New Organic Crystalline Material Close to Nodal-Line Materials: α′-STF2IBr2
Abstract
:1. Introduction
2. Materials and Methods
3. Results
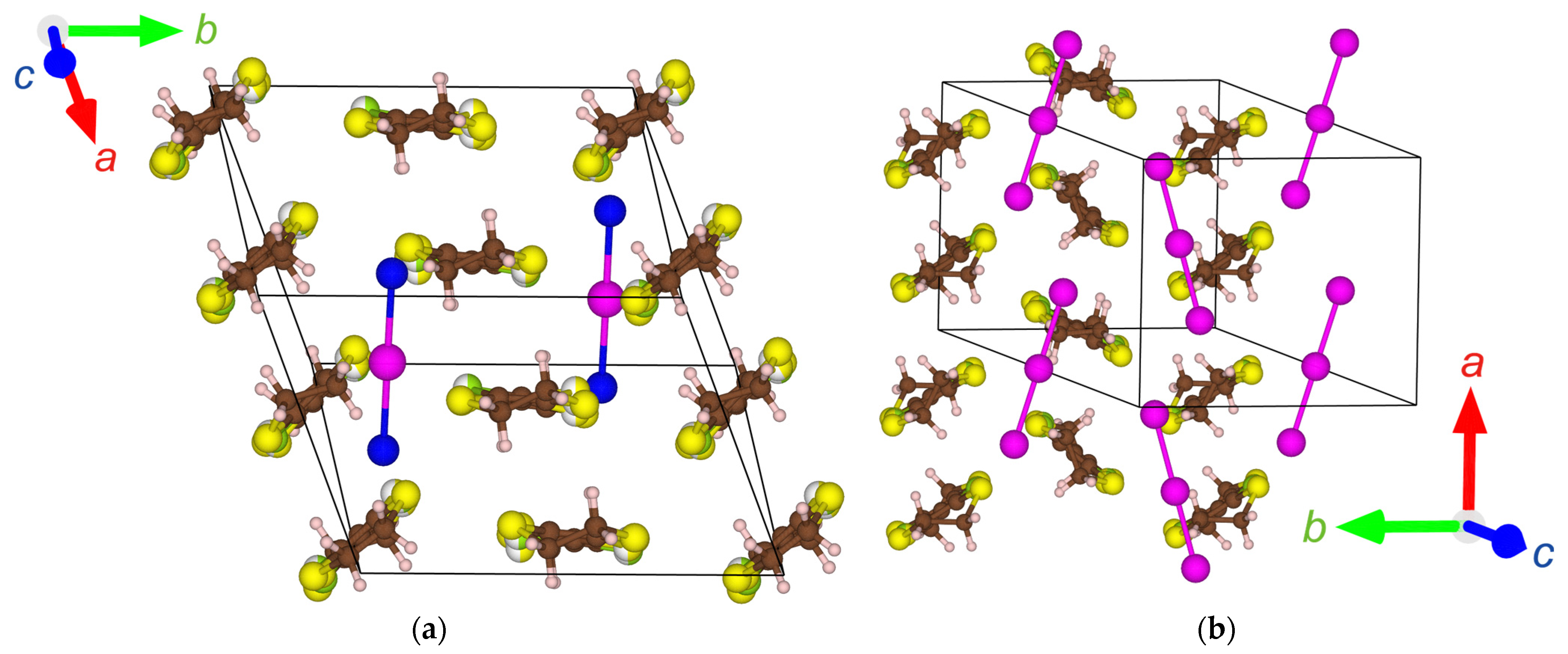
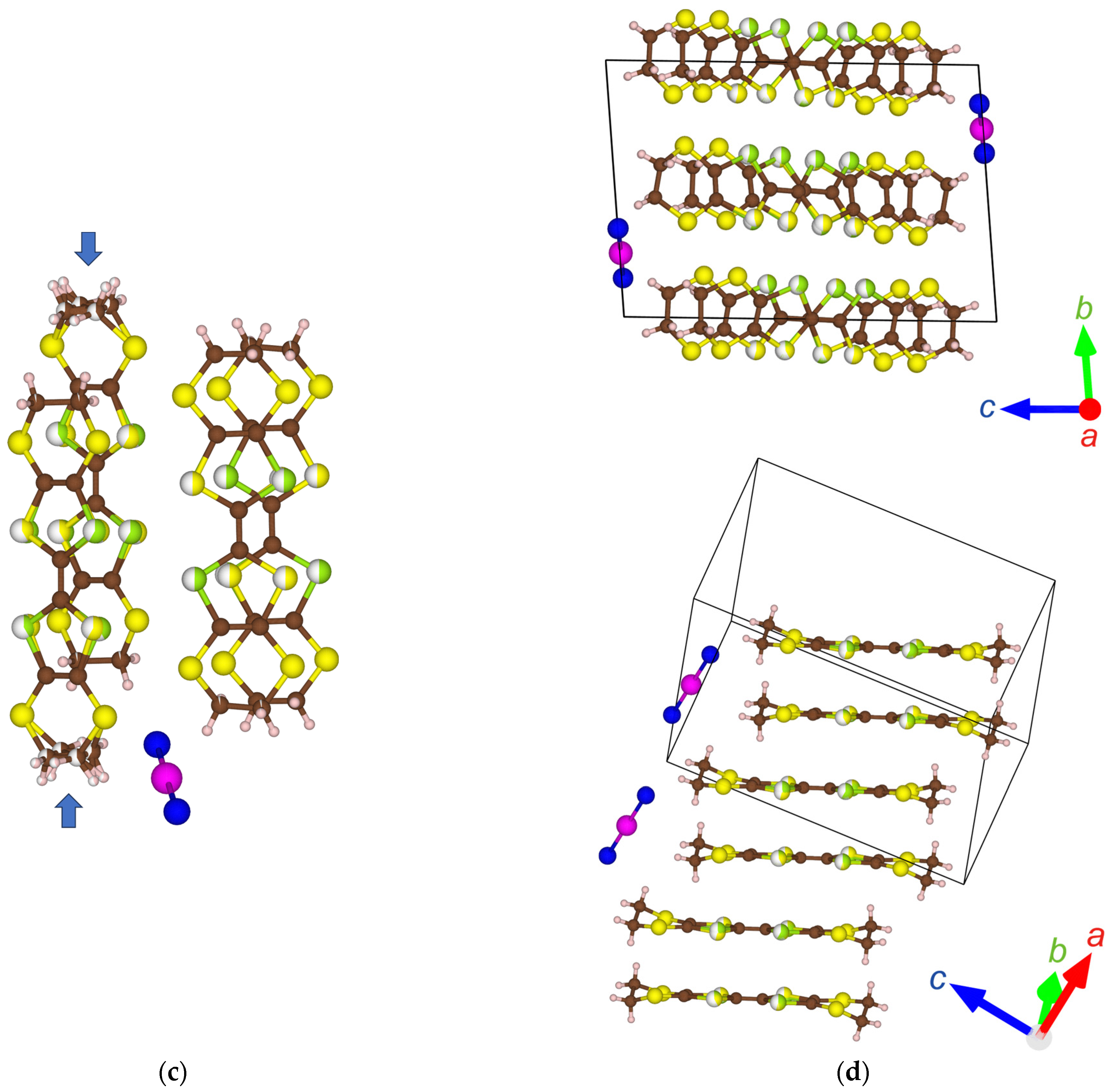
3.1. Stuructural Properties
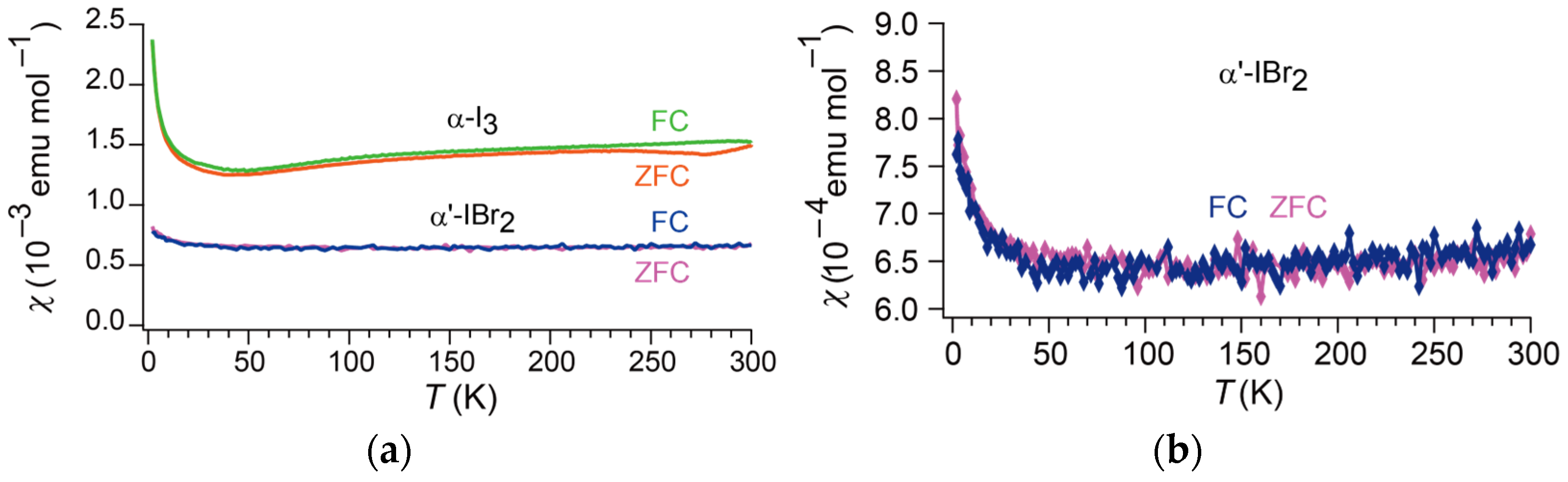
3.2. Electronic Properties
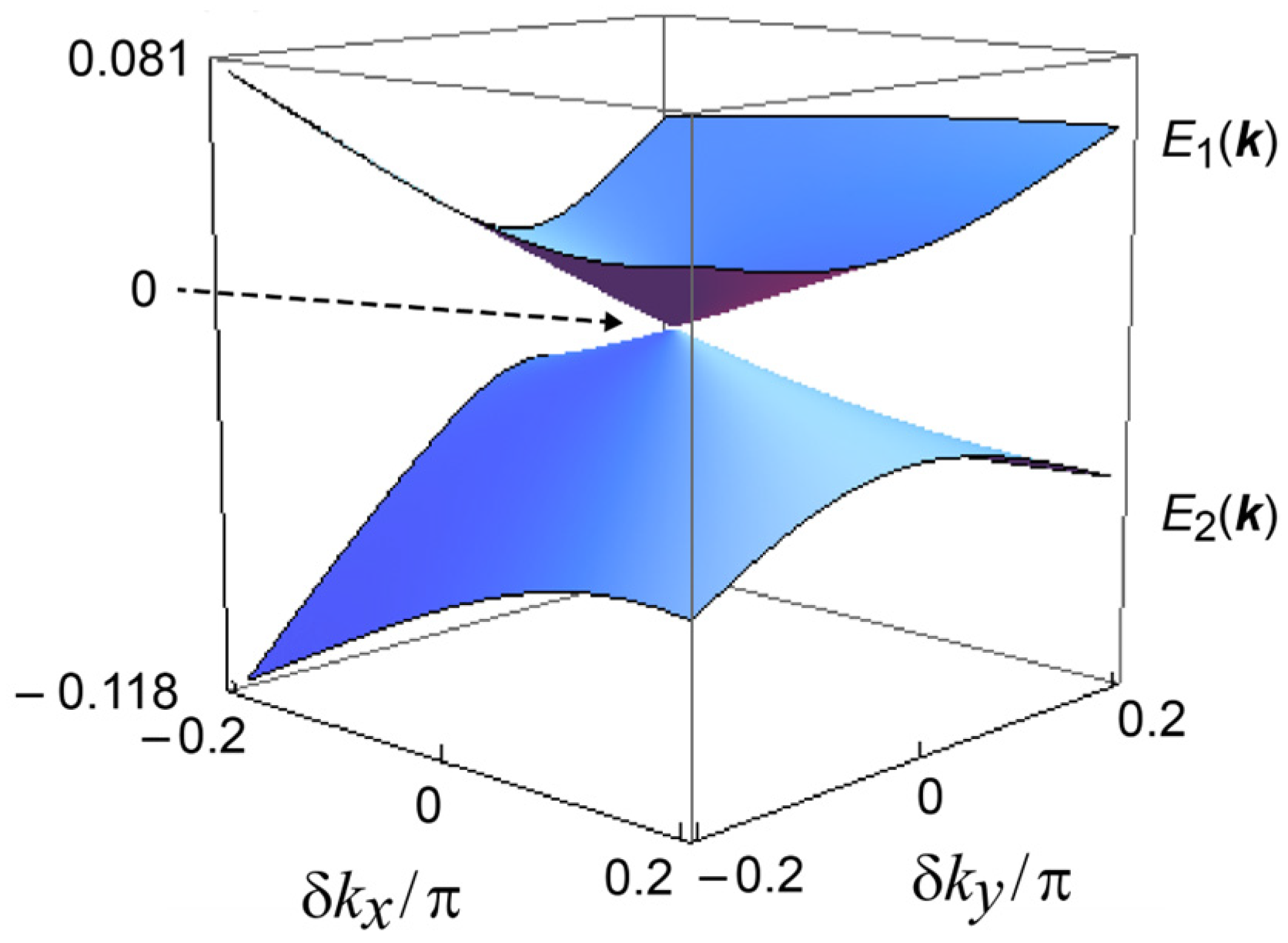
3.3. Calculated Band Structure
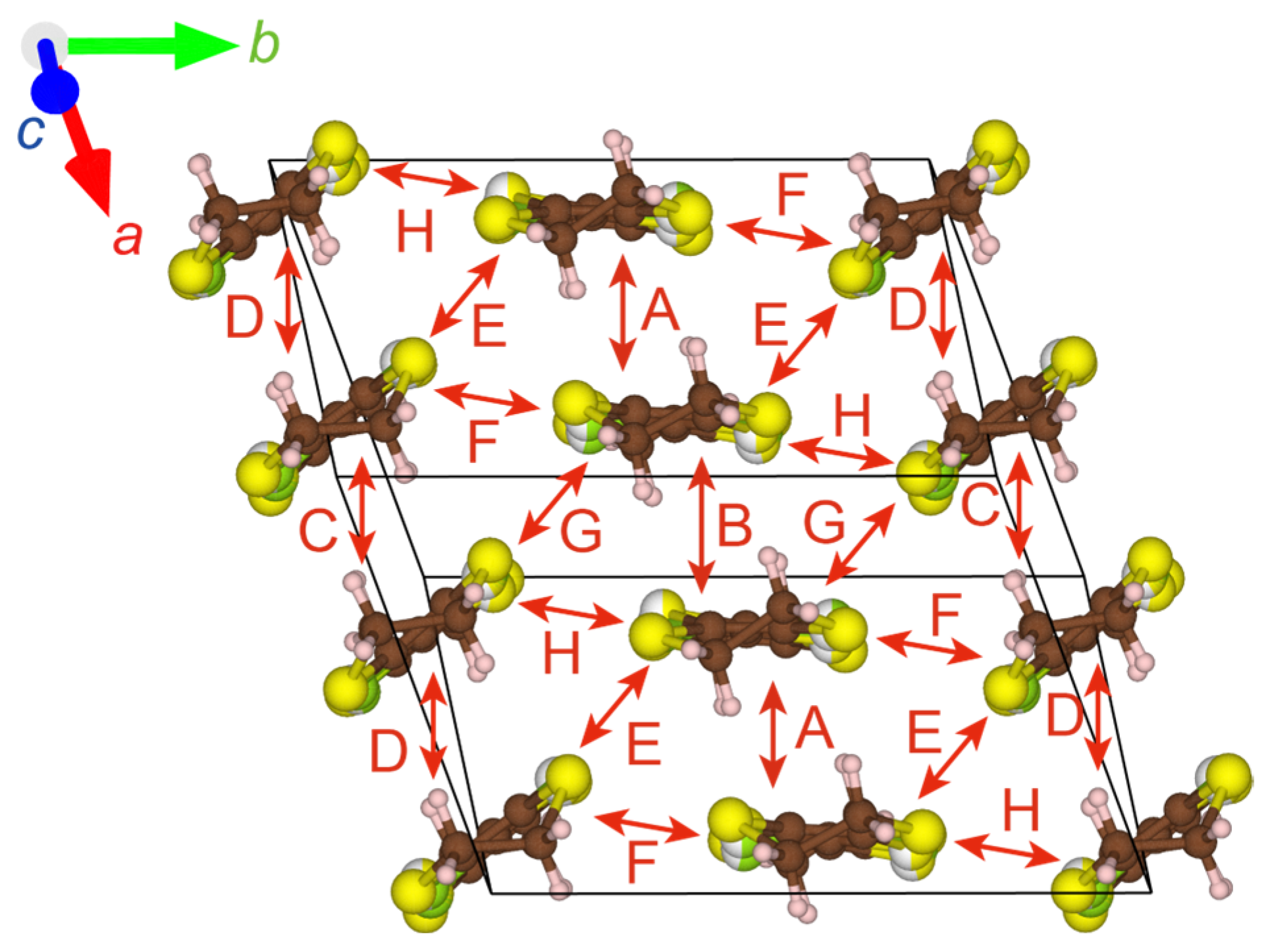
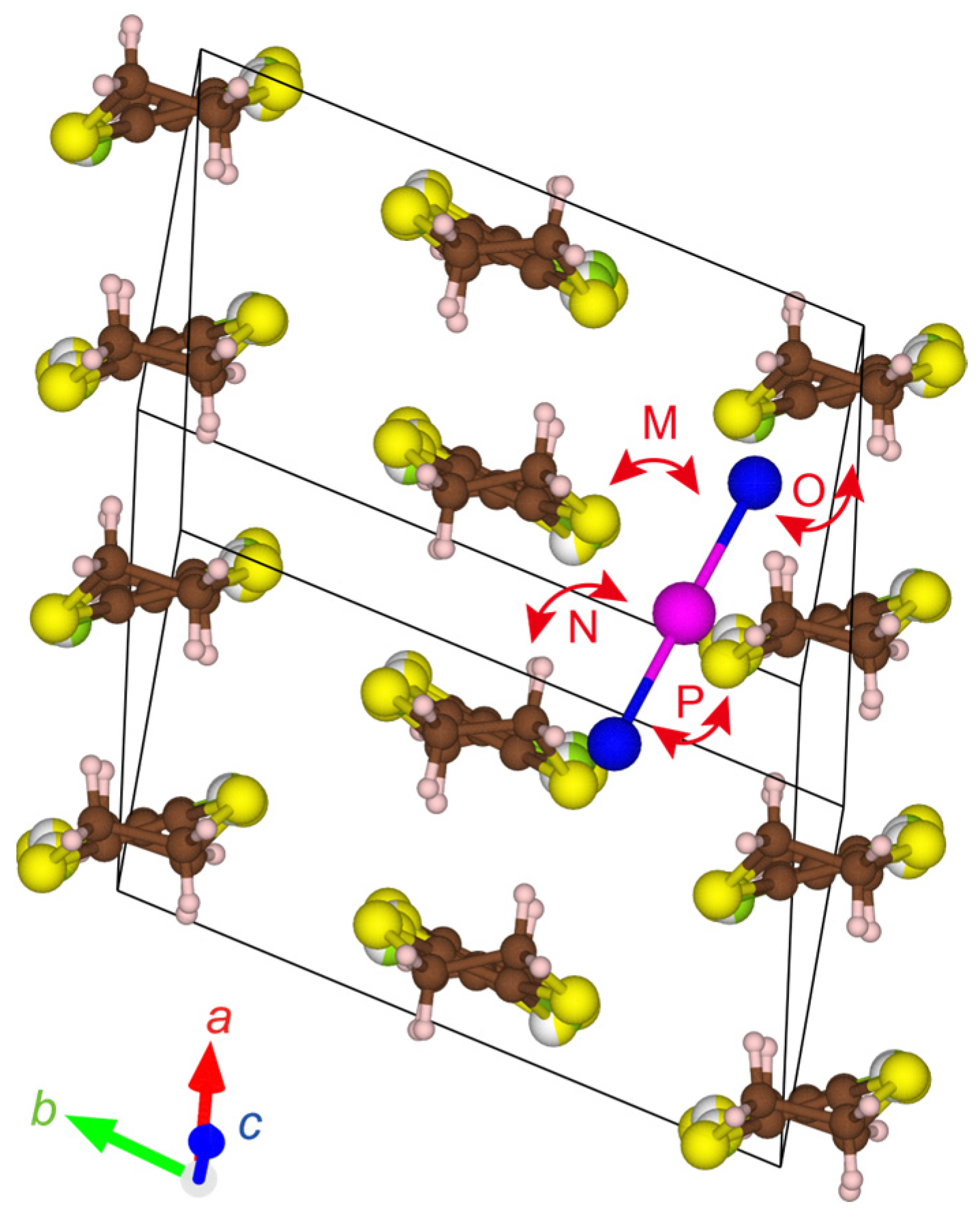
3.4. Calculated Intermolecular Interactions
3.4.1. STF–STF Interactions
3.4.2. IBr2–IBr2 and STF–IBr2 Interactions
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Yang, S.-Y.; Yang, H.; Derunova, E.; Parkin, S.S.P.; Yan, B.; Ali, M.N. Symmetry demanded topological nodal-line materials. Adv. Phys. X 2018, 3, 1414631. [Google Scholar] [CrossRef]
- Armitage, N.P.; Mele, E.J.; Vishwanath, A. Weyl and Dirac semimetals in three-dimensional solids. Rev. Mod. Phys. 2018, 90, 015001. [Google Scholar] [CrossRef]
- Hosen, M.M.; Dimitri, K.; Belopolski, I.; Maldonado, P.; Sanker, R.; Dhakal, N.; Dhakal, G.; Cole, T.; Oppeneer, P.M.; Kaczorowski, D.; et al. Tunability of the topological nodal-line semimetal phase in ZrSiX-type materials (X = S, Se, Te). Phys. Rev. B 2017, 95, 161101. [Google Scholar] [CrossRef]
- Bian, G.; Chang, T.-R.; Sanker, R.; Xu, S.-Y.; Zheng, H.; Neupert, T.; Chiu, C.-K.; Huang, S.-M.; Chang, G.; Belopolski, I.; et al. Topological nodal-line fermions in spin-orbit metal PbTaSe2. Nat. Commun. 2016, 7, 10556. [Google Scholar] [CrossRef]
- Schoop, L.M.; Ali, M.N.; Straβer, C.; Topp, A.; Varykhalov, A.; Marchenko, D.; Duppel, V.; Parkin, S.S.P.; Lotsch, B.V.; Ast, C.R. Dirac cone protected by non-symmorphic symmetry and three-dimensional Dirac line node in ZrSiS. Nat. Commun. 2016, 7, 11696. [Google Scholar] [CrossRef]
- Neupane, M.; Belopolski, I.; Hosen, M.M.; Sanchez, D.S.; Sanker, R.; Szlawska, M.; Xu, S.-Y.; Dimitri, K.; Dhakal, N.; Maldonado, P.; et al. Observation of topological nodal fermion semimetal phase in ZrSiS. Phys. Rev. B 2016, 93, 201104. [Google Scholar] [CrossRef]
- Takane, D.; Wang, Z.; Souma, S.; Nakayama, K.; Trang, C.X.; Sato, T.; Takahashi, T.; Ando, Y. Dirac-node arc in the topological line-node semimetal HfSiS. Phys. Rev. B 2016, 94, 121108. [Google Scholar] [CrossRef]
- Hu, J.; Tang, Z.; Liu, J.; Liu, X.; Zhu, Y.; Graf, D.; Myhro, K.; Tran, S.; Lau, C.N.; Wei, J.; et al. Evidence of topological nodal-line fermions in ZrSiSe and ZrSiTe. Phys. Rev. Lett. 2016, 117, 016602. [Google Scholar] [CrossRef]
- Kobayashi, A.; Suzumura, Y.; Fukuyama, H. Hall effect and orbital diamagnetism in zerogap state of molecular conductor α-(BEDT-TTF)2I3. J. Phys. Soc. Jpn. 2008, 77, 064718. [Google Scholar] [CrossRef]
- Kato, R.; Suzumura, Y. Novel Dirac electron in single-component molecular conductor [Pd(dddt)2] (dddt = 5,6-dihydro-1,4-dithiin-2,3-dithiolate). J. Phys. Soc. Jpn. 2017, 86, 064705. [Google Scholar] [CrossRef]
- Kato, R.; Cui, H.-B.; Tsumuraya, T.; Miyazaki, T.; Suzumura, Y. Emergence of the Dirac electron system in a single-component molecular conductor under high pressure. J. Am. Chem. Soc. 2017, 139, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Suzumura, Y.; Kato, R. Magnetic susceptibility of Dirac electrons in single-component molecular conductor [Pd(dddt)2] under pressure. Jpn. J. Appl. Phys. 2017, 56, 05FB02. [Google Scholar] [CrossRef]
- Suzumura, Y. Anisotropic conductivity of nodal line semimetal in single-component molecular conductor [Pd(dddt)2]. J. Phys. Soc. Jpn. 2017, 86, 124710. [Google Scholar] [CrossRef]
- Tsumuraya, T.; Kato, R.; Suzumura, Y. Effective hamiltonian of topological nodal line semimetal in single-component molecular conductor [Pd(dddt)2] from first-principles. J. Phys. Soc. Jpn. 2018, 87, 113701. [Google Scholar] [CrossRef]
- Suzumura, Y.; Cui, H.; Kato, R. Conductivity and resistivity of Dirac electrons in single-component molecular conductor [Pd(dddt)2]. J. Phys. Soc. Jpn. 2018, 87, 084702. [Google Scholar] [CrossRef]
- Zhou, B.; Ishibashi, S.; Ishii, T.; Sekine, T.; Takehara, R.; Miyagawa, K.; Kanoda, K.; Nishibori, E.; Kobayashi, A. Single-component molecular conductor [Pt(dmdt)2]—A three-dimensional ambient-pressure molecular Dirac electron system. Chem. Commun. 2019, 55, 3327–3330. [Google Scholar] [CrossRef]
- Suzumura, Y.; Kato, R.; Ogata, M. Electric transport of nodal line semimetals in single-component molecular conductors. Crystals 2020, 10, 862. [Google Scholar] [CrossRef]
- Kato, R.; Cui, H.; Minamidate, T.; Yeung, H.H.-M.; Suzumura, Y. Electronic structure of a single-component molecular conductor [Pd(dddt)2] (dddt = 5,6-dihydro-1,4-dithiin-2,3-dithiolate) under high pressure. J. Phys. Soc. Jpn. 2020, 89, 124706. [Google Scholar] [CrossRef]
- Cui, H.; Yeung, H.H.-M.; Kawasugi, Y.; Minamidate, T.; Saunders, L.K.; Kato, R. High-pressure crystal structure and unusual magnetoresistance of a single-component molecular conductor [Pd(dddt)2] (dddt = 5,6-dihydro-1,4-dithiin-2,3-dithiolate). Crystals 2021, 11, 534. [Google Scholar] [CrossRef]
- Matsuno, G.; Omori, Y.; Eguchi, T.; Kobayashi, A. Topological domain wall and valley Hall effect in charge ordered phase of molecular Dirac Fermion system α-(BEDT-TTF)2I3. J. Phys. Soc. Jpn. 2016, 85, 094710. [Google Scholar] [CrossRef]
- Tani, T.; Tajima, N.; Kobayashi, A. Field-angle dependence of interlayer magnetoresistance in organic Dirac electron system α-(BEDT-TTF)2I3. Crystals 2019, 9, 212. [Google Scholar] [CrossRef]
- Li, W.; Uykur, E.; Kuntscher, C.A.; Dressel, M. Optical signatures of energy gap in correlated Dirac fermions. Npj Quantum Mater. 2019, 4, 19. [Google Scholar] [CrossRef]
- Naito, T.; Doi, R.; Suzumura, Y. Exotic Dirac Cones on the Band Structure of α-STF2I3 at Ambient Temperature and Pressure. J. Phys. Soc. Jpn. 2020, 89, 023701. [Google Scholar] [CrossRef]
- Naito, T.; Doi, R. Band Structure and Physical Properties of α-STF2I3: Dirac Electrons in Disordered Conduction Sheets. Crystals 2020, 10, 270. [Google Scholar] [CrossRef]
- Kobara, R.; Igarashi, S.; Kawasugi, Y.; Doi, R.; Naito, T.; Tamura, M.; Kato, R.; Nishio, Y.; Kajita, K.; Tajima, N. Universal Behavior of Magnetoresistance in Organic Dirac Electron Systems. J. Phys. Soc. Jpn. 2020, 89, 113703. [Google Scholar] [CrossRef]
- Ohki, D.; Yoshimi, K.; Kobayashi, A. Transport properties of the organic Dirac electron system α-(BEDT-TSeF)2I3. Phys. Rev. B 2020, 102, 235116. [Google Scholar] [CrossRef]
- Tsumuraya, T.; Suzumura, Y. First-principles study of the effective Hamiltonian for Dirac fermions with spin-orbit coupling in two-dimensional molecular conductor α-(BETS)2I3. Eur. Phys. J. B 2021, 94, 17. [Google Scholar] [CrossRef]
- Kitou, S.; Tsumuraya, T.; Sawahata, H.; Ishii, F.; Hiraki, K.-I.; Nakamura, T.; Katayama, N.; Sawa, H. Ambient-pressure Dirac electron system in the quasi-two-dimensional molecular conductor α-(BETS)2I3. Phys. Rev. B 2021, 103, 035135. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Hosur, P.; Parameswaran, S.A.; Vishwanash, A. Charge transport in Weyl semimetals. Phys. Rev. Lett. 2012, 108, 046602. [Google Scholar] [CrossRef]
- Bácsi, Á.; Virosztek, A. Low-frequency optical conductivity in graphene and in other scale-invariant two-band systems. Phys. Rev. B 2013, 87, 125425. [Google Scholar] [CrossRef]
- Timusk, T.; Carbotte, J.P.; Homes, C.C.; Bosov, D.N.; Sharapov, S.G. Three-dimensional Dirac fermions in quasicrystals seen via optical conductivity. Phys. Rev. B 2013, 87, 235121. [Google Scholar] [CrossRef]
- Naito, T.; Kobayashi, H.; Kobayashi, A. The Electrical Behavior of Charge-Transfer Salts Based on an Unsymmetrical Donor Bis(ethylenedithio)diselenadithiafulvalene (STF): Disorder Effect on the Transport Properties. Bull. Chem. Soc. Jpn. 1997, 70, 107–114. [Google Scholar] [CrossRef]
- Wang, H.H.; Rust, P.R.; Mertzenich, C.; Firestone, M.A.; Webb, K.S.; Williams, J.M. Tetrabutylammonium dibromoiodide and superconducting bis(bisethylenedithiotetrathiafulvalenium) dibromoiodide. In Inorganic Syntheses; Grimes, R.N., Ed.; Wiley: New York, NY, USA, 1992; Volume 29, pp. 44–46. [Google Scholar]
- Ren, J.; Liang, W.; Whangbo, M.-H. Crystal and Electronic Structure Analysis Using CAESAR; PrimeColor Software, Inc.: Cary, NC, USA, 1998. [Google Scholar]
- Naito, T.; Suzumura, Y. Theoretical model for novel electronic state in a Dirac electron system close to merging: An imaginary element between sulfur and selenium. Crystals 2022, 12, 346. [Google Scholar] [CrossRef]
- Suzumura, Y.; Naito, T. Conductivity of two-dimensional Dirac electrons close to merging in organic conductor α-STF2I3 at ambient pressure. J. Phys. Soc. Jpn. 2022, 91, 064701. [Google Scholar] [CrossRef]
- Williams, J.M.; Wang, H.H.; Beno, M.A.; Emge, T.J.; Sowa, L.M.; Copps, P.T.; Behroozi, F.; Hall, L.N.; Carlson, K.D.; Crabtree, G.W. Ambient-pressure superconductivity at 2.7 K and higher temperatures in derivatives of (BEDT-TTF)2IBr2: Synthesis, structure, and detection of superconductivity. Inorg. Chem. 1984, 23, 3839–3841. [Google Scholar] [CrossRef]
- Yagubskiĭ, É.B.; Shchegolev, I.F.; Shibaeva, R.P.; Fedutin, D.N.; Rozenberg, L.P.; Sogomonyan, E.M.; Lobkovskaya, R.M.; Laukhin, V.N.; Ignat’ev, A.A.; Zvarykina, A.V.; et al. Organic conductors and superconductors: Mixed (IBr2−) polyhalides BEDT-TTF. JETP Lett. 1985, 42, 206–208. [Google Scholar]
- Beno, M.A.; Firestone, M.A.; Leung, P.C.W.; Sowa, L.M.; Wang, H.H.; Williams, J.M.; Whangbo, M.-H. Crystal and band structures of a new class of 2:1 organic conducting salts α′-(BEDT-TTF)2X, X− = Ag(CN)2−, Au(CN)2− and AuBr2−. Solid State Commun. 1986, 57, 735–739. [Google Scholar] [CrossRef]
- Kobayashi, H.; Kato, R.; Kobayashi, A.; Saito, G.; Tokumoto, M.; Anzai, H.; Ishiguro, T. Crystal structure of α′-(BEDT-TTF)2BrICl. Chem. Lett. 1986, 15, 93–96. [Google Scholar] [CrossRef]
- Tokumoto, M.; Anzai, H.; Ishiguro, T.; Saito, G.; Kobayashi, H.; Kato, R.; Kobayashi, A. Electrical and magnetic properties of organic semiconductors, (BEDT-TTF)2X (X = IBr2, IBrCl, and ICl2. Synth. Met. 1987, 19, 215–220. [Google Scholar] [CrossRef]
- Kurmoo, M.; Talham, D.R.; Day, P.; Howard, J.A.K.; Stringer, A.M.; Obertelli, D.S.; Friend, R.H. (BEDT-TTF)2CuCl2, a new conducting charge transfer salt. Synth. Met. 1988, 22, 415–418. [Google Scholar] [CrossRef]
- Ugawa, A.; Yakushi, K.; Kuroda, H.; Kawamoto, A.; Tanaka, J. Crystal structure and polarized reflectance spectra of α′-(bis(ethylenedithio)-tetrathiafulvalenium)2-bromoiodoaurate, α′-(BEDT-TTF)2IAuBr. Synth. Met. 1988, 22, 305–315. [Google Scholar] [CrossRef]
- Yue, Y.; Nakano, C.; Yamamoto, K.; Uruichi, M.; Wojciechowski, R.; Inokuchi, M.; Yakushi, K.; Kawamoto, A. Charge order-disorder phase transition in α′-[bis(ethylenedithio) tetrathiafulvalene]2IBr2 [α′-(BEDT-TTF)2IBr2]. J. Phys. Soc. Jpn. 2009, 78, 044701. [Google Scholar] [CrossRef]
- Hirose, S.; Kawamoto, A. 13C NMR study on the charge-ordering salt α′-(BEDT-TTF)2IBr2. Phys. Rev. B 2009, 80, 165103. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960; p. 260. [Google Scholar]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular Nonbonded Contact Distances in Organic Crystal Structures: Comparison with Distances Expected from van der Waals Radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Oka, R.; Ohara, K.; Konishi, K.; Yamane, I.; Shimada, T.; Naito, T. Band structure evolution during reversible interconversion between Dirac and standard fermions in organic charge-transfer salts. Magnetochemistry 2023, 9, 153. [Google Scholar] [CrossRef]
- Ferraro, J.R.; Beno, M.A.; Thorn, R.J.; Wang, H.H.; Webb, K.S.; Williams, J.M. Spectroscopic and structural characterization of tetrabutylammonium trihalides: N-Bu4NI3, n-Bu4NI2Br, n-Bu4NIBr2, and n-Bu4NAuI2; precursors to organic conducting salts. J. Phys. Chem. Solids 1986, 47, 301–308. [Google Scholar] [CrossRef]
- Tajima, N.; Kawasugi, Y.; Morinari, T.; Oka, R.; Naito, T.; Kato, R. Coherent interlayer coupling in quasi-two-dimension Dirac fermions in α-(BEDT-TTF)2I3. J. Phys. Soc. Jpn. 2022, 92, 013702. [Google Scholar] [CrossRef]


| Elements and Atomic Orbitals | Hii (eV) *1 | ζ1 | c1 | ζ2 | c2 |
|---|---|---|---|---|---|
| I 5s | −22.3 | 3.341 | 0.6869 | 2.046 | 0.4869 |
| I 5p | −10.9 | 2.920 | 0.6140 | 1.671 | 0.5258 |
| Br 4s | −26.8 | 3.361 | 0.6310 | 2.044 | 0.5050 |
| Br 4p | −12.3 | 2.920 | 0.5822 | 1.624 | 0.5472 |
| Im 3s *2 | −22.585 | 2.800 | 0.60765 | 1.800 | 0.51875 |
| Im 3p *2 | −11.325 | 2.325 | 0.57495 | 1.225 | 0.56685 |
| S 3s | −23.9 | 2.662 | 0.5990 | 1.688 | 0.5246 |
| S 3p | −11.9 | 2.338 | 0.5377 | 1.333 | 0.5615 |
| C 2s | −19.2 | 1.831 | 0.7931 | 1.153 | 0.2739 |
| C 2p | −11.8 | 2.730 | 0.2595 | 1.257 | 0.8026 |
| H 1s *3 | −13.6 | 1.3 | 1 | NA | NA |
| Materials | α′-STF2IBr2 | α-STF2I3 | |||
|---|---|---|---|---|---|
| T (K) | 296 | 150 | 90 | 296 | 93 |
| Space Group | P1 | ||||
| System | Triclinic | ||||
| a (Å) | 8.9175(3) | 8.6984(2) | 8.6555(2) | 9.2162(3) | 9.08536(18) |
| b (Å) | 11.9990(4) | 12.0537(3) | 12.0449(2) | 10.8222(3) | 10.6938(2) |
| c (Å) | 16.5862(6) | 16.5339(3) | 16.5069(3) | 17.6354(5) | 17.5875(4) |
| α (°) | 85.050(3) | 85.254(2) | 85.321(2) | 96.864(7) | 96.5821(17) |
| β (°) | 88.353(3) | 89.265(2) | 89.538(1) | 97.868(7) | 97.8330(18) |
| γ (°) | 70.791(3) | 71.102(2) | 71.173(2) | 90.692(6) | 90.9456(16) |
| V (Å3) | 1669.68(10) | 1634.30(7) | 1623.11(6) | 1729.19(10) | 1680.75(6) |
| Z | 2 | ||||
| CCDC deposit # | 2,298,691 | 2,299,346 | 2,261,968 | 2,121,344 | 2,261,185 |
| References | This work | [23,24] | |||
| Interacting Pairs | α′-STF2IBr2 (90 K) | |
|---|---|---|
| S (10−2) | t (10−1 eV) | |
| A | 2.05 | −0.3348 |
| B | 6.46 | −1.2825 |
| C | 3.45 | −0.6463 |
| D | 6.65 | −1.2821 |
| E | −0.83 | 0.1657 |
| F | −0.38 | 0.0850 |
| G | −0.12 | 0.0284 |
| H *1 | −0.65 | 0.1007 |
| Interacting Pairs | α′-STF2IBr2 (90 K) | |
|---|---|---|
| S (10−4) | t (10−1 eV) | |
| M | 2.0 | −1.7 |
| N | −1.0 | 1.5 |
| O | −3.0 | 6.7 |
| P | −1.0 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funatsu, K.; Oka, R.; Tajima, N.; Naito, T. New Organic Crystalline Material Close to Nodal-Line Materials: α′-STF2IBr2. Crystals 2023, 13, 1606. https://doi.org/10.3390/cryst13111606
Funatsu K, Oka R, Tajima N, Naito T. New Organic Crystalline Material Close to Nodal-Line Materials: α′-STF2IBr2. Crystals. 2023; 13(11):1606. https://doi.org/10.3390/cryst13111606
Chicago/Turabian StyleFunatsu, Koki, Ryuhei Oka, Naoya Tajima, and Toshio Naito. 2023. "New Organic Crystalline Material Close to Nodal-Line Materials: α′-STF2IBr2" Crystals 13, no. 11: 1606. https://doi.org/10.3390/cryst13111606





