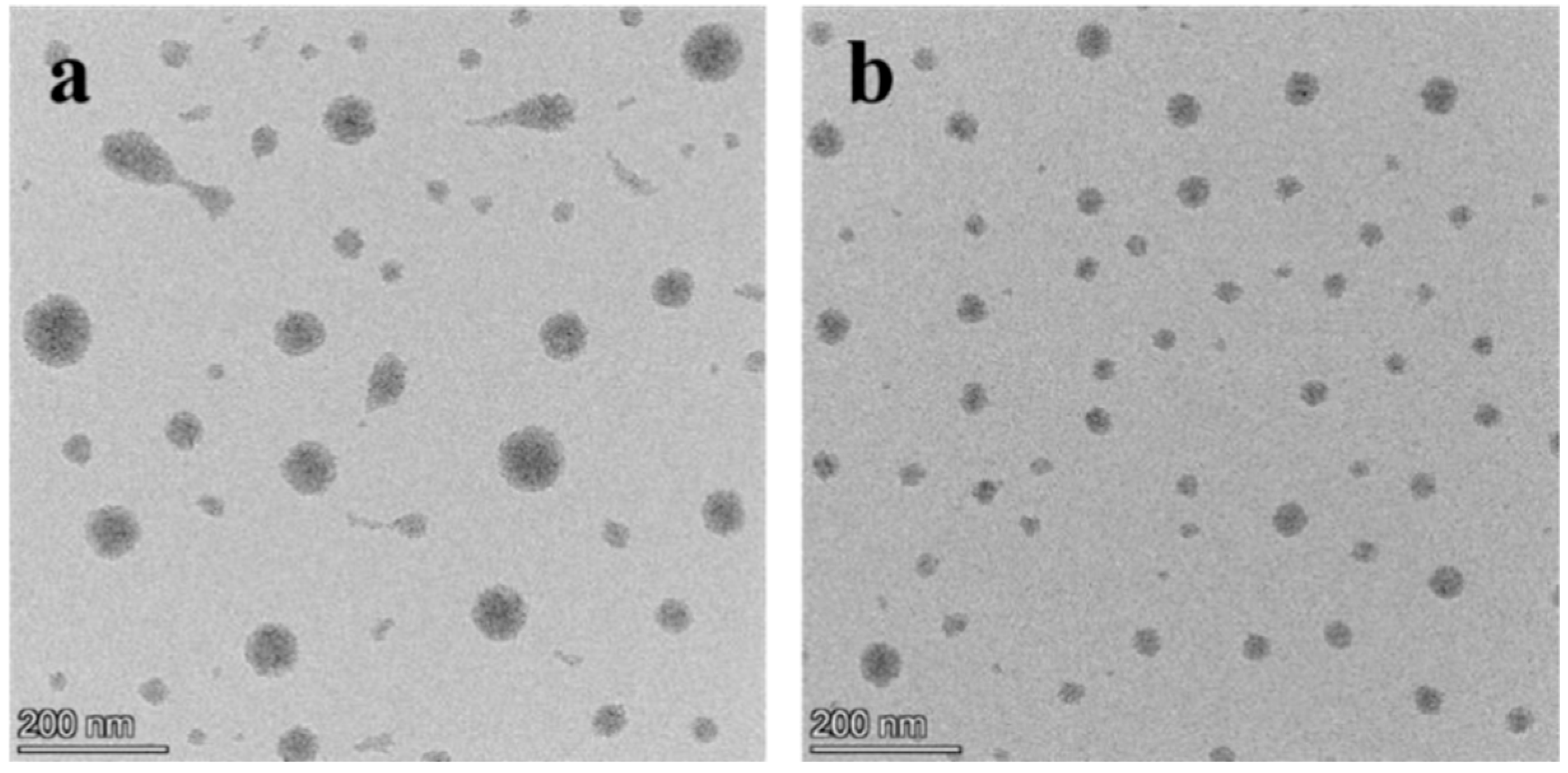
3.2.3. Morphology and Structure of Crystal Seeds
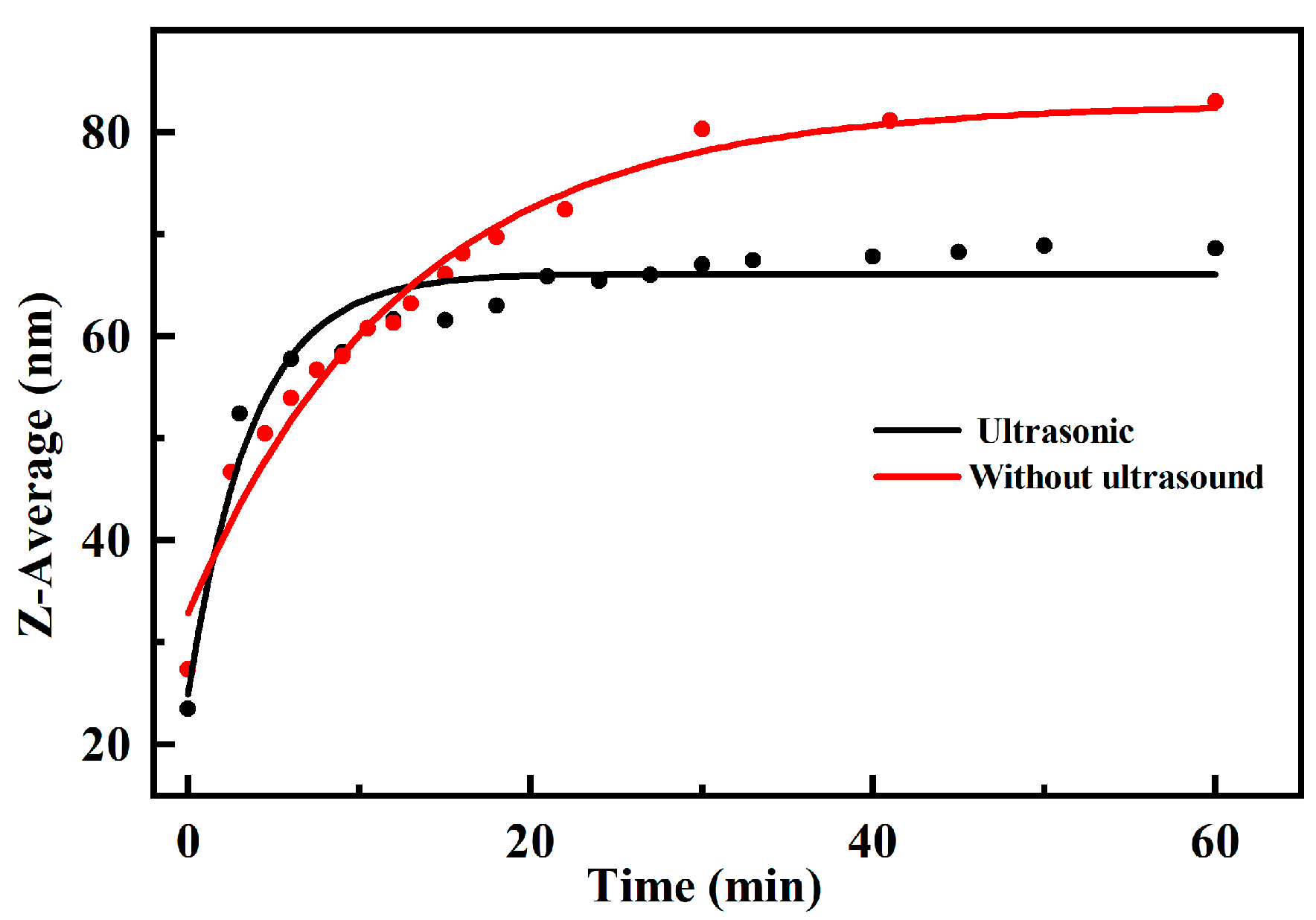
3.3. Growth Kinetics of Hydrolytic Crystal Seeds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haider, A.; Jameel, Z.; Al-Hussaini, I. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Ali, I.; Suhai, M.; Alothman, Z.; Alwarthan, A. Recent advances in syntheses, properties and applications of TiO2 nanostructures. RSC Adv. 2018, 8, 30125–30147. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, T.; Juan, J. An application of ultrasound technology in synthesis of titania-based photocatalyst for degrading pollutant. Chem. Eng. J. 2017, 317, 586–612. [Google Scholar]
- Zhang, W.; Ou, C.; Yuan, Z. Precipitation and growth behaviour of metatitanic acid partibles from titanium sulfate solution. Powder Technol. 2017, 315, 31–36. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Xue, T.; Li, J.; Chen, D.; Qi, T. Mechanism and kinetics of titanium hydrolysis in concentrated titanyl sulfate solution based on infrared and Raman spectra. Chem. Eng. Sci. 2015, 134, 196–204. [Google Scholar] [CrossRef]
- Wang, K.; Lu, R.; Dang, L.; Hao, L.; Wei, H. Online characterization of seed preparation and investigation on hydrolysis kinetics of TiOSO4 solution. Chem. Ind. Eng. 2022, 39, 50–56. (In Chinese) [Google Scholar]
- Hua, Q.; Lin, W. The Influence of crystal seeds on the particle size distribution of hydrolysis product of titanyl sulfate. Adv. Mater. Res. 2012, 396, 2042–2045. [Google Scholar] [CrossRef]
- Sathyamoorthy, S.; Moggridge, G.D.; Hounslow, M.J. Particle Formation during Anatase Precipitation of Seeded Titanyl Sulfate Solution. Cryst. Growth Des. 2001, 1, 123–129. [Google Scholar] [CrossRef]
- Sathyamoorthy, S.; Moggridge, G.D.; Hounslow, M.J. Controlling particle size during anatase precipitation. AIChE J. 2001, 47, 2012–2024. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, S.; Yuan, S.; Yue, H.; Liu, C.; Li, C.; Liang, B. Rutile TiO2 production by inductive hydrolysis of low concentration titaniferous solution with seed crystals prepared by microwave. Iron Steel Vanadium Titan. 2016, 37, 29–34. (In Chinese) [Google Scholar]
- Li, S.; Ma, K.; Tang, S.; Liu, C.; Yue, H.; Liang, B. Preparation of seed crystals by microwave assisted heating for the hydrolysis of the high concentration of titanium sulfate solution. Appl. Chem. Ind. 2019, 48, 2276–2280. [Google Scholar]
- Tang, S.; Zhang, Y.; Yuan, S.; Yue, H.; Liu, C.; Liu, C.; Li, C.; Liang, B. Microwave-assisted seed preparation for producing easily phase-transformed anatase to rutile. RSC Adv. 2017, 7, 45607–45614. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, K.; Zhu, J.; Zhou, X.; Lin, F. Influence of crystal seeds on hydrolysis process of rutile titanium dioxide and its brightness. Inorg. Chem. Ind. 2019, 51, 30–34. [Google Scholar]
- Ma, K.; Li, S.; Liao, L.; Tang, S.; Liu, C.; Yue, H.; Liang, B. Fabricating Dual-Activity Seed Crystals via Continuous Rapid Thermal Nucleation in a Coil Reactor for Low-Temperature Rutile TiO2 Production. ACS Sustain. Chem. Eng. 2021, 9, 9106–9113. [Google Scholar] [CrossRef]
- Kim, S.; Chang, T.; Shin, C. Enhancing effects of ultrasound treatment on the preparation of TiO2 Photocatalysts. Catal. Lett. 2007, 118, 224–230. [Google Scholar] [CrossRef]
- Xie, C.; Qiu, T.; Lu, H.; Yang, L. Morphological changes of calcium carbonate crystals under ultrasound action. J. South China Univ. Technol. 2007, 35, 62–65. (In Chinese) [Google Scholar]
- Amara, N.; Ratslmba, B.; Wilhelm, A.M.; Delmas, H. Crystallization of potash alum: Effect of power ultrasound. Ultrason. Sonochem. 2001, 8, 265–270. [Google Scholar] [CrossRef]
- Wu, Z.; Yin, Z.; Chen, Q.; Zhang, M.; Li, J. The Influence Mechanism of ultrasound effect on the crystallization process of ammonium molybdate in aqueous solution. Chin. J. Process Eng. 2002, 20, 27–30. (In Chinese) [Google Scholar]
- Lu, R.; Liu, C.; Wu, J.; Sun, W.; Sun, Q.; Dong, L. Process optimization of the extra-adding seeded hydrolysis of TiOSO4 to H2TiO3 by using the unenriched solution for the manufacture of TiO2 pigment. J. Cryst. Growth 2021, 572, 126268. [Google Scholar] [CrossRef]
- Lu, R.; Liu, C.; Wu, J.; Wu, Y.; Zhang, Q.; Sun, Q. Investigation on the structure evolution of rutile TiO2 during calcination of mixed-salt-treated metatitanic acid. J. Cryst. Growth 2023, 602, 126985. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Z.; Wang, J.; Guo, W.; Li, G. Degradation of reactive brilliant red in aqueous solution by ultrasonic cavitation. Ultrason. Sonochem. 2008, 15, 43–48. [Google Scholar] [CrossRef]
- Hanaor, D.; Sorrell, C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Hao, L.; Wei, H. On-line investigation of anatase precipitaiton from titianyl sulphate solution. Chem. Eng. Res. Des. 2010, 88, 1264–1271. [Google Scholar] [CrossRef]






| Total Titanium Content (g/L) | F Value | Fe/TiO2 | Ti3+ Content (g/L) |
|---|---|---|---|
| 175 | 1.88 | 0.30 | 0.59 |
| Ultrasonic Intensity (W) | Z-Average (nm) | D10 (nm) | D50 (nm) | D90 (nm) | Span |
|---|---|---|---|---|---|
| 40 | 479.7 | 311 | 402 | 522 | 0.525 |
| 60 | 420.8 | 287 | 371 | 487 | 0.539 |
| 80 | 410.6 | 278 | 359 | 458 | 0.501 |
| 100 | 431.4 | 291 | 377 | 498 | 0.549 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, R.; Li, F.; Li, X.; Dong, L. The Ultrasound-Assisted Preparation of Crystal Seeds for the Hydrolysis of TiOSO4 to H2TiO3. Crystals 2023, 13, 1553. https://doi.org/10.3390/cryst13111553
Lu R, Li F, Li X, Dong L. The Ultrasound-Assisted Preparation of Crystal Seeds for the Hydrolysis of TiOSO4 to H2TiO3. Crystals. 2023; 13(11):1553. https://doi.org/10.3390/cryst13111553
Chicago/Turabian StyleLu, Ruifang, Feifan Li, Xianglan Li, and Lichun Dong. 2023. "The Ultrasound-Assisted Preparation of Crystal Seeds for the Hydrolysis of TiOSO4 to H2TiO3" Crystals 13, no. 11: 1553. https://doi.org/10.3390/cryst13111553





