Abstract
V-bearing molten iron was obtained by adding Na2CO3 in the smelting process of vanadium titanomagnetite at low temperature. Two forms of V-rich carbides ((Fe,V)3C, VC) were detected in the V-bearing pig iron products. Once the smelting temperature was above 1300 °C, most of the V in the raw ore was reduced into molten iron. Owning to the high content of V, the unsteady (Fe,V)3C solid solution decomposed along with the precipitation of graphite and VC during the solidification process. The presence of VC cluster and VC precursor in (Fe,V)3C was detected by transmission electron microscopy, which confirmed the possibility of this transition process at the atomic perspective. The transformation dramatically affected the compositions and properties of V-bearing pig iron and had important guiding significance for the actual production process.
1. Introduction
Vanadium is an important strategic metal. In 2021, 89% of the global vanadium output came from vanadium titanomagnetite [1]. V-bearing molten iron is a key intermediate product, and its composition mainly depends on the raw material and smelting process. Under traditional blast furnace smelting conditions, the reduction temperature is about 1500 °C [2,3]. A large number of high-melting-point carbides such as TiC, TiN, VC, and VN are formed in the molten iron [4], leading to a series of production difficulties and quality problems [5,6,7]. Wang [8] studied the thermodynamic properties such as the solubility and activity coefficient of V and Ti elements in the molten iron. He [9] and Zhang [10] revealed the influence mechanism of molten iron fluidity by studying viscous flow properties focusing on the carbides of titanium. Hou [11] found through thermodynamic calculation that the precipitation amount of VC and VN in V-bearing molten iron increased sharply at 1200–1250 °C, which was consistent with the change trend of hot metal viscosity. The formation of V-rich carbonitride particles was observed by a confocal laser scanning high-temperature microscope. However, the precipitation process of V-rich carbides has not been thoroughly studied.
The research on carbides of vanadium was mainly concentrated in the field of high-V medium-/low-carbon steel [12,13,14], focusing on the morphology and distribution of carbides in the alloy structure and its modification control measures. Kesri [15] studied the phase diagram of Fe–C–V ternary system and determined the thermodynamic stable regions of four phases: austenite (A, γ-Fe ), ferrite (F, α-Fe), VCx type carbide, and M3C type carbide in the solidification process, in which the equilibrium temperature between the VCx and M3C stable regions was 1120 °C. Kawalec [16] and Fras [17] calculated the eutectic transformation process of VC in white cast iron, proposed the concept of the degree of eutectic saturation (Sc) in high-vanadium cast iron, and characterized the microstructure of eutectic VC. Recently, microalloyed steel with Nb, V, and Ti has received more and more attention with the increasing demand for high-quality steel [18,19]. The precipitation mechanism of the second-phase particles and their precipitation strengthening effect on the alloy structure have been studied extensively [20,21]. Pan [22] found five forms of V carbides in V-microalloyed medium-carbon steel. Liu [23] studied the effect of V content on VC precipitation in PD3 steel. Sayed [24] studied the effect of the heat-treatment parameters on the random vanadium precipitation in low-carbon steel. Javier [25] proposed a model to simulate the precipitation of V(CN). Li [26] revealed the hetero-nucleation effect of V(CN) during γ→α phase change. Wang [27] observed an intermediate coherent crystal structure within the α-Fe matrix through TEM, revealing a two-step nucleation and growth mechanism of V(CN) in microalloyed steels. However, all the above studies focused on the eutectoid process of steel. The precipitation process and mechanism of VC in V-bearing molten iron in the solidification process have not been fully explored.
Recently, a novel low-temperature smelting technology was developed by adding soda [28] or alkali [29] in the reduction smelting process of vanadium titanomagnetite. Chen’s research [30,31] showed that the oxide components in the slag were transformed into highly active sodium salt. This slag system has a low melting point and high fluidity, and it displays excellent impurity removal performance. Thus, this process can produce high-quality V-bearing molten iron at 1150–1300 °C. However, this molten iron has its own unique properties to be further investigated due to the existence of V-rich carbides. In this paper, V-bearing pig iron with different compositions was prepared. The morphology and structure of V-rich carbides and their precipitation characteristics in the pig iron structures were studied. This study is expected to provide technical support for the industrial practice of low-temperature smelting of vanadium titanomagnetite and the high-value utilization of V-bearing pig iron.
2. Experiment
2.1. Raw Materials and Experimental Procedures
The vanadium titanomagnetite used in the experiment came from a mine in Chaoyang, Liaoning Province, China (composition as shown in Table 1, average particle size 48 μm); the reducing agent anthracite (composition as shown in Table 2, crushed and screened to below 150 μm) and anhydrous Na2CO3 (AR, 99.8 wt%) were purchased from the Sinopharm Chemical Reagents Co., Ltd.(Shanghai, China) The smelting process was carried out in a box-type resistance furnace (Tianjin Zhonghuan Electric Furnace Co., Ltd., Tianjin, China, model: SX-D64163).

Table 1.
Chemical composition of vanadium titanomagnetite concentrate (wt.%).

Table 2.
Chemical composition of anthracite (wt.%).
Firstly, raw ore, anhydrous Na2CO3, and anthracite were mixed in a ratio of 10:6:3 and placed in a graphite clay crucible. When the temperature of the furnace rose to the setting value, it was held on for 10 min, and then the graphite clay crucible was quickly moved into the furnace chamber. The reaction timing began until the temperature stabilized again to the setting value. After the reaction, the crucible was taken out and cooled in the air. After cooling to room temperature, pig iron samples were separated from the slag for analysis. The flow sheet of the smelting process of vanadium titanomagnetite is shown in Figure 1.
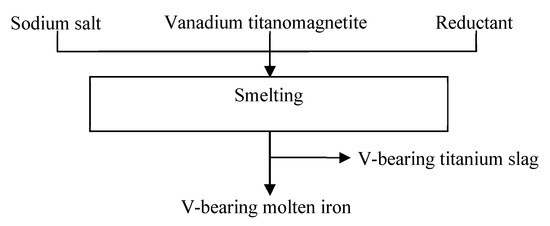
Figure 1.
Flow sheet of the smelting process of vanadium titanomagnetite.
By adding auxiliaries such as Na2CO3 or NaOH in the smelting process, high-quality V-bearing molten iron with a low content of Ti (<0.03 wt.%), Si (<0.03 wt.%), S (<0.003 wt.%), and P (<0.005 wt.%) was produced. In view of the fact that the contents of C (4–5 wt.%) and V (0.3–2 wt.%) elements in the pig iron, the low-temperature property of the molten iron was mainly affected by these two elements due to the formation of V-rich carbides with a high melting point.
2.2. Theory Background
The Fe–C phase diagram has been studied quite thoroughly [32,33]. However, it is still controversial in some aspects, such as the specific temperature point and C content of the eutectic and eutectoid transformation lines. In general [34,35,36], the highest content of C in the F phase is 0.0218 wt.%, in the A phase is 2.11 wt.%, and in cementite (Fe3C) is 6.69 wt.%.
The natural cooling rate is very fast in laboratory conditions. For hypereutectic white cast iron (wt.% (C) = 4.3–6.69%), the phase transition follows the diagram of metastable equilibrium Fe–Fe3C [33,36]. Firstly, primary cementate (Fe3CI) is crystallized from the liquid phase. Then, the isothermal eutectic transformation occurs at the eutectic point (1148 °C, wt.% (C) = 4.3%). Eutectic A phase is precipitated with eutectic Fe3C; these two phase form ledeburite cast (Ld), and its form at room temperature is L’d. After the liquid phase disappears, the secondary cementate (Fe3CII) is precipitated from the A phase. Upon approaching the eutectoid point (723 °C, wt.% (C) = 0.77%), the eutectoid F phase is precipitated with eutectoid Fe3C. These two phases form pearlite (P). Lastly, the third cementate (Fe3CIII) is precipitated from the F phase. Therefore, the equilibrium microstructure of cast iron at room temperature is as follows: primary cementate (Fe3CI) + L’d (eutectic Fe3C + Fe3CII + P (eutectoid Fe3C + Fe3CIII + F)).
Fe3C is an interstitial solid solution with the broad homogeneity range [35,36]. There are still some aspects under be explored, especially the carbide section of the diagram. The situation becomes even more confusing when the V element is present in the system.
2.3. Test Method
The carbon content in pig iron was determined using a CS-2800 carbon sulfur analyzer (NCS Testing Technology CO., Ltd., Beijing, China). The content of vanadium in pig iron was determined by high-sensitivity XRF (PHECDA-PRO, Beijing Ancoren Technology Co., Ltd., Beijing, China). The morphology and composition of the various phases in the pig iron samples were analyzed using a mineral dissociation analyzer (MLA250, FEI Company, Hillsboro, Oregon, United States). Furthermore, the pig iron was thinned by focused ion beam scanning electron microscopy (Helios G4 PFIB, Thermo Scientific™, Waltham, MA, USA), and the fine structure of the V-rich carbides and pig iron matrix was characterized by high-resolution field-emission transmission electron microscopy (JEM-F200, JEOL, Tokyo, Japan).
3. Results and Discussion
3.1. C and V Content of the Pig Iron
Several samples of V-bearing pig iron were prepared under different smelting conditions. The effects of different melting temperature and reaction time on the contents of C and V elements in the samples were investigated. The results are shown in Figure 2.
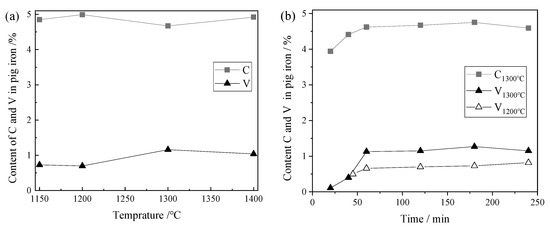
Figure 2.
Content of C and V elements in pig iron at different smelting parameters: (a) effect of smelting temperature; (b) effect of smelting time.
As shown in Figure 2a, the C content in pig iron at different melting temperatures had little change with an average content of about 4.85 wt.%. However, the V content in pig iron increased with the increase in temperature. The V content was 0.70 wt.% at 1200 °C and 1.16 wt.% at 1300 °C.
The variation trend of V content in pig iron was almost consistent with that of C in Figure 2b. When the smelting temperature was 1300 °C, the iron was separated with the slag at 20 min, the content of C was 3.94 wt.%, and the content of V was only 0.11 wt.%. Then, the content of C and V increased rapidly. After 60 min of reaction, the content of C and V tended to change gently, and the content of C and V remained at about 4.8 wt.% and 1.1 wt.%, respectively. When the smelting temperature was 1200 °C, it took about 45 min for the separation of iron and slag to be completed. At this time, the content of V was 0.5 wt.%, and then it slowly increased to 0.7–0.9%.
It could be inferred that the solubility of V element in pig iron was greatly affected by the C content. The significant difference in V content in pig iron at 1200 °C and 1300 °C may be due to the transformation of the V-rich carbides in pig iron.
3.2. Morphology and Composition
The content of C was different in various phases; thus, it could be inferred that the content of V in different pig iron structures was also changing. For hypereutectic white cast iron, it depended on its solubility in the Fe3C phase and the F phase, as well as the proportion of the two phases in the structure. The microstructure of V-bearing pig iron can be further analyzed in Figure 3.
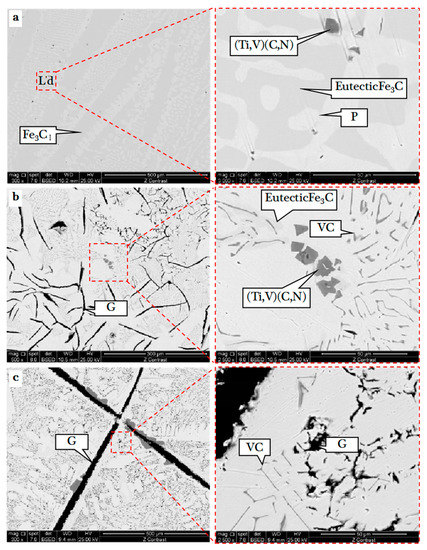
Figure 3.
SEM images of the V-bearing pig iron at different smelting temperature: (a) 1200 °C, 120 min; (b) 1300 °C, 120 min; (c) 1400 °C, 120 min.
As shown in Figure 3a, V-bearing pig iron at 1200 °C was mainly composed of the dark-gray long-strip Fe3CI phase and light and dark L’d phase. The bright color phase was the P phase, and the gray phase was a mixture of eutectic Fe3C and Fe3CII. There were dark-gray particles in different L’d phases. The L’d structure almost disappeared at 1300 °C (Figure 3b), and there appeared a blacker fine flake graphite phase (G) which separated the iron matrix, while the P phase region expanded substantially. The original eutectic Fe3C region was transformed into a large number of gray worm-like and rod-shaped particles with centripetal converging distribution, while the dark-gray cube particles were in the core. Figure 3c showed the appearance of large flake G-phase and large cube particles over 100 μm at 1400 °C. The number of gray worm-like and rod-shaped particles increased, and the distribution was more regular, indicating that the phase precipitation had better crystallinity with the increase in the smelting temperature. The components of different phases in pig iron at 1200 °C were analyzed by EDS point scanning as shown in Figure 4 and Table 3.
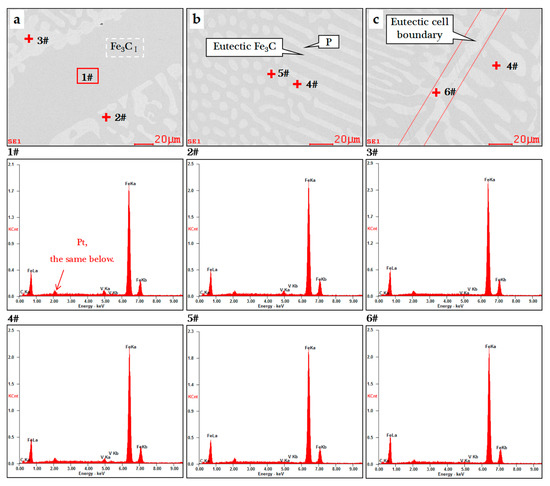
Figure 4.
SEM-EDS images of the V-bearing pig iron at 1200 °C: (a) Fe3CI and adjacent L’d phases; (b) L’d phase consisting of eutectic Fe3C and pearlite phases; (c) eutectic cell boundary region.

Table 3.
EDS results of the V-bearing pig iron at 1200 °C.
The results showed that the highest content of V was 2.18 wt.% (Figure 4a), which existed in the Fe3CI, while the lowest V content in P was 0.31 wt.% (Figure 4c). Although EDS results had a large deviation, it was obvious that the Fe3C phase could enrich V, and the solubility of V in the A phase was limited. Assuming that all Fe3C had the same V content, it could be inferred that the V content in F phase was very low; the value reported in the literature [22] was only 0.09 wt.%.
According to the relevant theories in Section 2.2, Fe3CI was the first phase precipitated from the V-bearing molten iron; accordingly, it had the highest content of V. Then the V content in the subsequent precipitated eutectic Fe3C gradually decreased due to the insufficient content of V. According to the lever rule, the content of Fe3CI in the V-bearing pig iron is presented in Equation (1).
Then, the LD phase accounted for 87%. The theoretical content of eutectic Fe3C precipitated from LD is presented in Equation (2).
Theoretically, for this hypereutectic white cast iron product, the content of Fe3C phase was 78.9% and that of F phase was 21.1%. The chemical formula of this V-rich Fe3C could be written as (Fe1−x,Vx)3C, (0 ≤ x ≤ 1) (for short, (Fe,V)3C).
The components of different phases in pig iron at 1300 °C were analyzed by EDS point scanning and mapping scanning, as shown in Figure 5 and Table 4.
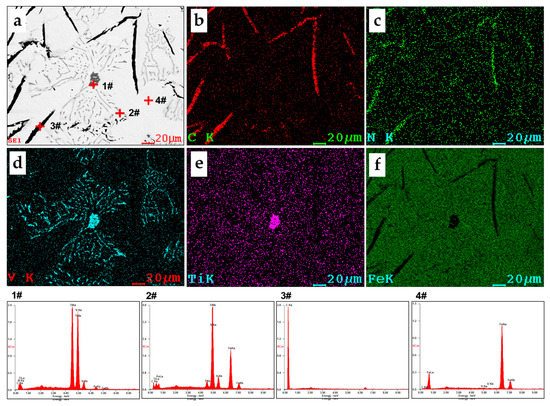
Figure 5.
SEM-EDS images of the V-bearing pig iron at 1300 °C: (a) (Ti,V)(C,N),VC, graphite, and austenite phases in pig iron; (b–f) mapping scanning measurement of C, N, V, Ti, and Fe elements.

Table 4.
EDS results of the V-bearing pig iron at 1300 °C.
In Figure 5a, the content of V and Ti in regular cube particles was 35.70 wt.% and 41.51 wt.%, respectively. These secondary phase particles were (Ti,V)(C,N). The worm-like and rod-like gray particles were mainly composed of VC, with a small amount of Ti. Due to the small size of the particle, EDS scanning could inevitably identify a relatively high content of matrix Fe. After removing Fe, the content of V in VC phase reached 65 wt.%. Considering that there was no V in the G phase and (Ti,V)(C,N) particles were few, the precipitation of VC almost enriched the majority of V from the molten iron.
By further comparison of Figure 3a,c, it can be seen that the eutectic (Fe,V)3C region originally in L’d was greatly reduced, while a large amount of VC phase appeared in the gap of the G phase, indicating that (Fe,V)3C solid solution was in a kind of metastable phase, which would decompose into the G phase and generate VC under certain conditions. The morphology of VC was consistent with the literature [15,16,17,18]. It also showed that excessive element V, a strongly graphitized element, could reduce the stability of Fe3C.
To sum up, the V content in molten iron was low below 1300 °C. The liquid iron had good fluidity, and the impurity content was very low. The obtained pig iron was mainly composed of (Fe,V)3C and P phase. Above 1300 °C, the V content in molten iron increased by more than 1 wt.%; (Fe,V)3C would lost its stability and decomposed along with the precipitation of graphite and VC during the solidification process.
3.3. Interface Structures and Phase Transformation Process
High-resolution structures of pig iron were detected by FIB-TEM. Figure 6a shows the FIB section of the pig iron sample. In Figure 6b, it can be seen that the V-bearing pig iron structure contained three phases: (Fe,V)3C, VC, and F.
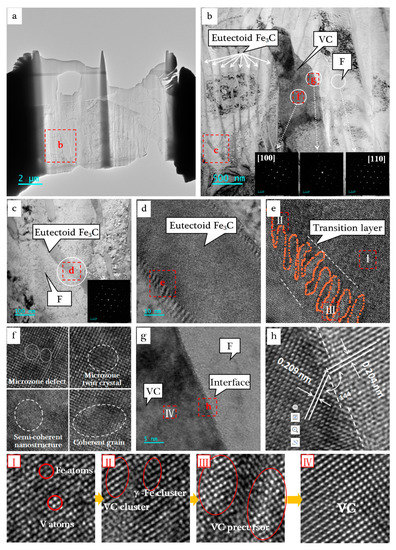
Figure 6.
TEM image and electron diffraction pattern of vanadium-bearing pig iron. (a) FIB section; (b) VC and pearlite matrix; (c) eutectic cementite and ferrite matrix; (d) (Fe,V)3C; (e) interface fluctuation zone; (f) nanoparticles in VC phase; (g) interface between VC and ferrite matrix; (h) interface structure; (I) (Fe,V)3C solid solution structure; (II) VC cluster; (III) VC grain; (Ⅳ) VC.
Figure 6c shows the α-Fe matrix and eutectic Fe3C in the P phase. According to the above analysis, the eutectoid Fe3C was also enriched with V. Surprisingly, the atomic structure of the (Fe,V)3C solid solution was perfectly preserved due to the eutectoid transition from A to P being a solid phase transition process. We can clearly see the obvious interface undulating transition layer in the Figure 6d,e.
Figure 6f shows the high-resolution structure of VC phases, which were full of various microdefects, microtwins, semi-colattice nanostructures, and colattice nanoparticles. Figure 6g,h shows the interface structure of VC and F phases. It can be seen that there were small fluctuations of about 3–4 atomic layers thickness at the interface, from which the mismatches of semi-coherent interface micro-regions and noncoherent dislocations can be seem. The VC and A phases were eutectic precipitated from the (Fe,V)3C matrix. When the temperature was above 723 °C, both phases had an FCC structure. Therefore, the interface should be a coherent or semi-coherent interface. When the temperature was below 723 °C, the A phase transformed into the F phase. Its structure changed from FCC to BCC, during which the atomic rearrangement led to the mismatches of the interfacial lattice. As can be seen from the Figure 6h, there was a difference of 0.005 nm in the crystal plane spacing between VC and F phase; thus, it showed periodic mismatches, forming a stepwise dislocation.
As shown in Figure 6I, there were many vacancies and defects in the complex orthogonal lattice structure of Fe3C. When the V atoms were solidly dissolved into the Fe3C lattice, they would partially displace the Fe atom and formed (Fe,V)3C solid solution. In Figure 6II, we can see the VC cluster structure formed by more than six V atoms converging together. The VC precursor crystal nucleus is shown in Figure 6III. Figure 6IV shows the local structure of VC particles. The process of VC precipitation from (Fe,V)3C is clearly displayed at the atomic level through the TEM images.
The clear images of the transition process provided by TEM helped in explaining the whole process of VC phase precipitation from the (Fe,V)3C phase during the solidification of the V-bearing molten iron.
- (a)
- The nucleation induction stage. With the decrease in the temperature, the first crystallized (Fe,V)3CI phase was unstable. It decomposed into large GI flakes and released V element into the liquid phase. As a result, C content decreased while V content increased in the adjacent liquid phase. Then, the A phase was precipitated out of the GI phase as the heterogeneous nucleation interface [37]. C and V elements were released into the adjacent liquid phase due to their low solubility in A phase, which then induced the formation of (Fe,V)3CI and further decomposed into GI.
- (b)
- The nucleation pregnant stage. With the continuous progress of the process (1), the content of C in the residual liquid phase dropped to the eutectic component, while the content of V element increased and dissolved into the eutectic (Fe,V)3C phase. The size effect caused lattice distortion with the increase in solid solubility, leading to a gradual decrease in the stability of the (Fe,V)3C phase. Then, many VC cluster structures were formed due to the fluctuation of V concentration and energy.
- (c)
- The nucleus formation stage. When the total distortion energy reached the critical value, these clusters first adsorbed to the A phase matrix interface under induction of the FCC structure. Then, they further absorbed the adjacent VC clusters. Finally, they grew into a VC precursor nucleus which started from the A phase matrix and protruded into the (Fe,V)3C phase. For the eutectoid process, due to the difficulty of diffusion in the solid phase transition process and the absence of redundant V atoms, the VC precursor crystal nucleus could not be further grown; finally, the two-phase undulating interface structure was formed. Wang [27] made a similar discovery in V-microalloyed steels.
- (d)
- The precipitation and growth stage. For the eutectic process, VC precursor crystal nuclei further converged and grew, forming the VC primary crystal nuclei. With the progress of the reaction, the content of C and V elements adjacent to the crystal nuclei decreased, while the content outside was higher, thus forming a concentration gradient of C and V elements decreased to the grain center. Therefore, the growth direction of VC deviated from the center of the sphere. Under the restriction of the separation of the A phase and G phase, the VC phase was precipitated continuously and finally formed a radiating eutectic cell [13]. If there were (Ti,V)(C,N) second-phase particles or other impurity particles precipitated earlier in the molten iron, they would become the core of VC eutectic cell as the heterogeneous nucleated crystal species.
In this process, the large precipitation of (Fe,V)3CI and VC with a high melting point led to poor fluidity of the V-bearing molten iron. On the surface of molten iron, (Fe,V)3CI decomposed and a large amount of GI precipitated due to the rapid temperature drop. The eutectic precipitation temperature of the G phase was 1154 °C [32], and the solidification temperature of the molten iron actually increased. Furthermore, G burned and released a large amount of CO2 after contact with air, resulting in huge pores on the surface of cast iron and large number of small pores in the interior. This seriously affected the composition and quality of the pig iron.
4. Conclusions
V-bearing molten iron was obtained by adding Na2CO3 in the smelting process of vanadium titanomagnetite at low temperature. The morphology and structure of V-rich carbides in V-bearing pig iron were studied, and the precipitation characteristics of V-rich carbides in molten iron were discussed.
- (1)
- Vanadium trended to form carbide in iron and steel structure. There were two main forms of V-rich carbides in low-temperature V-bearing pig iron: (Fe,V)3C solid solution and VC solid solution.
- (2)
- The microstructure of pig iron and the existence form of V-rich carbides changed at different melting temperatures. In the temperature range of 1150 °C to 1250 °C, the metallographic morphology were P and (Fe,V)3C phase. When the temperature exceeded 1300 °C, the P, G, and VC phases were mainly present.
- (3)
- When V atoms were solidly dissolved into the Fe3C lattice, they partially displaced the Fe atoms and formed (Fe,V)3C solid solution. In the eutectic transition process, the supersaturated V element destroyed the stability of (Fe,V)3C and promoted the precipitation of G and VC. The existence of VC clusters and VC precursor nuclei were observed by TEM, which confirmed the possibility of this transition process at the atomic perspective.
Above 1300 °C, the rapid crystallization of (Fe,V)3C and its transition to the G and VC phases led to an increase in viscosity and poor fluidity of the V-bearing molten iron, resulting in a large number of porosity defects. The process seriously affects the composition and quality of the pig iron product. Therefore, it is recommended that the melting temperature should be controlled at no more than 1300 °C.
Author Contributions
Conceptualization, L.C. and D.C.; funding acquisition, D.C., H.Z., Y.Z., L.W. and T.Q.; investigation, L.C. and X.S.; methodology, L.C., H.Z. and Y.Z.; project administration, D.C., H.Z., Y.Z. and L.W.; resources, D.C., L.W. and T.Q.; supervision, D.C., H.Z., L.W. and T.Q.; validation, L.C., D.C., X.S. and L.W.; visualization, L.C., Y.Z., Y.L. and F.M.; writing—original draft, L.C., Y.L. and F.M.; writing—review and editing, L.C., D.C., H.Z. and L.W. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this research was provided by Strategic Priority Research Program of the Chinese Academy of Sciences (Funder: Desheng Chen, Funding number: XDC04010102, and Funder: Lina Wang, Funding number: XDC04010100), the Special Project for Transformation of Major Technological Achievements in HeBei province (Funder: Desheng Chen, Funding number: 19044012Z), the science and technology program of Hengshui (Funder: Hongxin Zhao, Funding number: 2020016004B), the Province Key R&D Program of Hebei (Funder: Yulan Zhen, Funding number: 20374105D), and the National Natural Science Foundation of China (Funder: Yahui Liu, Funding number: 22078343).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, Y.; Chen, D.H.; Liu, W.H.; Sun, Z.H.; Zhang, B.X.; He, R. Global vanadium industry development report 2021. Iron Steel Va-Nadium Titan. 2022, 43, 1–9. [Google Scholar] [CrossRef]
- Xie, H.E.; Hu, P.; Zheng, K.; Zhu, F.X. Study on phase and chemical composition of V-Ti sinter during softening, melting and dripping process. Iron Steel Vanadium Titan. 2022, 43, 107–117. [Google Scholar] [CrossRef]
- Husslage, W.M.; Bakker, T.; Steeghs, A.G.S.; Reuter, M.; Heerema, R.H. Flow of molten slag and iron at 1500 °C to 1600 °C through packed coke beds. Met. Mater. Trans. B 2005, 36, 765–776. [Google Scholar] [CrossRef]
- Wen, G.Y.; Yan, Y.Z.; Zhao, S.Z.; Huang, J.J.; Jiang, G.H.; Yang, X.M. Properties of liquid iron containing vanadium and titanium. Iron Steel 1996, 31, 6–11. [Google Scholar]
- Yu, T.; Jiang, T.; Wen, J.; Sun, H.; Li, M.; Peng, Y. Effect of chemical composition on the element distribution, phase composition and calcification roasting process of vanadium slag. Int. J. Miner. Met. Mater. 2022, 29, 2144–2151. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Xue, Z. An Environment-Friendly Process Featuring Calcified Roasting and Precipitation Purification to Prepare Vanadium Pentoxide from the Converter Vanadium Slag. Metals 2019, 9, 21. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Song, W.-C.; Li, H. Vanadium extraction and dephosphorization from V-bearing hot metal with fluxes containing CaO. J. Central South Univ. 2015, 22, 2887–2893. [Google Scholar] [CrossRef]
- Wang, H.C.; Chen, E.B.; Dong, Y.C.; Li, W.C. Analysis of the thermodynamic Property of Fe-C-V melt. J. Univ. Sci. Technol. Beijing 2000, 22, 312–315. [Google Scholar]
- He, Y.Y.; Liu, Q.C.; Yang, J.; Chen, Q.X.; Ao, W.Z. Experimental Investigation on Fluidity of Hot Metal Bearing Titanium. Iron Steel Vanadium Titan. 2010, 31, 10–14. [Google Scholar]
- Zhang, J.L.; Wei, M.F.; Guo, H.W.; Mao, R.; Hu, Z.W.; Zhao, Y.B. Effect of Ti and Si on the viscosity and solidification properties of molten iron. J. Univ. Sci. Technol. Beijing 2013, 35, 994–999. [Google Scholar] [CrossRef]
- Hou, P.; Yu, W.Z.; Bai, C.G.; Pan, C.; Yuan, W.N.; Li, T. Viscous flow properties and influencing factors of vanadium-titanium magnetite smelting iron. Iron Steel 2022, 57, 57–65. [Google Scholar] [CrossRef]
- Hwang, K.C.; Lee, S.; Lee, H.C. Effects of alloying elements on microstructure and fracture properties of cast high speed steel rolls: Part I: Microstructural analysis. Mater. Sci. Eng. A 1998, 254, 282–295. [Google Scholar] [CrossRef]
- Wei, S.Z.; Ni, F.; Zhu, J.H.; Long, R.; Xu, L.J. Solidification process of Fe-C alloy containing rich vanadium. J. Iron Steel Res. 2005, 17, 56–64. [Google Scholar]
- Xu, L.J.; Li, Z.; Wei, S.Z. VC precipitation and retained austenite transformation of high-vanadium high-speed steel during tem-pering. Heat Treat. Met. 2016, 41, 6–11. [Google Scholar] [CrossRef]
- Kesri, R.; Durand-Charre, M. Metallurgical structure and phase diagram of Fe–C–V system: Comparison with other systems forming MC carbides. Mater. Sci. Technol. 1988, 4, 692–699. [Google Scholar] [CrossRef]
- Kawalec, M.; Fraś, E. Structure, Mechanical Properties and Wear Resistance of High-vanadium Cast Iron. ISIJ Int. 2008, 48, 518–524. [Google Scholar] [CrossRef]
- Fras, E.; Kawalec, M.; Lopez, H. Solidification microstructures and mechanical properties of high-vanadium Fe–C–V and Fe–C–V–Si alloys. Mater. Sci. Eng. A 2009, 524, 193–203. [Google Scholar] [CrossRef]
- Tian, Y.; Yu, H.; Zhou, T.; Wang, K.; Zhu, Z. Revealing morphology rules of MX precipitates in Ti-V-Nb multi-microalloyed steels. Mater. Charact. 2022, 188, 111919. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Liu, L.; Deng, X.; Wang, Z. The formation mechanism of complex carbides in Nb-V microalloyed steel. Mater. Lett. 2022, 311, 131544. [Google Scholar] [CrossRef]
- Hu, B.-H.; Cai, Q.-W.; Wu, H.-B. Kinetics of Precipitation Behavior of Second Phase Particles in Ferritic Ti-Mo Microalloyed Steel. J. Iron Steel Res. Int. 2013, 20, 69–77. [Google Scholar] [CrossRef]
- Wang, K.; Wang, L.J.; Wang, Q.; He, J.C.; Liu, C.M. Grain growth of deformation induced ferrite in V microalloyed low carbon steel during controlled cooling process. J. Iron Steel Res. 2011, 18, 377–382. [Google Scholar] [CrossRef]
- Liu, T.M.; Zhou, S.Z.; Zuo, R.L.; Mei, D.S.; Deng, J.H. Effect of vanadium content on VC precipitation in PD3 steel. J. Chongqing Univ. 2001, 6, 78–81. [Google Scholar] [CrossRef]
- Pan, X.-L.; Umemoto, M. Precipitation Characteristics and Mechanism of Vanadium Carbides in a V-Microalloyed Medium-Carbon Steel. Acta Met. Sin. English Lett. 2018, 31, 1197–1206. [Google Scholar] [CrossRef]
- Hashemi, S.G.; Eghbali, B. Analysis of the formation conditions and characteristics of interphase and random vanadium precipitation in a low-carbon steel during isothermal heat treatment. Int. J. Miner. Met. Mater. 2018, 25, 339–349. [Google Scholar] [CrossRef]
- Aldazabal, J.; Garcia-Mateo, C.; Capdevila, C. Simulation of V(CN) Precipitation in Steels Allowing for Local Concentration Fluctuations. Mater. Trans. 2006, 47, 2732–2736. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Wang, X.; Zhao, Y. Precipitation and hetero-nucleation effect of V(C, N) in V-microalloyed steel. J. Wuhan Univ. Technol. Sci. Ed. 2008, 23, 844–849. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Detemple, E.; Eggeler, G. Revealing the two-step nucleation and growth mechanism of vanadium carbonitrides in microalloyed steels. Scr. Mater. 2020, 187, 350–354. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Yi, L.-Y.; Wang, L.-N.; Chen, D.-S.; Wang, W.-J.; Liu, Y.-H.; Zhao, H.-X.; Qi, T. A novel process for the recovery of iron, titanium, and vanadium from vanadium-bearing titanomagnetite: Sodium modification–direct reduction coupled process. Int. J. Miner. Met. Mater. 2017, 24, 504–511. [Google Scholar] [CrossRef]
- Shi, L.-Y.; Zhen, Y.-L.; Chen, D.-S.; Wang, L.-N.; Qi, T. Carbothermic Reduction of Vanadium-Titanium Magnetite in Molten NaOH. ISIJ Int. 2018, 58, 627–632. [Google Scholar] [CrossRef]
- Chen, L.; Zhen, Y.; Zhang, G.; Chen, D.; Wang, L.; Zhao, H.; Meng, F.; Qi, T. Carbothermic reduction of vanadium titanomagnetite with the assistance of sodium carbonate. Int. J. Miner. Met. Mater. 2022, 29, 239–247. [Google Scholar] [CrossRef]
- Chen, L.-M.; Zhen, Y.-L.; Zhang, G.-H.; Chen, D.; Wang, L.; Zhao, H.; Liu, Y.; Meng, F.; Wang, M.; Qi, T. Mechanism of Sodium Carbonate-Assisted Carbothermic Reduction of Titanomagnetite Concentrate. Met. Mater. Trans. B 2022, 53, 2272–2292. [Google Scholar] [CrossRef]
- Zhukov, A.A. Phase diagram of alloys of the system Fe-C. Met. Sci. Heat Treat. 1988, 30, 249–255. [Google Scholar] [CrossRef]
- Okamoto, H. The C-Fe (carbon-iron) system. J. Phase Equilibria Diffus. 1992, 13, 543–565. [Google Scholar] [CrossRef]
- Raghavan, V. C-Fe-V (carbon-iron-vanadium). J. Phase Equilibria Diffus. 1993, 14, 622–623. [Google Scholar] [CrossRef]
- Davydov, S.V. Phase Equilibria in the Carbide Region of Iron–Carbon Phase Diagram. Steel Transl. 2020, 50, 888–896. [Google Scholar] [CrossRef]
- Leineweber, A.; Shang, S.; Liu, Z. C-vacancy concentration in cementite, Fe3C1−, in equilibrium with α-Fe[C] and γ-Fe[C]. Acta Mater. 2015, 86, 374–384. [Google Scholar] [CrossRef]
- Zhai, Q.Y.; Xu, J.F.; Yuan, S. Formation mechanism of the austenite shell around nodular graphite in the hypereutectic nodular iron. Foundry 2001, 1, 18–21. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).