Micro-Vickers Hardness of Cu and Cu2O Dual Phase Composite Films Electrodeposited from Acidic Aqueous Solutions Containing Polyethylene Glycol
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Cathodic Polarization Behavior during Cu and Cu2O Dual Phase Composite Electrodeposition
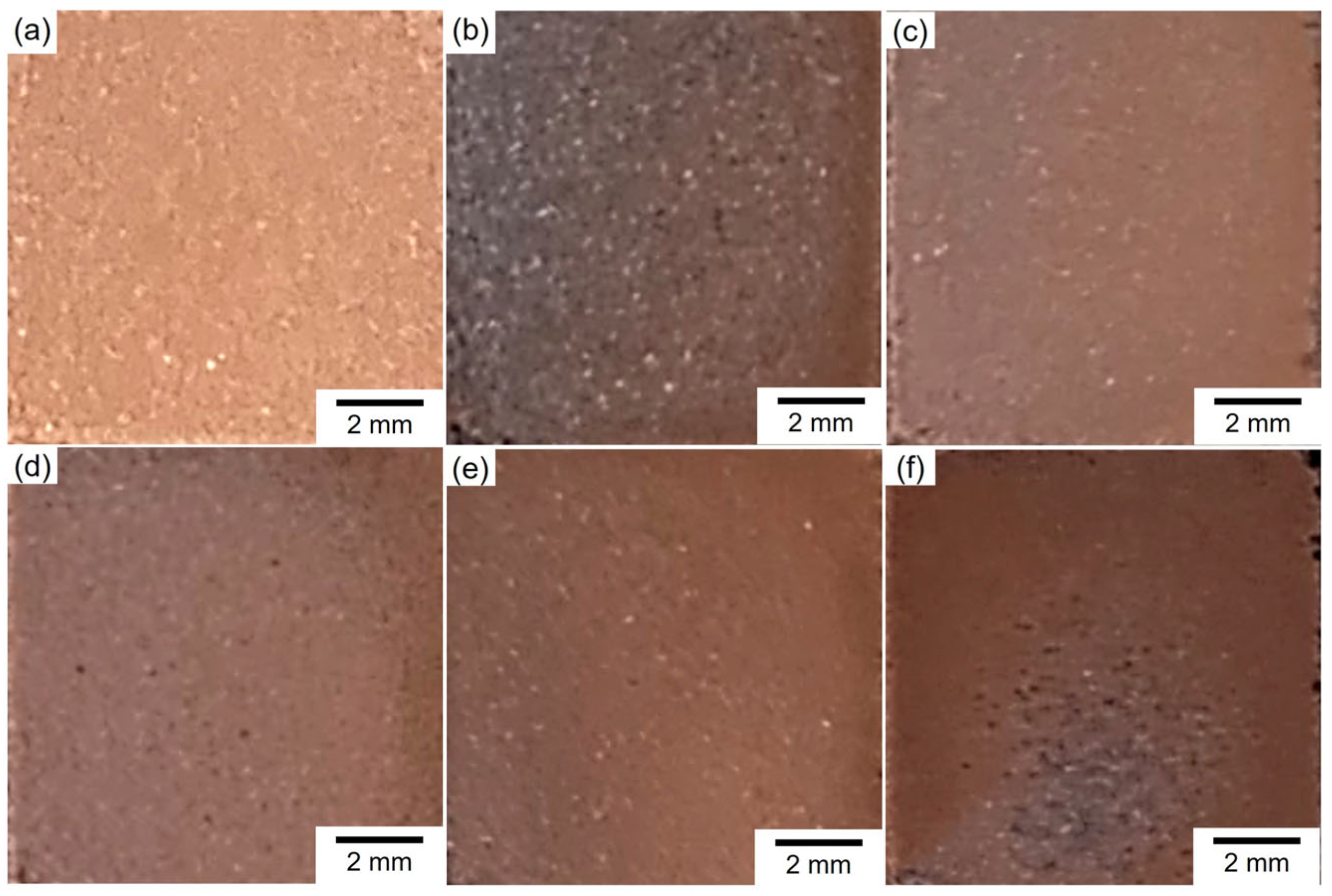
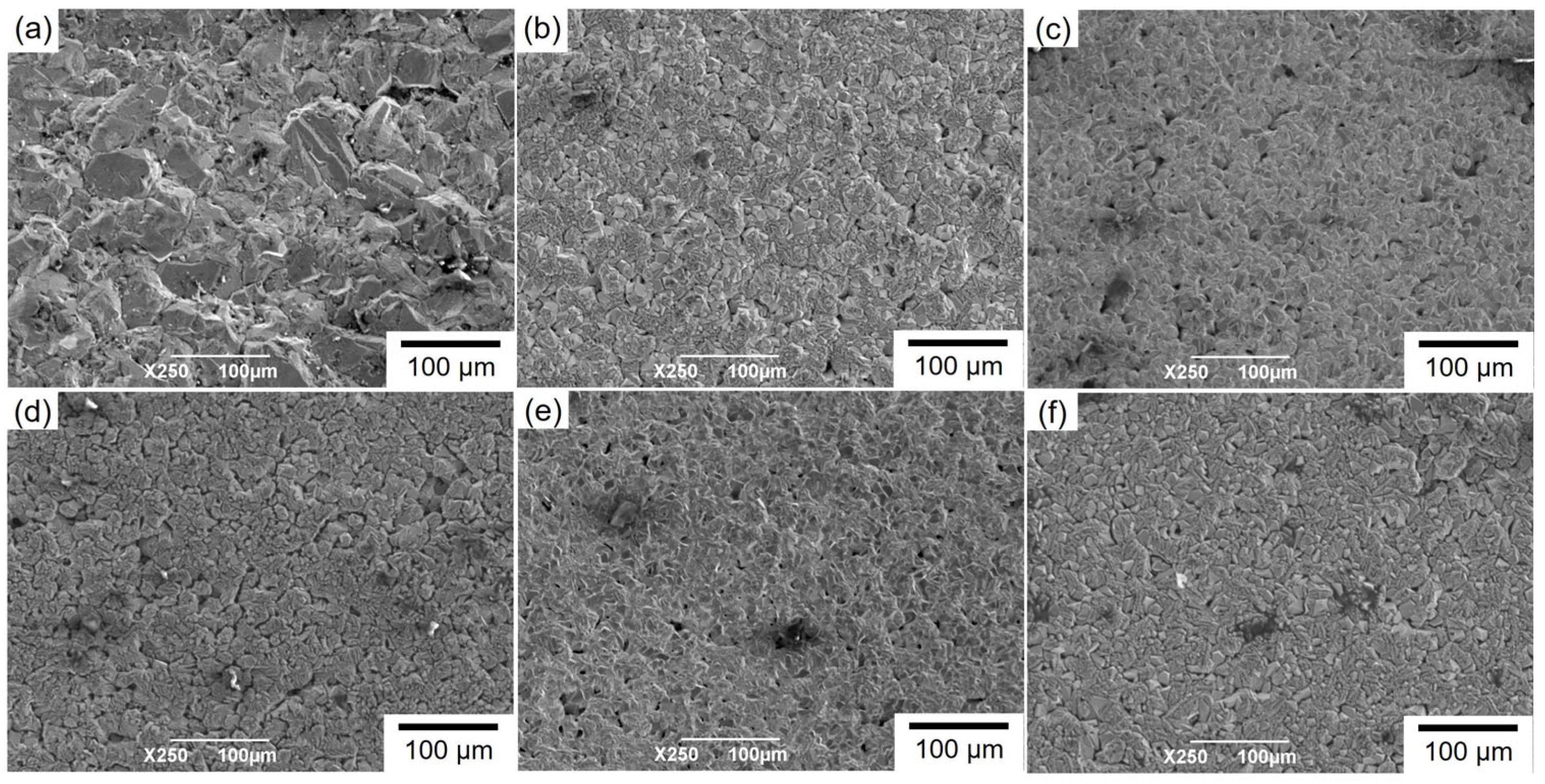
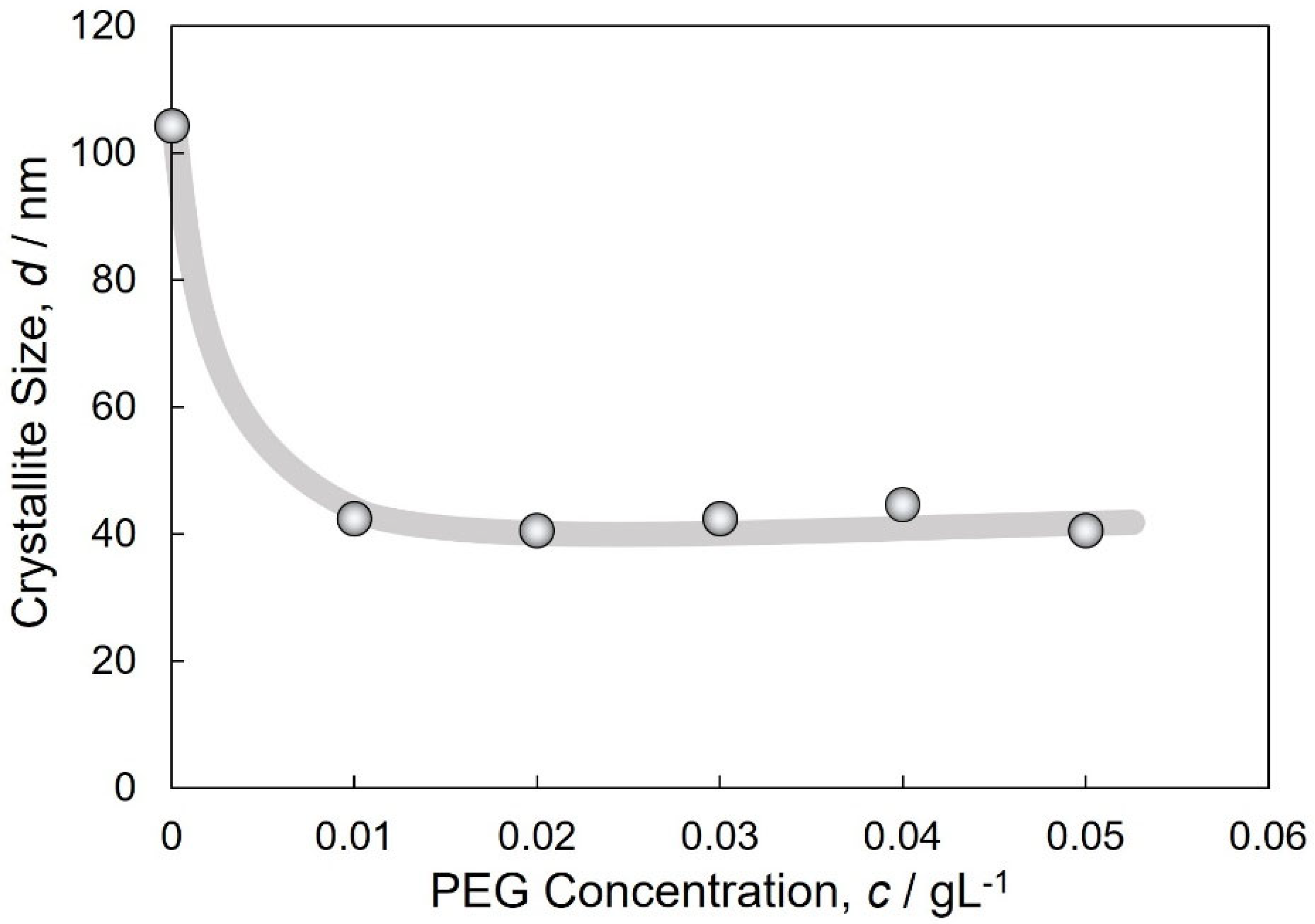
3.2. Structure of Electrodeposited Cu and Cu2O Dual Phase Composite Films
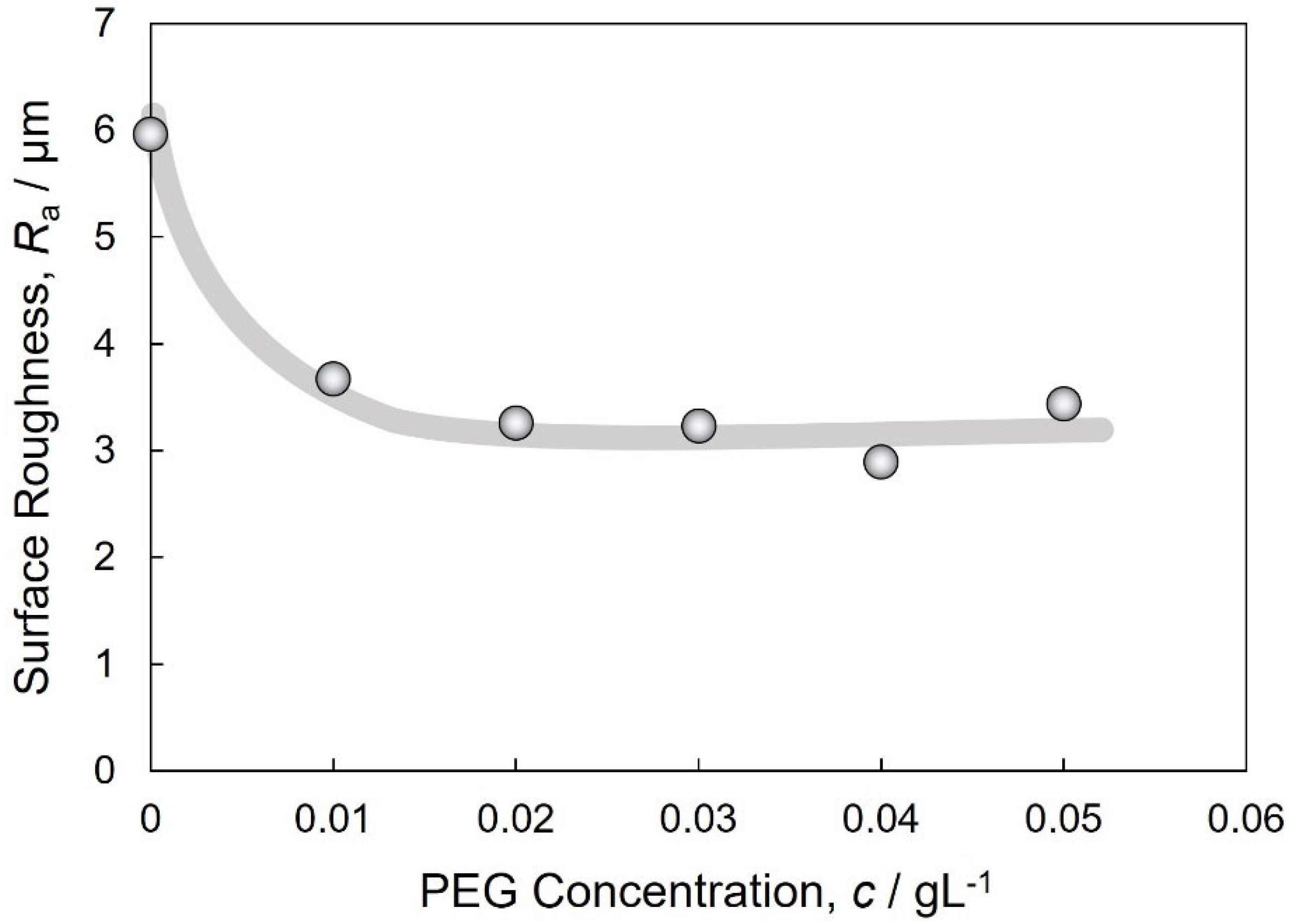
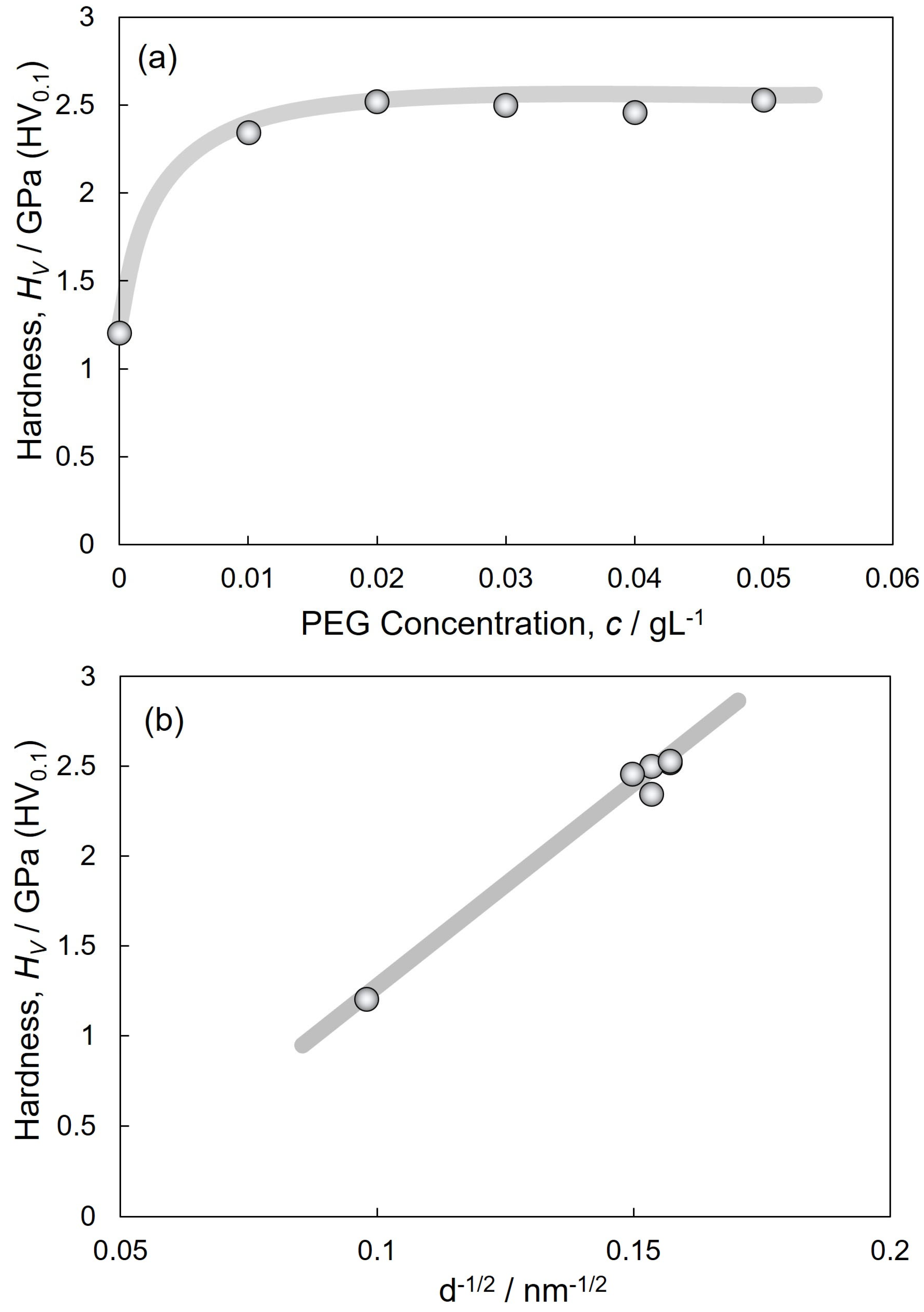
3.3. Micro-Vickers Hardness of Electrodeposited Cu and Cu2O Dual Phase Composite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bai, S.; Liu, J.; Cui, M.; Luo, R.; He, J.; Chen, A. Two-Step Electrodeposition to Fabricate the p-n Heterojunction of a Cu2O/BiVO4 Photoanode for the Enhancement of Photoelectrochemical Water Splitting. Dalt. Trans. 2018, 47, 6763–6771. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.A.; Shen, L.; Zheng, J.; Xu, C. Boosting Light Harvesting and Charge Separation of WO3 via coupling with Cu2O/CuO towards Highly Efficient Tandem Photoanodes. RSC Adv. 2021, 11, 13513–13520. [Google Scholar] [CrossRef] [PubMed]
- Kimizuka, N.; Mohri, T. Spinel, YbFe2O4, and Yb2Fe3O7 types of structures for compounds in the In2O3 and Sc2O3 A2O3 BO systems [A: Fe, Ga, or Al; B: Mg, Mn, Fe, Ni, Cu, or Zn] at temperatures over 1000 °C. J. Solid State Chem. 1985, 60, 382–384. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Ueda, K.; Kamiya, T.; Hirano, M.; Hosono, H. Thin-film transistor fabricated in single-crystalline transparent oxide semiconductor. Science 2003, 300, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Ohta, H.; Takagi, A.; Kamiya, T.; Hirano, M.; Hosono, H. Room-temperature fabrication of transparent flexible thin-film transistors using amorphous oxide semiconductors. Nature 2004, 432, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.T.; Zhao, Z.Y.; Shen, H.L. Stability and Fundamental Properties of CuXO1-X as Optoelectronic Functional Materials. RSC Adv. 2022, 12, 3755–3762. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yang, Q.; Liang, S.; Yang, Z. Hollow CuXO (x = 2, 1) Micro/Nanostructures: Synthesis, Fundamental Properties and Applications. CrystEngComm 2017, 19, 6225–6251. [Google Scholar] [CrossRef]
- Shyamal, S.; Hajra, P.; Mandal, H.; Bera, A.; Sariket, D.; Satpati, A.K.; Kundu, S.; Bhattacharya, C. Benign Role of Bi on an Electrodeposited Cu2O Semiconductor towards Photo-Assisted H2 Generation from Water. J. Mater. Chem. A 2016, 4, 9244–9252. [Google Scholar] [CrossRef]
- Guo, Y.; Lei, H.; Xiong, L.; Li, B.; Chen, Z.; Wen, J.; Yang, G.; Li, G.; Fang, G. Single Phase, High Hole Mobility Cu2O Films as an Efficient and Robust Hole Transporting Layer for Organic Solar Cells. J. Mater. Chem. A 2017, 5, 11055–11062. [Google Scholar] [CrossRef]
- Barreca, D.; Comini, E.; Gasparotto, A.; Maccato, C.; Sada, C.; Sberveglieri, G.; Tondello, E. Chemical Vapor Deposition of Copper Oxide Films and Entangled Quasi-1D Nanoarchitectures as Innovative Gas Sensors. Sens. Actuators B Chem. 2009, 141, 270–275. [Google Scholar] [CrossRef]
- Neetzel, C.; Muench, F.; Matsutani, T.; Jaud, J.C.; Broetz, J.; Ohgai, T.; Ensinger, W. Facile Wet-Chemical Synthesis of Differently Shaped Cuprous Oxide Particles and a Thin Film: Effect of Catalyst Morphology on the Glucose Sensing Performance. Sens. Actuators B Chem. 2015, 214, 189–196. [Google Scholar] [CrossRef]
- Al-Jawhari, H.A. A Review of Recent Advances in Transparent P-Type Cu2O-Based Thin Film Transistors. Mater. Sci. Semicond. Process. 2015, 40, 241–252. [Google Scholar] [CrossRef]
- Xue, J.; Dieckmann, R. The Non-Stoichiometry and the Point Defect Structure of Cuprous Oxide (Cu2-δO). J. Phys. Chem. Solids 1990, 51, 1263–1275. [Google Scholar] [CrossRef]
- Shimizu, K.; Kobayashi, K.; Thompson, G.E.; Wood, G.C. High Resolution Cross-Sectional Transmission Electron Microscopy of Thermal Oxide Films on Copper. Corros. Sci. 1994, 36, 621–629. [Google Scholar] [CrossRef]
- Reyes-Vallejo, O.; Escorcia-García, J.; Sebastian, P.J. Effect of Complexing Agent and Deposition Time on Structural, Morphological, Optical and Electrical Properties of Cuprous Oxide Thin Films Prepared by Chemical Bath Deposition. Mater. Sci. Semicond. Process. 2022, 138, 106242. [Google Scholar] [CrossRef]
- Chen, Y.J.; Li, M.H.; Huang, J.C.A.; Chen, P. The Cu/Cu2O Nanocomposite as a p-Type Transparent-Conductive-Oxide for Efficient Bifacial-Illuminated Perovskite Solar Cells. J. Mater. Chem. C 2018, 6, 6280–6286. [Google Scholar] [CrossRef]
- Kamimura, H.; Hayashida, M.; Ohgai, T. CPP-GMR Performance of Electrochemically Synthesized Co/Cu Multilayered Nanowire Arrays with Extremely Large Aspect Ratio. Nanomaterials 2020, 10, 5. [Google Scholar] [CrossRef]
- Saeki, R.; Mizoguchi, S.; Kamimura, H.; Hayashida, M.; Ohgai, T. CPP-GMR Performance of Electrodeposited Metallic Multilayered Nanowires with a Wide Range of Aspect Ratios. J. Magn. Magn. Mater. 2021, 529, 167849. [Google Scholar] [CrossRef]
- Rajeshwar, K. Electrosynthesized Thin Films of Group II–VI Compound Semiconductors, Alloys and Superstructures. Adv. Mater. 1992, 4, 23–29. [Google Scholar] [CrossRef]
- Lincot, D. Electrodeposition of Semiconductors. Thin Solid Films 2005, 487, 40–48. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Shi, S.; Zhang, Y.; Liu, Z.; Zhang, X.; Yin, S.; Li, L. Structural, Optical and Photoelectrical Properties of Cu2O Films Electrodeposited at Different PH. RSC Adv. 2016, 6, 4422–4428. [Google Scholar] [CrossRef]
- Huang, M.C.; Wang, T.; Chang, W.S.; Lin, J.C.; Wu, C.C.; Chen, I.C.; Peng, K.C.; Lee, S.W. Temperature Dependence on P-Cu2O Thin Film Electrochemically Deposited onto Copper Substrate. Appl. Surf. Sci. 2014, 301, 369–377. [Google Scholar] [CrossRef]
- Saeki, R.; Ohgai, T. Determination of Cathode Current Efficiency for Electrodeposition of Ferromagnetic Cobalt Nanowire Arrays in Nanochannels with Extremely Large Aspect Ratio. Results Phys. 2019, 15, 102658. [Google Scholar] [CrossRef]
- Sai Guru Srinivasan, S.; Govardhanan, B.; Ashok, M.; Santhosh Kumar, M.C. Influence of Deposition Time on the Visible-Light-Driven Photocatalytic Activity of Cu2O Thin Films by Reactive Sputtering at Room Temperature. Mater. Lett. 2021, 284, 128980. [Google Scholar] [CrossRef]
- Ismail, W.; El-Shafai, N.M.; El-Shaer, A.; Abdelfatah, M. Impact of Substrate Type on the Surface and Properties of Electrodeposited Cu2O Nanostructure Films as an Absorber Layer for Solar Cell Applications. Mater. Sci. Semicond. Process. 2020, 120, 105335. [Google Scholar] [CrossRef]
- Li, Q.; Hu, J.; Zhang, J.; Yang, P.; Hu, Y.; An, M. Screening of Electroplating Additive for Improving Throwing Power of Copper Pyrophosphate Bath via Molecular Dynamics Simulation. Chem. Phys. Lett. 2020, 757, 137848. [Google Scholar] [CrossRef]
- Zhou, Y.; Switzer, J.A. Electrochemical Deposition and Microstructure of Copper (I) Oxide Films. Scr. Mater. 1998, 38, 1731–1738. [Google Scholar] [CrossRef]
- Wang, L.C.; de Tacconi, N.R.; Chenthamarakshan, C.R.; Rajeshwar, K.; Tao, M. Electrodeposited Copper Oxide Films: Effect of Bath PH on Grain Orientation and Orientation-Dependent Interfacial Behavior. Thin Solid Films 2007, 515, 3090–3095. [Google Scholar] [CrossRef]
- Ohgai, T.; Hoffer, X.; Gravier, L.; Ansermet, J.-P. Electrochemical Surface Modification of Aluminium Sheets for Application to Nano-Electronic Devices: Anodization Aluminium and Electrodeposition of Cobalt-Copper. J. Appl. Electrochem. 2004, 34, 1007–1012. [Google Scholar] [CrossRef]
- Ohgai, T.; Hjort, K.; Spohr, R.; Neumann, R. Electrodeposition of Cobalt Based Ferro-Magnetic Metal Nanowires in Polycarbonate Films with Cylindrical Nanochannels Fabricated by Heavy-Ion-Track Etching. J. Appl. Electrochem. 2008, 38, 713–719. [Google Scholar] [CrossRef]
- Li, Z.; Soroka, I.L.; Min, F.; Jonsson, M. PH-Control as a Way to Fine-Tune the Cu/Cu2O Ratio in Radiation Induced Synthesis of Cu2O Particles. Dalt. Trans. 2018, 47, 16139–16144. [Google Scholar] [CrossRef]
- Oh, Y.-J.; Park, G.-S.; Chung, C.-H. Planarization of Copper Layer for Damascene Interconnection by Electrochemical Polishing in Alkali-Based Solution. J. Electrochem. Soc. 2006, 153, G617. [Google Scholar] [CrossRef]
- Han, J.; Chang, J.; Wei, R.; Ning, X.; Li, J.; Li, Z.; Guo, H.; Yang, Y. Mechanistic Investigation on Tuning the Conductivity Type of Cuprous Oxide (Cu2O) Thin Films via Deposition Potential. Int. J. Hydrogen Energy 2018, 43, 13764–13777. [Google Scholar] [CrossRef]
- Zeng, T.W.; Yen, S.C. Effects of Additives in an Electrodeposition Bath on the Surface Morphologic Evolution of Electrodeposited Copper. Int. J. Electrochem. Sci. 2021, 16, 210245. [Google Scholar] [CrossRef]
- Hebert, K.R.; Adhikari, S.; Houser, J.E. Chemical Mechanism of Suppression of Copper Electrodeposition by Poly(Ethylene Glycol). J. Electrochem. Soc. 2005, 152, C324. [Google Scholar] [CrossRef]
- Grujicic, D.; Pesic, B. Electrodeposition of copper: The nucleation mechanisms. Electrochim. Acta 2002, 47, 2901–2912. [Google Scholar] [CrossRef]
- Tromans, D.; Sun, R.H. Anodic polarization behavior of copper in aqueous chloride/benzotriazole solutions. J. Electrochem. Soc. 1991, 138, 3235. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, W.; Zhong, C.; Deng, Y.; Liu, L.; Wu, Y. Preparation of submicron-sized cuprous oxide crystallites by electrodeposition with polyethylene glycol as additive. J. Cryst. Growth 2012, 354, 193–197. [Google Scholar] [CrossRef]
- Rasmussen, A.A.; Jensen, J.A.D.; Horsewell, A.; Somers, M.A.J. Microstructure in Electrodeposited Copper Layers; the Role of the Substrate. Electrochim. Acta 2001, 47, 67–74. [Google Scholar] [CrossRef]
- Sayem Rahman, A.S.M.; Islam, M.A.; Shorowordi, K.M. Electrodeposition and Characterization of Copper Oxide Thin Films for Solar Cell Applications. Procedia Eng. 2015, 105, 679–685. [Google Scholar] [CrossRef]
- Chen, T.; Kitada, A.; Fukami, K.; Murase, K. Determination of Stability Constants of Copper (II)–Lactate Complexes in Cu2O Electrodeposition Baths by UV-VIS Absorption Spectra Factor Analysis. J. Electrochem. Soc. 2019, 166, D761. [Google Scholar] [CrossRef]
- Saeki, R.; Ohgai, T. Determination of Activation Overpotential during the Nucleation of Hcp-Cobalt Nanowires Synthesized by Potentio-Static Electrochemical Reduction. Materials 2018, 11, 2355. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Biswas, K. Effect of Thiourea on Grain Refinement and Defect Structure of the Pulsed Electrodeposited Nanocrystalline Copper. Surf. Coat. Technol. 2013, 214, 8–18. [Google Scholar] [CrossRef]
- Ibanez, A.; Fatas, E. Mechanical and structural properties of electrodeposited copper and their relation with the electrodeposition parameters. Surf. Coat. Technol. 2005, 191, 7–16. [Google Scholar] [CrossRef]
- Bata, V.; Pereloma, E.V. An alternative physical explanation of the Hall–Petch relation. Acta Mater. 2004, 52, 657–665. [Google Scholar] [CrossRef]
- Kumar, K.S.; Van Swygenhoven, H.; Suresh, S. Mechanical Behavior of Nanocrystalline Metals and Alloys. Acta Mater. 2003, 51, 5743–5774. [Google Scholar] [CrossRef]
- Hakamada, M.; Nakamoto, Y.; Matsumoto, H.; Iwasaki, H.; Chen, Y.; Kusuda, H.; Mabuchi, M. Relationship between hardness and grain size in electrodeposited copper films. Mater. Sci. Eng. A 2007, 457, 120–126. [Google Scholar] [CrossRef]
- Dow, W.P.; Yen, M.Y.; Lin, W.B.; Ho, S.W. Influence of molecular weight of polyethylene glycol on microvia filling by copper electroplating. J. Electrochem. Soc. 2005, 152, C769. [Google Scholar] [CrossRef]
- Ko, S.L.; Lin, J.Y.; Wang, Y.Y.; Wan, C.C. Effect of the molecular weight of polyethylene glycol as single additive in copper deposition for interconnect metallization. Thin Solid Films 2008, 516, 5046–5051. [Google Scholar] [CrossRef]
- Song, S.J.; Choi, S.R.; Kim, J.G.; Kim, H.G. Effect of molecular weight of polyethylene glycol on copper electrodeposition in the presence of bis-3-sulfopropyl-disulfide. Int. J. Electrochem. Sci. 2016, 151, 10067–10079. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawakami, R.; Saeki, R.; Munetoh, S.; Ohgai, T. Micro-Vickers Hardness of Cu and Cu2O Dual Phase Composite Films Electrodeposited from Acidic Aqueous Solutions Containing Polyethylene Glycol. Crystals 2023, 13, 1654. https://doi.org/10.3390/cryst13121654
Kawakami R, Saeki R, Munetoh S, Ohgai T. Micro-Vickers Hardness of Cu and Cu2O Dual Phase Composite Films Electrodeposited from Acidic Aqueous Solutions Containing Polyethylene Glycol. Crystals. 2023; 13(12):1654. https://doi.org/10.3390/cryst13121654
Chicago/Turabian StyleKawakami, Reina, Ryusei Saeki, Shinji Munetoh, and Takeshi Ohgai. 2023. "Micro-Vickers Hardness of Cu and Cu2O Dual Phase Composite Films Electrodeposited from Acidic Aqueous Solutions Containing Polyethylene Glycol" Crystals 13, no. 12: 1654. https://doi.org/10.3390/cryst13121654




