5-Fluoro-1-Methyl-Pyrazol-4-yl-Substituted Nitronyl Nitroxide Radical and Its 3d Metal Complexes: Synthesis, Structure, and Magnetic Properties
Abstract
:1. Introduction
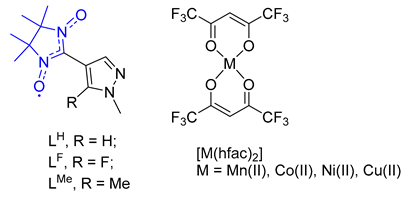
2. Materials and Methods
2.1. Materials and Spectral Measurements
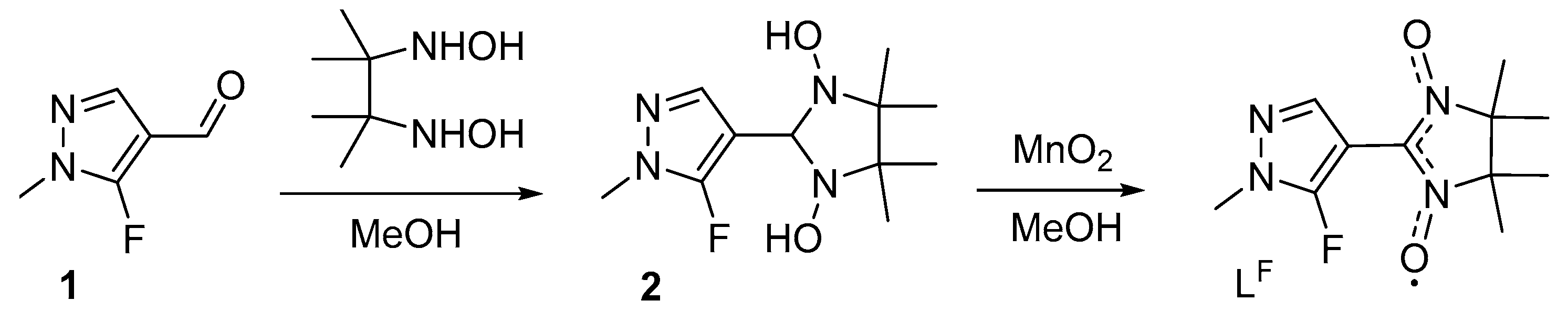
2.2. Preparation of Paramagnetic Ligand LF (the Synthetic Procedure Is Similar to That Described Earlier) [33]
2.3. Preparation of Complexes
2.4. X-ray Crystallographic Data and Refinement Details
2.5. Powder XRD Data
2.6. Magnetic Measurements
3. Results and Discussion
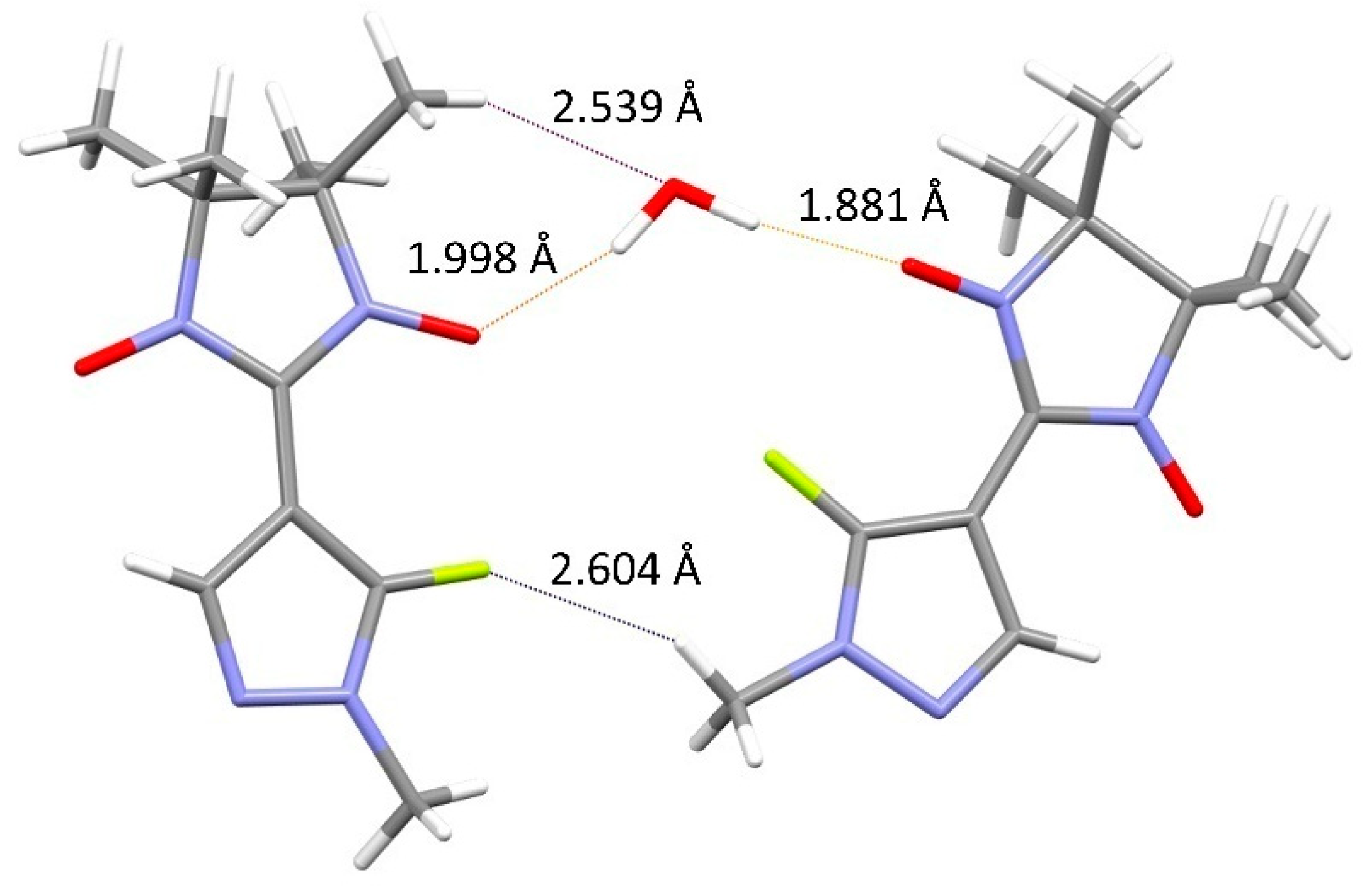
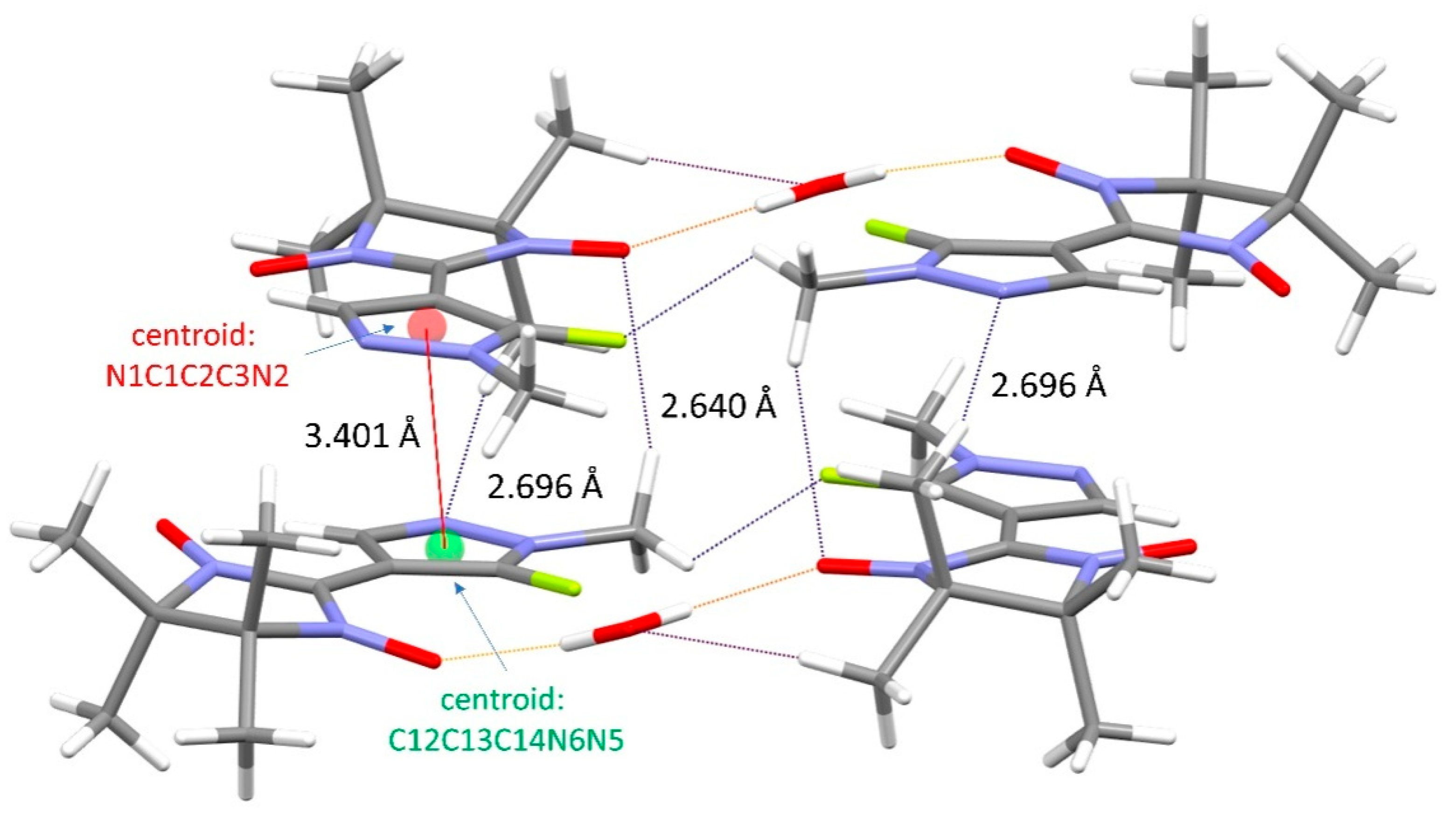
3.1. Synthesis and Structure of the Organic Radical LF
3.2. Synthesis and Structures of Complexes
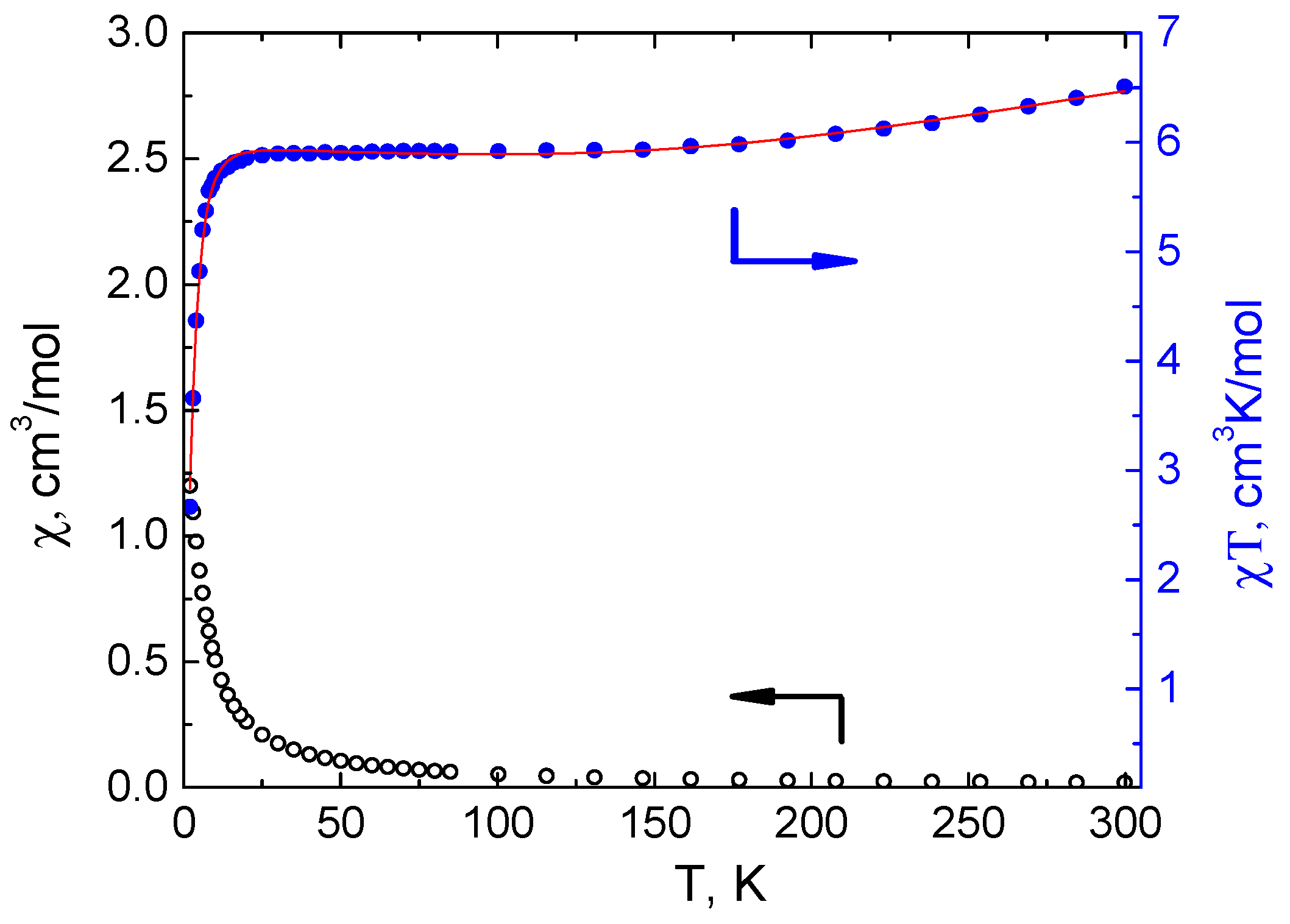
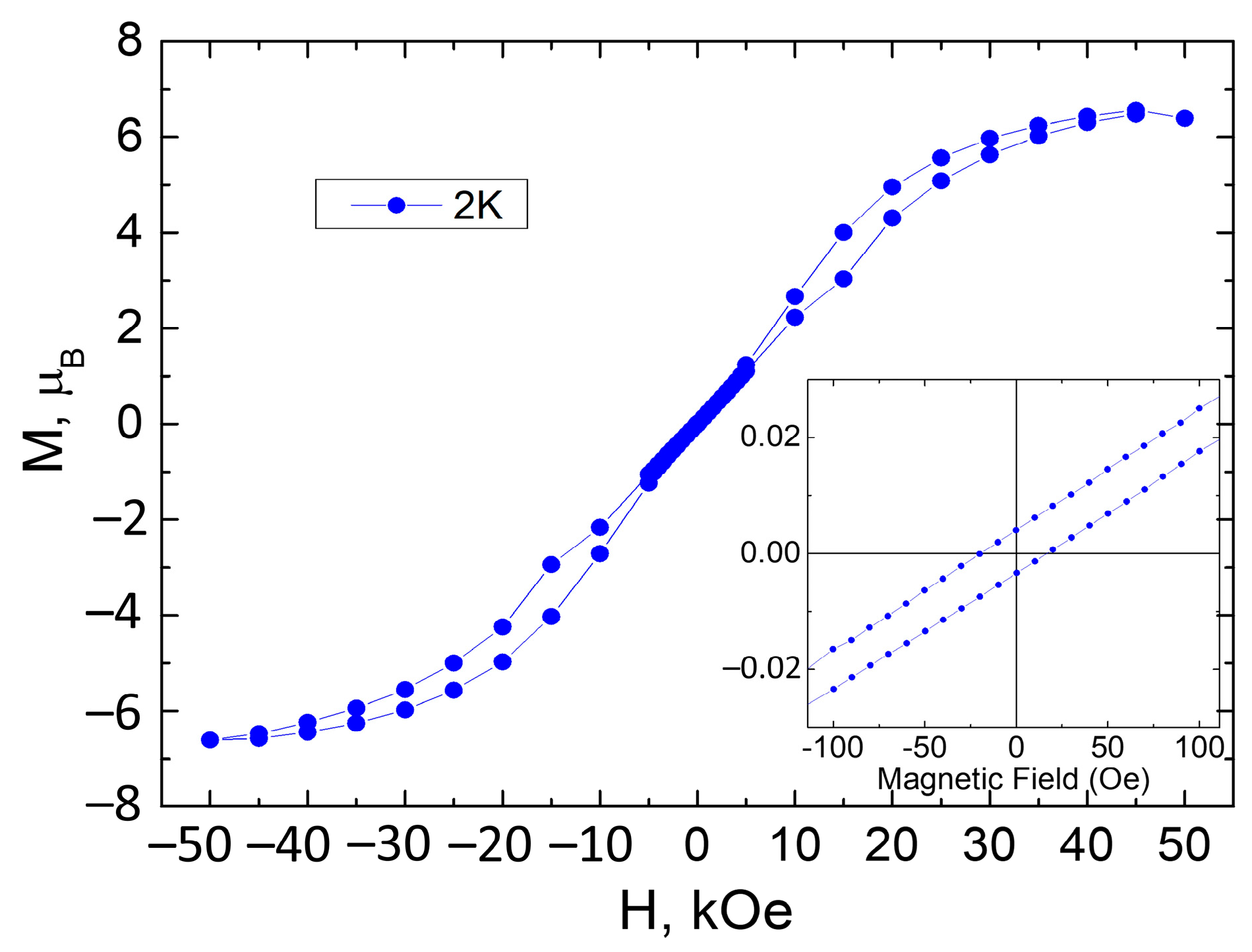
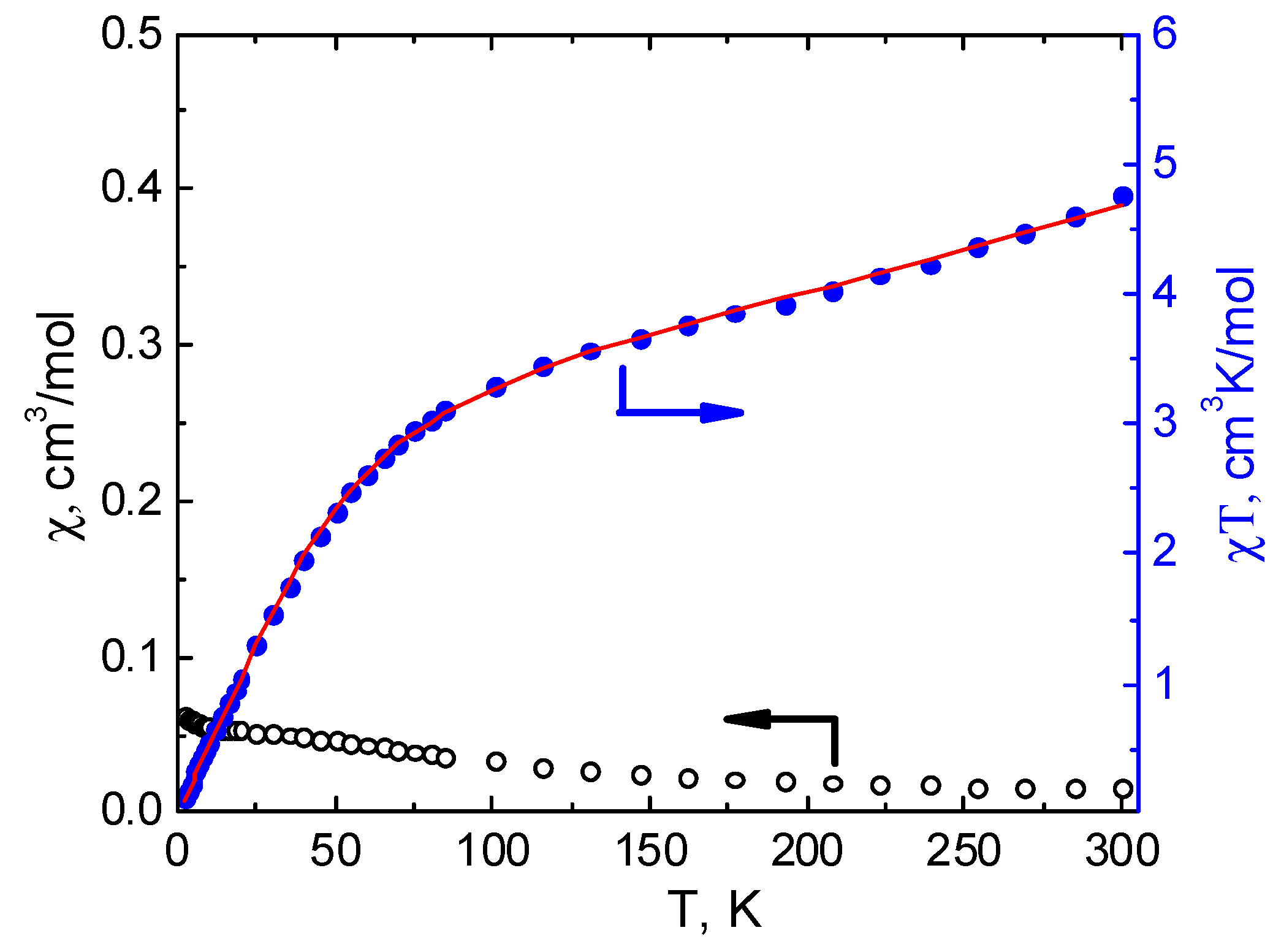
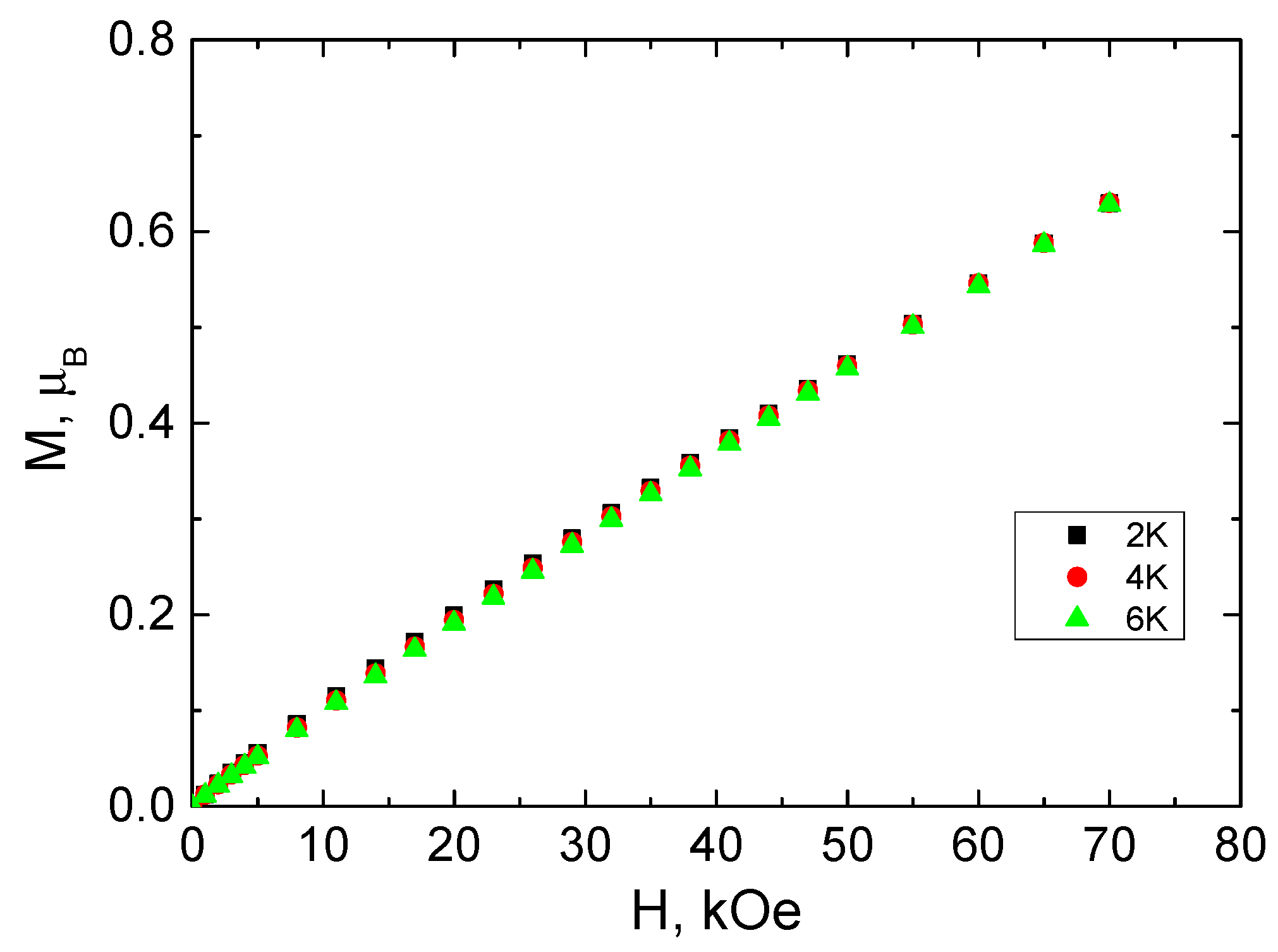
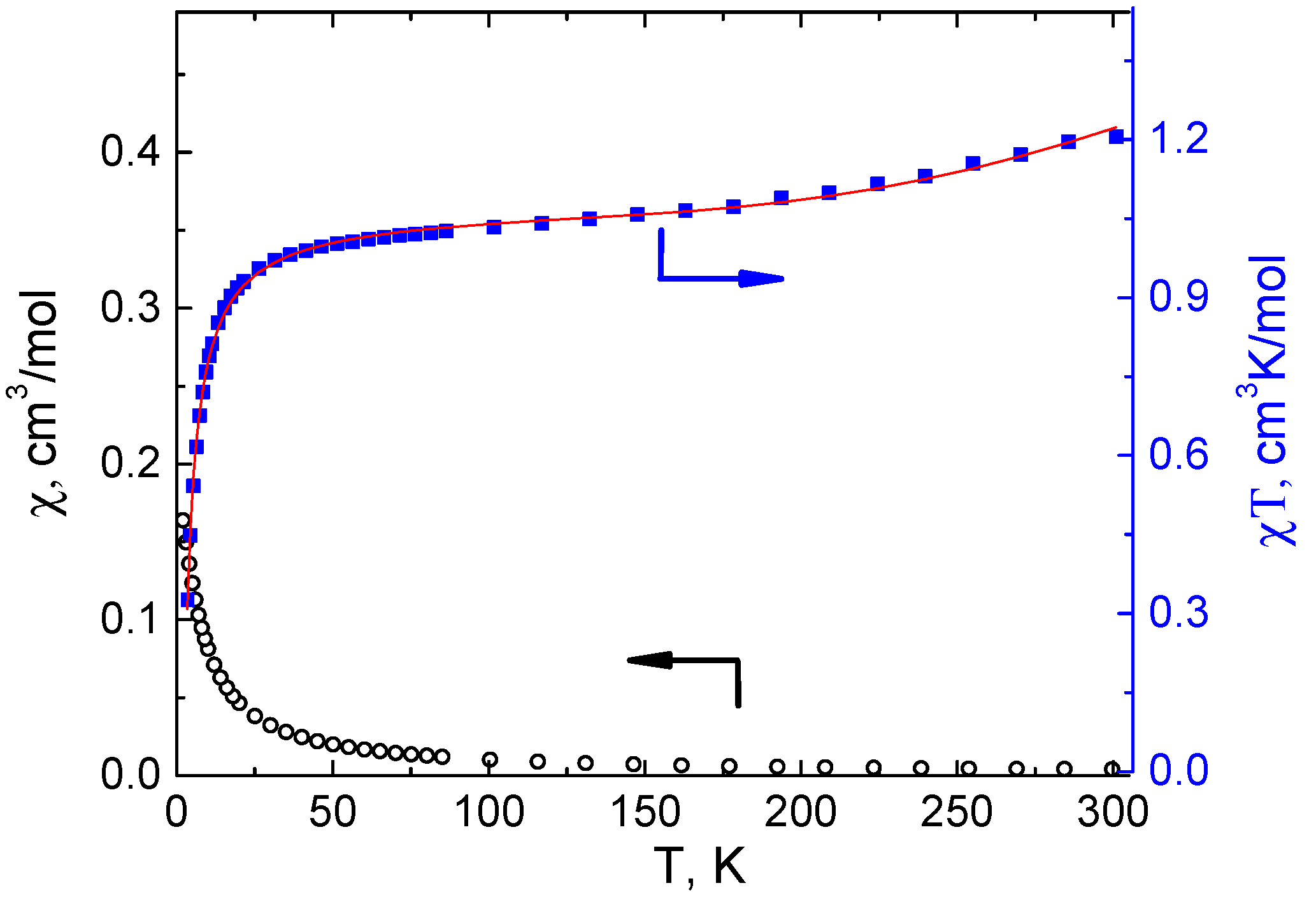
3.3. Magnetochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benelli, C.; Gatteschi, D. Magnetism of Lanthanides in Molecular Materials with Transition-Metal Ions and Organic Radicals. Chem. Rev. 2002, 102, 2369–2388. [Google Scholar] [CrossRef] [PubMed]
- Fedin, M.V.; Veber, S.L.; Bagryanskaya, E.G.; Ovcharenko, V.I. Electron paramagnetic resonance of switchable copper-nitroxide-based molecular magnets: An indispensable tool for intriguing systems. Coord. Chem. Rev. 2015, 289, 341–356. [Google Scholar] [CrossRef]
- Demir, S.; Jeon, I.-R.; Long, J.R.; Harris, T.D. Radical ligand-containing single-molecule magnets. Coord. Chem. Rev. 2015, 149, 289–290. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular Magnetism, Quo Vadis? A Historical Perspective from a Coordination Chemist Viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Meng, X.; Shi, W.; Cheng, P. Magnetism in one-dimensional metal–nitronyl nitroxide radical system. Coord. Chem. Rev. 2019, 378, 134–150. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Ovcharenko, V.I.; Terent’ev, A.O.; Krylov, I.B.; Magdesieva, T.V.; Mazhukin, D.G.; Gritsan, N.P. Conjugated nitroxides. Russ. Chem. Rev. 2022, 91, RCR5025. [Google Scholar] [CrossRef]
- Sun, J.; Yang, M.; Xi, L.; Ma, Y.; Li, L.C. Magnetic relaxation in [Ln(hfac)4]− anions with [Cu(hfac)-radical]nn+ cation chains as counterions. Dalton Trans. 2018, 47, 8142–8148. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.F.; Guo, J.N.; Yang, M.; Xi, L.; Li, L.C.; Sutter, J.P. Two-dimensional Co–Ln networks bridged by phenyl pyrimidyl substituted nitronyl nitroxides: Structural and magnetic properties. Dalton Trans. 2018, 47, 4672–4677. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, V.; Romanenko, G.; Polushkin, A.; Letyagin, G.; Bogomyakov, A.; Fedin, M.; Maryunina, K.; Nishihara, S.; Inoue, K.; Petrova, M.; et al. Pressure-Controlled Migration of Paramagnetic Centers in a Heterospin Crystal. Inorg. Chem. 2019, 58, 9187–9194. [Google Scholar] [CrossRef]
- Maryunina, K.Y.; Zhang, X.; Nishihara, S.; Inoue, K.; Morozov, V.A.; Romanenko, G.V.; Ovcharenko, V.I. A Heterospin Pressure Sensor. J. Mater. Chem. C 2015, 3, 7788–7791. [Google Scholar] [CrossRef]
- Stein, B.W.; Tichnell, C.R.; Chen, J.; Shultz, D.A.; Kirk, M.L. Excited State Magnetic Exchange Interactions Enable Large Spin Polarization Effects. J. Am. Chem. Soc. 2018, 140, 2221–2228. [Google Scholar] [CrossRef] [PubMed]
- Vostrikova, K.E.; Luneau, D.; Wernsdorfer, W.; Rey, P.; Verdaguer, M. A S = 7 ground spin-state cluster built from three shells of different spin carriers ferromagnetically coupled, transition-metal ions and nitroxide free radicals. J. Am. Chem. Soc. 2000, 122, 718–719. [Google Scholar] [CrossRef]
- Lecourt, C.; Izumi, Y.; Khrouz, L.; Toche, F.; Chiriac, R.; Bélanger-Desmarais, N.; Reber, C.; Fabelo, O.; Inoue, K.; Desroches, C.; et al. Thermally-induced hysteretic valence tautomeric conversions in the solid state via two-step labile electron transfers in manganese-nitronyl nitroxide 2D-frameworks. Dalton Trans. 2020, 49, 15646–15662. [Google Scholar] [CrossRef] [PubMed]
- Li, H.D.; Sun, Z.; Sun, J.; Xi, L.; Guo, J.N.; Sun, G.F.; Xie, J.; Ma, Y.; Li, L.C. Single-molecule magnet behavior in a CuII-decorated {DyIII2} complex with nitronyl nitroxide biradicals. J. Mater. Chem. C 2018, 6, 2060–2068. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Romanenko, G.V.; Veber, S.L.; Fedin, M.V.; Polushkin, A.V.; Tkacheva, A.O.; Ovcharenko, V.I. Cu(hfac)2 complexes with nitronyl ketones structurally mimicking nitronyl nitroxides in breathing crystals. Aust. J. Chem. 2015, 68, 970–980. [Google Scholar] [CrossRef]
- Lannes, A.; Suffren, Y.; Tommasino, J.B.; Chiriac, R.; Toche, F.; Khrouz, L.; Molton, F.; Duboc, C.; Kieffer, I.; Hazemann, J.L.; et al. Room Temperature Magnetic Switchability Assisted by Hysteretic Valence Tautomerism in a Layered Two-Dimensional Manganese-Radical Coordination Framework. J. Am. Chem. Soc. 2016, 138, 16493–16501. [Google Scholar] [CrossRef]
- Shultz, D.A.; Kirk, M.L.; Zhang, J.; Stasiw, D.E.; Wang, G.; Yang, J.; Habel-Rodriguez, D.; Stein, B.W.; Sommer, R.D. Spectroscopic Signatures of Resonance Inhibition Reveal Differences in Donor-Bridge and Bridge-Acceptor Couplings. J. Am. Chem. Soc. 2020, 142, 4916–4924. [Google Scholar] [CrossRef]
- Artiukhova, N.A.; Romanenko, G.V.; Bogomyakov, A.S.; Barskaya, I.Y.; Veber, S.L.; Fedin, M.V.; Maryunina, K.Y.; Inoue, K.; Ovcharenko, V.I. Cu(II) complex with nitronyl nitroxide whose paramagnetism is suppressed by temperature decrease and/or pressure increase. J. Mater. Chem. C 2016, 4, 11157–11163. [Google Scholar] [CrossRef]
- Novitchi, G.; Shova, S.; Train, C. Investigation by Chemical Substitution within 2p-3d-4f Clusters of the Cobalt(II) Role in the Magnetic Behavior of [vdCoLn]2 (vd = Verdazyl Radical). Inorg. Chem. 2022, 61, 17037–17048. [Google Scholar] [CrossRef]
- Ishii, N.; Okamura, Y.; Chiba, S.; Nogami, T.; Ishida, T. Giant coercivity in a one-dimensional cobalt-radical coordination magnet. J. Am. Chem. Soc. 2008, 130, 24–25. [Google Scholar] [CrossRef]
- Maria, G.F.V.; Cassaro, R.A.A.; Akpinar, H.; Schlueter, J.A.; Lahti, P.M.; Novak, M.A. A Cobalt Pyrenylnitronylnitroxide Single-Chain Magnet with High Coercivity and Record Blocking Temperature. Chem. Eur. J. 2014, 20, 5460–5467. [Google Scholar]
- Fedin, M.V.; Veber, S.L.; Sagdeev, R.Z.; Ovcharenko, V.I.; Bagryanskaya, E.G. EPR spectroscopy of thermally induced and light-induced spin transitions in heterospin exchange clusters of compounds Cu(hfac)2LR. Russ. Chem. Bull. 2010, 59, 1065–1079. [Google Scholar] [CrossRef]
- Dong, X.; Lorenc, M.; Tretyakov, E.V.; Ovcharenko, V.I.; Fedin, M.V. Light-Induced Spin State Switching in Copper(II)-Nitroxide-Based Molecular Magnet at Room Temperature. J. Phys. Chem. Lett. 2017, 8, 5587–5592. [Google Scholar] [CrossRef]
- Tretyakov, E.; Fedyushin, P.; Bakuleva, N.; Korlyukov, A.; Dorovatovskii, P.; Gritsan, N.; Dmitriev, A.; Akyeva, A.; Syroeshkin, M.; Stass, D.; et al. Series of Fluorinated Benzimidazole-Substituted Nitronyl Nitroxides: Synthesis, Structure, Acidity, Redox Properties, and Magnetostructural Correlations. J. Org. Chem. 2023, 88, 10355–10370. [Google Scholar] [CrossRef] [PubMed]
- Luneau, D. Coordination Chemistry of Nitronyl Nitroxide Radicals Has Memory. Eur. J. Inorg. Chem. 2020, 2020, 597–604. [Google Scholar] [CrossRef]
- Fedyushin, P.A.; Serykh, A.A.; Vinogradov, A.S.; Mezhenkova, T.V.; Platonov, V.E.; Nasyrova, D.I.; Samigullina, A.I.; Fedin, M.V.; Zayakin, I.A.; Tretyakov, E.V. Biradical with a polyfluorinated terphenylene backbone. Russ. Chem. Bull. 2022, 71, 1670–1678. [Google Scholar] [CrossRef]
- Fedyushin, P.A.; Akyeva, A.Y.; Syroeshkin, M.A.; Rybalova, T.V.; Stass, D.V.; Korolev, V.A.; Tretyakov, E.V.; Egorov, M.P. Synthesis, structure, and properties of tert-butyl perfluorobiphenyl nitroxide. Russ. Chem. Bull. 2022, 71, 1474–1482. [Google Scholar] [CrossRef]
- Politanskaya, L.V.; Fedyushin, P.A.; Rybalova, T.V.; Bogomyakov, A.S.; Asanbaeva, N.B.; Tretyakov, E.V. Fluorinated Organic Paramagnetic Building Blocks for Cross-Coupling Reactions. Molecules 2020, 25, 5427. [Google Scholar] [CrossRef]
- Fedyushin, P.; Rybalova, T.; Asanbaeva, N.; Bagryanskaya, E.; Dmitriev, A.; Gritsan, N.; Kazantsev, M.; Tretyakov, E. Synthesis of Nitroxide Diradical Using a New Approach. Molecules 2020, 25, 2701. [Google Scholar] [CrossRef]
- Tretyakov, E.; Fedyushin, P.; Panteleeva, E.; Gurskaya, L.; Rybalova, T.; Bogomyakov, A.; Zaytseva, E.; Kazantsev, M.; Shundrina, I.; Ovcharenko, V. Aromatic SNF-Approach to Fluorinated Phenyl tert-Butyl Nitroxides. Molecules 2019, 24, 4493. [Google Scholar] [CrossRef]
- Gulyaev, D.; Serykh, A.; Tretyakov, E.; Akyeva, A.; Syroeshkin, M.; Gorbunov, D.E.; Maltseva, S.V.; Gritsan, N.P.; Romanenko, G.; Bogomyakov, A. Effects of Difluorophenyl Substituents on Structural, Redox, and Magnetic Properties of Blatter Radicals. Catalysts 2023, 13, 1206. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Fokin, S.V.; Romanenko, G.V.; Shvedenkov, Y.G.; Ikorskii, V.N.; Tretyakov, E.V.; Vasilevskii, S.F. Nonclassical Spin Transitions. J. Struct. Chem. 2002, 43, 153–167. [Google Scholar] [CrossRef]
- Serykh, A.; Tretyakov, E.; Fedyushin, P.; Ugrak, B.; Dutova, T.; Lalov, A.; Korlyukov, A.; Akyeva, A.; Syroeshkin, M.; Bogomyakov, A.; et al. N-Fluoroalkylpyrazolyl-substituted Nitronyl Nitroxides. J. Mol. Struct. 2022, 1269, 133739. [Google Scholar] [CrossRef]
- Kudryavtseva, E.; Serykh, A.; Ugrak, B.; Dutova, T.; Nasyrova, D.; Korlyukov, A.; Zykin, M.; Efimov, N.; Bogomyakov, A.; Tretyakov, E. An Influence of Fluorinated Alkyl Substituents on Structure and Magnetic Properties of Mn(II) Complexes with Pyrazolyl-Substituted Nitronyl Nitroxides. Crystals 2023, 13, 1528. [Google Scholar] [CrossRef]
- Ovcharenko, V.I.; Fokin, S.V.; Romanenko, G.V.; Korobkov, I.V.; Rey, P. Synthesis of vicinal bishydroxylamine. Russ. Chem. Bull. 1999, 48, 1519–1525. [Google Scholar] [CrossRef]
- Porter, L.C.; Dickman, M.H.; Doedens, R.J. Bis(nitroxyl) adducts of cobalt and nickel hexafluoroacetylacetonates. Preparation, structures, and magnetic properties of M(F6acac)2(proxyl)2 (M = Co2+, Ni2+). Inorg. Chem. 1988, 27, 1548–1552. [Google Scholar] [CrossRef]
- Cotton, F.A.; Holm, R.H. H. Magnetic investigations of spin-free cobaltous complexes. III. On the existence of planar complexes. J. Am. Chem. Soc. 1960, 82, 2979–2983. [Google Scholar] [CrossRef]
- Bertrand, J.A.; Kaplan, R.I. A study of bis(hexafluoroacetylacetonato) copper(II). Inorg. Chem. 1966, 5, 489–491. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.41.106a. Rigaku Oxford Diffraction. Rigaku: Tokyo, Japan, 2021.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 229–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Software for Empirical Absorption Correction, SADABS Version 2.10; University of Göttingen: Göttingen, Germany, 2002. [Google Scholar]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Ullman, E.F.; Osiecki, J.H.; Boocock DG, B.; Darcy, R. Stable free radicals. X. Nitronyl nitroxide monoradicals and biradicals as possible small molecule spin labels. J. Am. Chem. Soc. 1972, 94, 7049. [Google Scholar] [CrossRef]
- Fokin, S.; Ovcharenko, V.; Romanenko, G.; Ikorskii, V. Problem of a Wide Variety of Products in the Cu(hfac)2−Nitroxide System. Inorg. Chem. 2004, 43, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Beppu, S.; Tanaka, K.; Kuratsu, M.; Furuichi, K.; Kozaki, M.; Suzuki, S.; Shiomi, D.; Sato, K.; Takui, T.; et al. Preparation, structure, and magnetic interaction of a Mn(hfac)2-bridged [2-(3-pyridyl)(nitronyl nitroxide)–Mn(hfac)2]2 chain complex. Chem. Commun. 2007, 24, 2485–2487. [Google Scholar] [CrossRef]
- Field, L.M.; Lahti, P.M.; Palacio, F. 1∶1 Complexes of 5-(4-[N-tert-butyl-N-aminoxyl]phenyl)pyrimidine with manganese(II) and copper(II) hexafluoroacetonylacetonate. Chem. Commun. 2002, 6, 636–637. [Google Scholar] [CrossRef]
- Wang, H.-M.; Liu, Z.-L.; Liu, C.-M.; Zhang, D.-Q.; Lu, Z.-L.; Geng, H.; Shuai, Z.-G.; Zhu, D.-B. Coordination Complexes of 2-(4-Quinolyl)nitronyl Nitroxide with M(hfac)2 [M = Mn(II), Co(II), and Cu(II)]: Syntheses, Crystal Structures, and Magnetic Characterization. Inorg. Chem. 2004, 43, 4091–4098. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.L.; Ma, Y.; Wang, Q.L.; Li, L.C.; Liao, D.Z. A Four-spin Cobalt-radical Complex: Structure and Magnetic Properties. Chem. Lett. 2012, 41, 1523–1525. [Google Scholar] [CrossRef]
- Liu, R.-N.; Li, L.-C.; Xing, X.-Y.; Liao, D.-Z. Cyclic metal–radical complexes based on 2-[4-(1-imidazole)phenyl]-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide: Syntheses, crystal structures and magnetic properties. Inorganica Chim. Acta 2009, 362, 2253–2258. [Google Scholar] [CrossRef]
- Ma, J.-K.; Chen, J.; Zhang, Y.-J.; Wang, Q.-L. Two New Cobalt(II) Heterotrispin Dimers Bridged by Functional Nitronyl Nitroxides: Structure and Magnetic Properties. Sci. Adv. Mater. 2023, 15, 799–806. [Google Scholar] [CrossRef]
- Vasilevsky, S.F.; Tretyakov, E.V.; Usov, O.M.; Molin, Y.N.; Fokin, S.V.; Shwedenkov, Y.G.; Ikorskii, V.N.; Romanenko, G.V.; Sagdeev, R.Z.; Ovcharenko, V.I. A new family of stable 2-imidazoline nitroxides. Mendeleev Commun. 1998, 8, 216–218. [Google Scholar] [CrossRef]
- Romanenko, G.V.; Fokin, S.V.; Vasilevskii, S.F.; Tret’yakov, E.V.; Shvedenkov, Y.G.; Ovcharenko, V.I. Dimeric Complexes of Manganese(II) and Nickel(II) Hexafluoroacetylacetonates with Pyrazole-Containing Nitronylnitroxyl Radicals. Russ. J. Coord. Chem. 2001, 27, 360–367. [Google Scholar] [CrossRef]
- Rancurel, C.; Leznoff, D.B.; Sutter, J.-P.; Golhen, S.; Ouahab, L.; Kliava, J.; Kahn, O. Synthesis, Structure and Magnetism of Mono- and Binuclear Manganese(II) Compounds of Nitronyl Nitroxide Substituted Phosphine Oxides. Inorg. Chem. 1999, 38, 4753–4758. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Sessoli, R. Magnetic properties of a layered molecular material comprising manganese hexafluoroacetylacetonate and nitronyl nitroxide radicals. Inorg. Chem. 1993, 32, 4612–4616. [Google Scholar] [CrossRef]
- Okada, K.; Nagao, O.; Mori, H.; Kozaki, M.; Shiomi, D.; Sato, K.; Takui, T.; Kitagawa, Y.; Yamaguchi, K. Preparation and Magnetic Properties of Mn(hfac)2-Complexes of 2-(5-Pyrimidinyl)- and 2-(3-Pyridyl)-Substituted Nitronyl Nitroxides. Inorg. Chem. 2003, 42, 3221–3228. [Google Scholar] [CrossRef]
- Guo, J.N.; Wang, J.J.; Sun, G.F.; Li, H.D.; Li, L.C. A novel nitronyl nitroxide radical containing thiophene and pyridine rings and its manganese(II) complex: Synthesis, structure, and magnetic properties. J. Coord. Chem. 2017, 70, 1926–1935. [Google Scholar] [CrossRef]
- Luneau, D.; Rey, P.; Laugier, J.; Fries, P.; Caneschi, A.; Gatteschi, D.; Sessoli, R. Nitrogen-bonded copper (II)-imino nitroxide complexes exhibiting large ferromagnetic interactions. J. Am. Chem. Soc. 1991, 113, 1245–1251. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Rey, P. Toward molecular magnets: The metal-radical approach. Acc. Chem. Res. 1989, 22, 392–398. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Xiang, R.; Lan, L.; Dong, X.; Sakiyama, H.; Muddassir, M. Auxiliary linkers-induced assembly of two 2D Co(II)-based coordination polymers with different interpenetrating fashion: Structure and magnetism. Polyhedron 2023, 244, 116625. [Google Scholar] [CrossRef]
- Yang, M.; Xie, S.; Liang, X.; Zhang, Y.; Dong, W. A novel functional nitronyl nitroxide and its manganese and cobalt complexes: Synthesis, structures and magnetic properties. Polyhedron 2019, 161, 132–136. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.N.; Zhang, S.L.; Zhao, X.H.; Shao, D.; Wang, X.Y. Syntheses and magnetic properties of a pyrimidyl-substituted nitronyl nitroxide radical and its cobalt (II) complexes. Chem. Commun. 2016, 52, 5033–5036. [Google Scholar] [CrossRef] [PubMed]
- Zheludev, A.; Barone, V.; Bonnet, M.; Delley, B.; Grand, A.; Ressouche, E.; Rey, P.; Subra, R.; Schweizer, J. Spin density in a nitronyl nitroxide free radical. Polarized neutron diffraction investigation and ab initio calculations. J. Am. Chem. Soc. 1994, 116, 2019–2027. [Google Scholar] [CrossRef]
- Kahn, O.; Briat, B. Exchange interaction in polynuclear complexes. Part 1—Principles, model and application to the binuclear complexes of chromium(III). J. Chem. Soc. Faraday Trans. 2 1976, 72, 268–281. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, T.; Xiang, R.; Dong, X.; Sakiyama, H.; Muddassir, M.; Pan, Y. Impact of N-donor auxiliary ligands on three new Co(II)-based coordination polymers with symmetrical tetracarboxylate ligands: A magnetism study. New J. Chem. 2023, 47, 20426–20434. [Google Scholar] [CrossRef]
- Balachandran, C.; Haribabu, J.; Jeyalakshmi, K.; Bhuvanesh, N.S.P.; Karvembu, R.; Emi, N.; Awale, S. Nickel(II) bis(isatin thiosemicarbazone) complexes induced apoptosis through mitochondrial signaling pathway and G0/G1 cell cycle arrest in IM-9 cells. J. Inorg. Biochem. 2018, 182, 208–221. [Google Scholar] [CrossRef]
- Rahman, K.N.A.; Haribabu, J.; Balachandran, C.; Bhuvanesh, N.S.P.; Karvembu, R.; Sreekanth, A. Copper, nickel and zinc complexes of 3-acetyl coumarin thiosemicarbazone: Synthesis, characterization and in vitro evaluation of cytotoxicity and DNA/protein binding properties. Polyhedron 2017, 135, 26–35. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, T.; Liu, Y.; An, N.; Afzal, M.; Alarifi, A.; Muddassir, M.; Sakiyama, H.; Mohanty, A.; Dong, X. Structures and magnetic studies of four new Ni(II) coordination polymers built using symmetrical tetracarboxylate and N-donor linkers. New J. Chem. 2023, 47, 21214–21224. [Google Scholar] [CrossRef]
- Caneschi, A.; Gatteschi, D.; Grand, A.; Laugier, J.; Pardi, L.; Rey, P. Moderate Ferromagnetic Exchange between Copper(II) and a Nitronyl Nitroxide in a Square-Pyramidal Adduct. MO Interpretation of the Mechanism of Exchange in Copper(II)–Nitroxide Complexes. Inorg. Chem. 1988, 27, 1031–1035. [Google Scholar] [CrossRef]
- Iwahori, F.; Markosyan, A.S.; Inoue, K. Structures and Magnetic Properties of the Complexes Made up by Cu(hfac)2 and Bisnitroxide Radical Derivatives. Mol. Cryst. Liq. Cryst. 2002, 376, 449–454. [Google Scholar] [CrossRef]



| Complex | M–ONO | M–N | M–Ohfac | ∠MONON | N–OM N–O | ONO⋯ONO |
|---|---|---|---|---|---|---|
| [Mn(hfac)2LF]2 296 K | 2.127(4) | 2.289(5) | 2.137(5)– 2.166(5) | 129.4(4) | 1.315(6) 1.263(7) | 5.547(9) |
| [Mn(hfac)2LF]2 100 K | 2.126(2) | 2.273(2) | 2.148(2)– 2.174(2) | 125.3(1) | 1.303(3) 1.270(3) | 5.922(3) |
| [Co(hfac)2LF]2 | 2.032(2) | 2.150(2) | 2.038(2)– 2.109(2) | 121.8(1) | 1.308(2) 1.266(3) | 3.715(3) |
| [Ni(hfac)2LF]2 | 2.034(3) | 2.097(4) | 2.011(3)– 2.051(3) | 122.3(3) | 1.303(5) 1.276(5) | 3.407(6) |
| [Cu(hfac)2LF]n | 2.448(2) | 2.469(2) | 1.933(2)– 1.968(2) | 131.9(2) | 1.292(3) 1.269(3) | 4.163(7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudryavtseva, E.; Serykh, A.; Ugrak, B.; Dutova, T.; Nasyrova, D.; Aleshin, D.; Efimov, N.; Dorovatovskii, P.; Bogomyakov, A.; Fokin, S.; et al. 5-Fluoro-1-Methyl-Pyrazol-4-yl-Substituted Nitronyl Nitroxide Radical and Its 3d Metal Complexes: Synthesis, Structure, and Magnetic Properties. Crystals 2023, 13, 1655. https://doi.org/10.3390/cryst13121655
Kudryavtseva E, Serykh A, Ugrak B, Dutova T, Nasyrova D, Aleshin D, Efimov N, Dorovatovskii P, Bogomyakov A, Fokin S, et al. 5-Fluoro-1-Methyl-Pyrazol-4-yl-Substituted Nitronyl Nitroxide Radical and Its 3d Metal Complexes: Synthesis, Structure, and Magnetic Properties. Crystals. 2023; 13(12):1655. https://doi.org/10.3390/cryst13121655
Chicago/Turabian StyleKudryavtseva, Ekaterina, Andrey Serykh, Bogdan Ugrak, Tatyana Dutova, Darina Nasyrova, Dmitrii Aleshin, Nikolay Efimov, Pavel Dorovatovskii, Artem Bogomyakov, Sergey Fokin, and et al. 2023. "5-Fluoro-1-Methyl-Pyrazol-4-yl-Substituted Nitronyl Nitroxide Radical and Its 3d Metal Complexes: Synthesis, Structure, and Magnetic Properties" Crystals 13, no. 12: 1655. https://doi.org/10.3390/cryst13121655





