Direct Evidence for Phase Transition Process of VC Precipitation from (Fe,V)3C in Low-Temperature V-Bearing Molten Iron
Abstract
:1. Introduction
2. Experiment
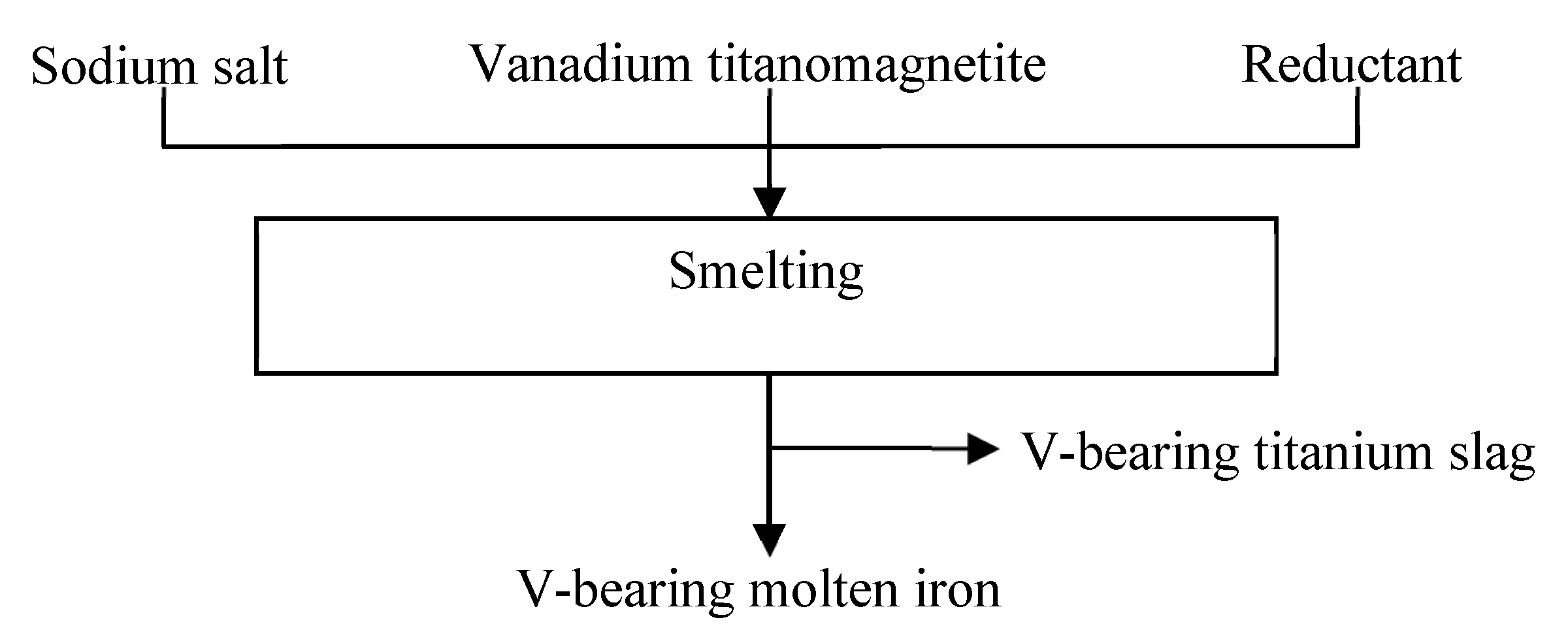
2.1. Raw Materials and Experimental Procedures
2.2. Theory Background
2.3. Test Method
3. Results and Discussion
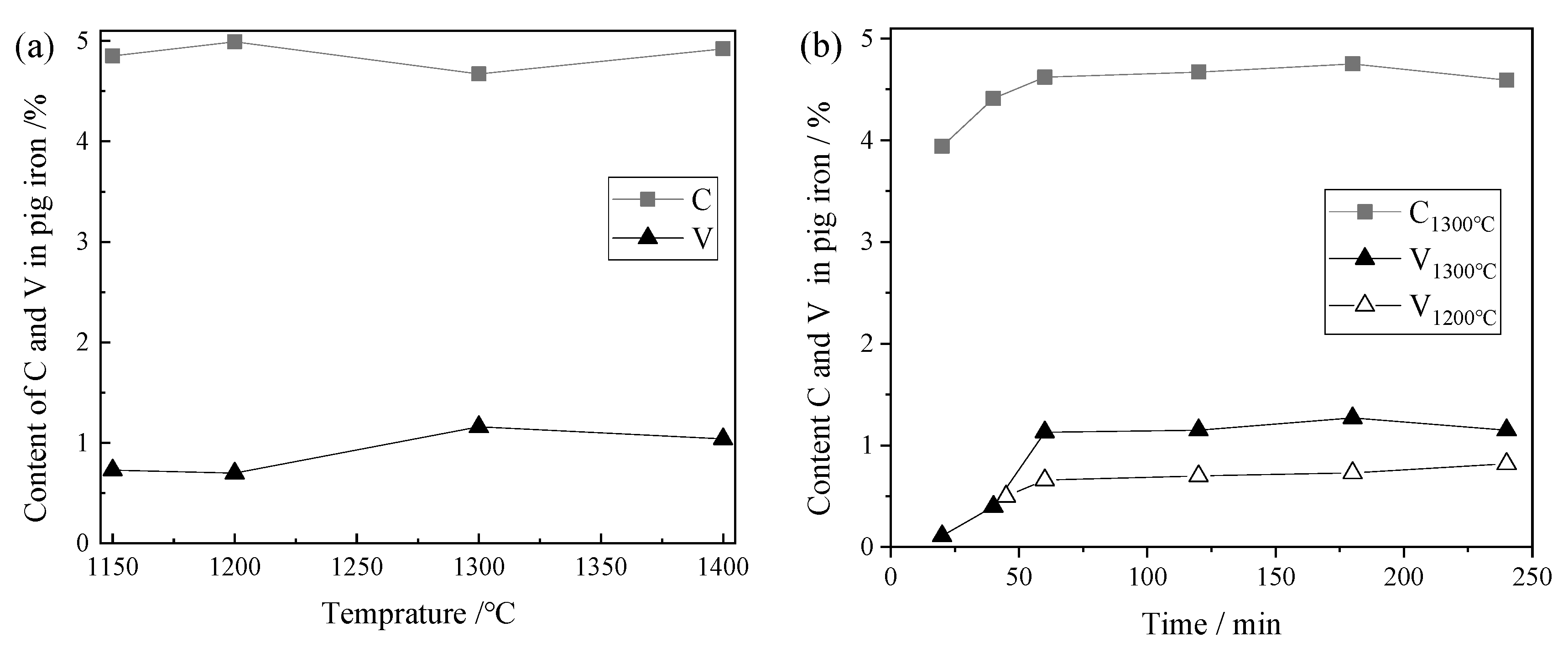
3.1. C and V Content of the Pig Iron
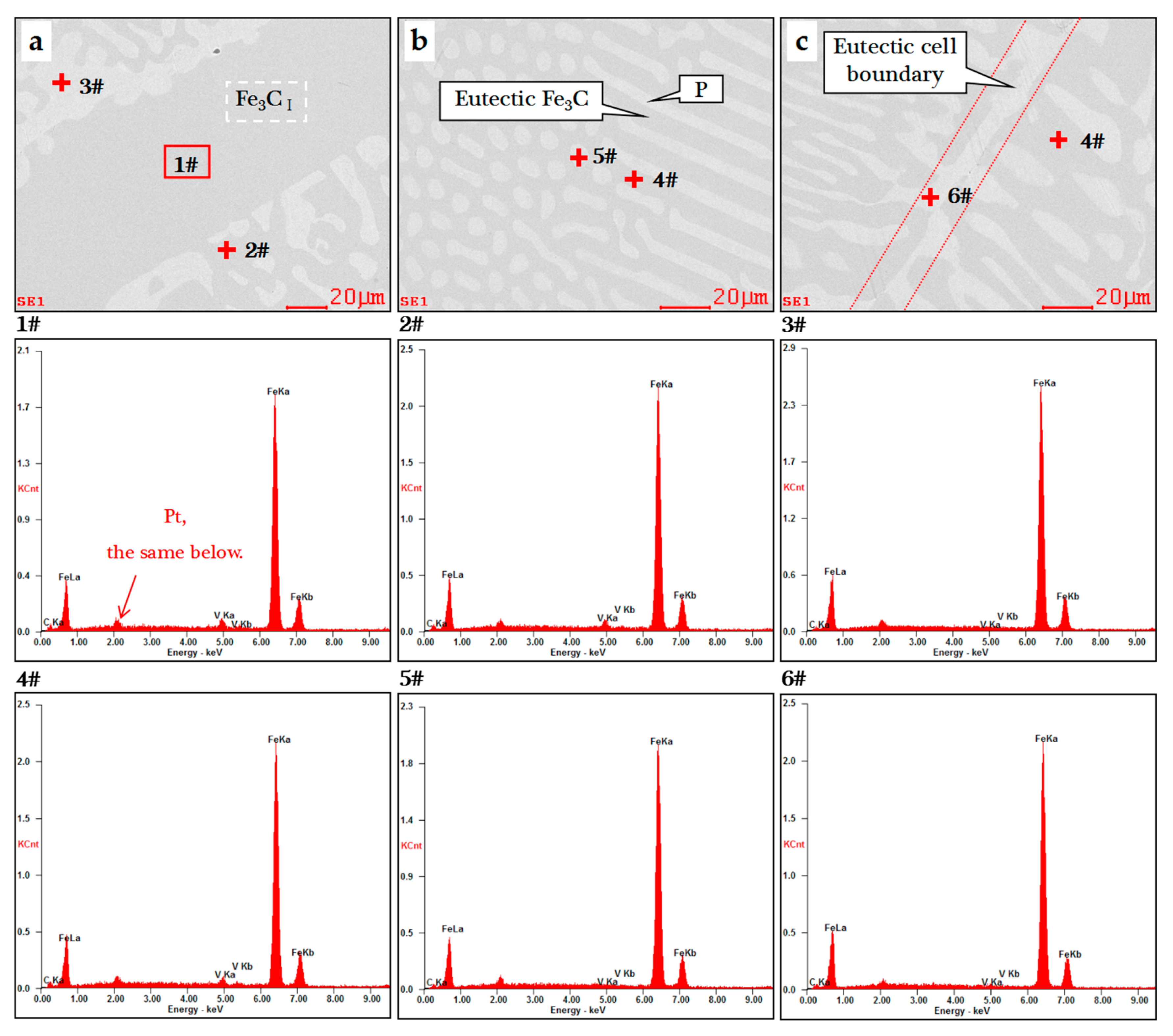
3.2. Morphology and Composition
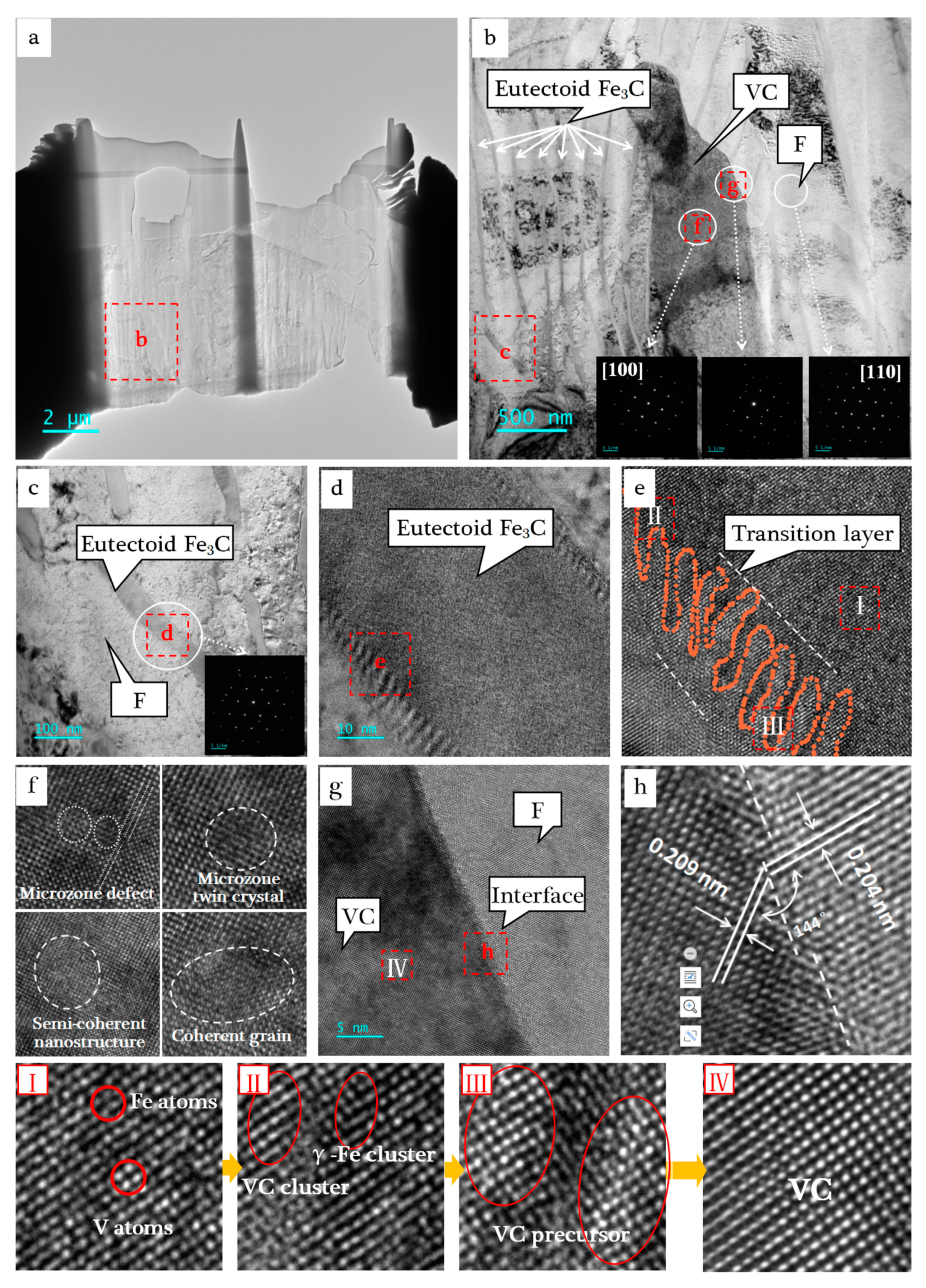
3.3. Interface Structures and Phase Transformation Process
- (a)
- The nucleation induction stage. With the decrease in the temperature, the first crystallized (Fe,V)3CI phase was unstable. It decomposed into large GI flakes and released V element into the liquid phase. As a result, C content decreased while V content increased in the adjacent liquid phase. Then, the A phase was precipitated out of the GI phase as the heterogeneous nucleation interface [37]. C and V elements were released into the adjacent liquid phase due to their low solubility in A phase, which then induced the formation of (Fe,V)3CI and further decomposed into GI.
- (b)
- The nucleation pregnant stage. With the continuous progress of the process (1), the content of C in the residual liquid phase dropped to the eutectic component, while the content of V element increased and dissolved into the eutectic (Fe,V)3C phase. The size effect caused lattice distortion with the increase in solid solubility, leading to a gradual decrease in the stability of the (Fe,V)3C phase. Then, many VC cluster structures were formed due to the fluctuation of V concentration and energy.
- (c)
- The nucleus formation stage. When the total distortion energy reached the critical value, these clusters first adsorbed to the A phase matrix interface under induction of the FCC structure. Then, they further absorbed the adjacent VC clusters. Finally, they grew into a VC precursor nucleus which started from the A phase matrix and protruded into the (Fe,V)3C phase. For the eutectoid process, due to the difficulty of diffusion in the solid phase transition process and the absence of redundant V atoms, the VC precursor crystal nucleus could not be further grown; finally, the two-phase undulating interface structure was formed. Wang [27] made a similar discovery in V-microalloyed steels.
- (d)
- The precipitation and growth stage. For the eutectic process, VC precursor crystal nuclei further converged and grew, forming the VC primary crystal nuclei. With the progress of the reaction, the content of C and V elements adjacent to the crystal nuclei decreased, while the content outside was higher, thus forming a concentration gradient of C and V elements decreased to the grain center. Therefore, the growth direction of VC deviated from the center of the sphere. Under the restriction of the separation of the A phase and G phase, the VC phase was precipitated continuously and finally formed a radiating eutectic cell [13]. If there were (Ti,V)(C,N) second-phase particles or other impurity particles precipitated earlier in the molten iron, they would become the core of VC eutectic cell as the heterogeneous nucleated crystal species.
4. Conclusions
- (1)
- Vanadium trended to form carbide in iron and steel structure. There were two main forms of V-rich carbides in low-temperature V-bearing pig iron: (Fe,V)3C solid solution and VC solid solution.
- (2)
- The microstructure of pig iron and the existence form of V-rich carbides changed at different melting temperatures. In the temperature range of 1150 °C to 1250 °C, the metallographic morphology were P and (Fe,V)3C phase. When the temperature exceeded 1300 °C, the P, G, and VC phases were mainly present.
- (3)
- When V atoms were solidly dissolved into the Fe3C lattice, they partially displaced the Fe atoms and formed (Fe,V)3C solid solution. In the eutectic transition process, the supersaturated V element destroyed the stability of (Fe,V)3C and promoted the precipitation of G and VC. The existence of VC clusters and VC precursor nuclei were observed by TEM, which confirmed the possibility of this transition process at the atomic perspective.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Chen, D.H.; Liu, W.H.; Sun, Z.H.; Zhang, B.X.; He, R. Global vanadium industry development report 2021. Iron Steel Va-Nadium Titan. 2022, 43, 1–9. [Google Scholar] [CrossRef]
- Xie, H.E.; Hu, P.; Zheng, K.; Zhu, F.X. Study on phase and chemical composition of V-Ti sinter during softening, melting and dripping process. Iron Steel Vanadium Titan. 2022, 43, 107–117. [Google Scholar] [CrossRef]
- Husslage, W.M.; Bakker, T.; Steeghs, A.G.S.; Reuter, M.; Heerema, R.H. Flow of molten slag and iron at 1500 °C to 1600 °C through packed coke beds. Met. Mater. Trans. B 2005, 36, 765–776. [Google Scholar] [CrossRef]
- Wen, G.Y.; Yan, Y.Z.; Zhao, S.Z.; Huang, J.J.; Jiang, G.H.; Yang, X.M. Properties of liquid iron containing vanadium and titanium. Iron Steel 1996, 31, 6–11. [Google Scholar]
- Yu, T.; Jiang, T.; Wen, J.; Sun, H.; Li, M.; Peng, Y. Effect of chemical composition on the element distribution, phase composition and calcification roasting process of vanadium slag. Int. J. Miner. Met. Mater. 2022, 29, 2144–2151. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Xue, Z. An Environment-Friendly Process Featuring Calcified Roasting and Precipitation Purification to Prepare Vanadium Pentoxide from the Converter Vanadium Slag. Metals 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-N.; Song, W.-C.; Li, H. Vanadium extraction and dephosphorization from V-bearing hot metal with fluxes containing CaO. J. Central South Univ. 2015, 22, 2887–2893. [Google Scholar] [CrossRef]
- Wang, H.C.; Chen, E.B.; Dong, Y.C.; Li, W.C. Analysis of the thermodynamic Property of Fe-C-V melt. J. Univ. Sci. Technol. Beijing 2000, 22, 312–315. [Google Scholar]
- He, Y.Y.; Liu, Q.C.; Yang, J.; Chen, Q.X.; Ao, W.Z. Experimental Investigation on Fluidity of Hot Metal Bearing Titanium. Iron Steel Vanadium Titan. 2010, 31, 10–14. [Google Scholar]
- Zhang, J.L.; Wei, M.F.; Guo, H.W.; Mao, R.; Hu, Z.W.; Zhao, Y.B. Effect of Ti and Si on the viscosity and solidification properties of molten iron. J. Univ. Sci. Technol. Beijing 2013, 35, 994–999. [Google Scholar] [CrossRef]
- Hou, P.; Yu, W.Z.; Bai, C.G.; Pan, C.; Yuan, W.N.; Li, T. Viscous flow properties and influencing factors of vanadium-titanium magnetite smelting iron. Iron Steel 2022, 57, 57–65. [Google Scholar] [CrossRef]
- Hwang, K.C.; Lee, S.; Lee, H.C. Effects of alloying elements on microstructure and fracture properties of cast high speed steel rolls: Part I: Microstructural analysis. Mater. Sci. Eng. A 1998, 254, 282–295. [Google Scholar] [CrossRef]
- Wei, S.Z.; Ni, F.; Zhu, J.H.; Long, R.; Xu, L.J. Solidification process of Fe-C alloy containing rich vanadium. J. Iron Steel Res. 2005, 17, 56–64. [Google Scholar]
- Xu, L.J.; Li, Z.; Wei, S.Z. VC precipitation and retained austenite transformation of high-vanadium high-speed steel during tem-pering. Heat Treat. Met. 2016, 41, 6–11. [Google Scholar] [CrossRef]
- Kesri, R.; Durand-Charre, M. Metallurgical structure and phase diagram of Fe–C–V system: Comparison with other systems forming MC carbides. Mater. Sci. Technol. 1988, 4, 692–699. [Google Scholar] [CrossRef]
- Kawalec, M.; Fraś, E. Structure, Mechanical Properties and Wear Resistance of High-vanadium Cast Iron. ISIJ Int. 2008, 48, 518–524. [Google Scholar] [CrossRef] [Green Version]
- Fras, E.; Kawalec, M.; Lopez, H. Solidification microstructures and mechanical properties of high-vanadium Fe–C–V and Fe–C–V–Si alloys. Mater. Sci. Eng. A 2009, 524, 193–203. [Google Scholar] [CrossRef]
- Tian, Y.; Yu, H.; Zhou, T.; Wang, K.; Zhu, Z. Revealing morphology rules of MX precipitates in Ti-V-Nb multi-microalloyed steels. Mater. Charact. 2022, 188, 111919. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Liu, L.; Deng, X.; Wang, Z. The formation mechanism of complex carbides in Nb-V microalloyed steel. Mater. Lett. 2022, 311, 131544. [Google Scholar] [CrossRef]
- Hu, B.-H.; Cai, Q.-W.; Wu, H.-B. Kinetics of Precipitation Behavior of Second Phase Particles in Ferritic Ti-Mo Microalloyed Steel. J. Iron Steel Res. Int. 2013, 20, 69–77. [Google Scholar] [CrossRef]
- Wang, K.; Wang, L.J.; Wang, Q.; He, J.C.; Liu, C.M. Grain growth of deformation induced ferrite in V microalloyed low carbon steel during controlled cooling process. J. Iron Steel Res. 2011, 18, 377–382. [Google Scholar] [CrossRef]
- Liu, T.M.; Zhou, S.Z.; Zuo, R.L.; Mei, D.S.; Deng, J.H. Effect of vanadium content on VC precipitation in PD3 steel. J. Chongqing Univ. 2001, 6, 78–81. [Google Scholar] [CrossRef]
- Pan, X.-L.; Umemoto, M. Precipitation Characteristics and Mechanism of Vanadium Carbides in a V-Microalloyed Medium-Carbon Steel. Acta Met. Sin. English Lett. 2018, 31, 1197–1206. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, S.G.; Eghbali, B. Analysis of the formation conditions and characteristics of interphase and random vanadium precipitation in a low-carbon steel during isothermal heat treatment. Int. J. Miner. Met. Mater. 2018, 25, 339–349. [Google Scholar] [CrossRef]
- Aldazabal, J.; Garcia-Mateo, C.; Capdevila, C. Simulation of V(CN) Precipitation in Steels Allowing for Local Concentration Fluctuations. Mater. Trans. 2006, 47, 2732–2736. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhao, L.; Wang, X.; Zhao, Y. Precipitation and hetero-nucleation effect of V(C, N) in V-microalloyed steel. J. Wuhan Univ. Technol. Sci. Ed. 2008, 23, 844–849. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Detemple, E.; Eggeler, G. Revealing the two-step nucleation and growth mechanism of vanadium carbonitrides in microalloyed steels. Scr. Mater. 2020, 187, 350–354. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Yi, L.-Y.; Wang, L.-N.; Chen, D.-S.; Wang, W.-J.; Liu, Y.-H.; Zhao, H.-X.; Qi, T. A novel process for the recovery of iron, titanium, and vanadium from vanadium-bearing titanomagnetite: Sodium modification–direct reduction coupled process. Int. J. Miner. Met. Mater. 2017, 24, 504–511. [Google Scholar] [CrossRef]
- Shi, L.-Y.; Zhen, Y.-L.; Chen, D.-S.; Wang, L.-N.; Qi, T. Carbothermic Reduction of Vanadium-Titanium Magnetite in Molten NaOH. ISIJ Int. 2018, 58, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhen, Y.; Zhang, G.; Chen, D.; Wang, L.; Zhao, H.; Meng, F.; Qi, T. Carbothermic reduction of vanadium titanomagnetite with the assistance of sodium carbonate. Int. J. Miner. Met. Mater. 2022, 29, 239–247. [Google Scholar] [CrossRef]
- Chen, L.-M.; Zhen, Y.-L.; Zhang, G.-H.; Chen, D.; Wang, L.; Zhao, H.; Liu, Y.; Meng, F.; Wang, M.; Qi, T. Mechanism of Sodium Carbonate-Assisted Carbothermic Reduction of Titanomagnetite Concentrate. Met. Mater. Trans. B 2022, 53, 2272–2292. [Google Scholar] [CrossRef]
- Zhukov, A.A. Phase diagram of alloys of the system Fe-C. Met. Sci. Heat Treat. 1988, 30, 249–255. [Google Scholar] [CrossRef]
- Okamoto, H. The C-Fe (carbon-iron) system. J. Phase Equilibria Diffus. 1992, 13, 543–565. [Google Scholar] [CrossRef]
- Raghavan, V. C-Fe-V (carbon-iron-vanadium). J. Phase Equilibria Diffus. 1993, 14, 622–623. [Google Scholar] [CrossRef]
- Davydov, S.V. Phase Equilibria in the Carbide Region of Iron–Carbon Phase Diagram. Steel Transl. 2020, 50, 888–896. [Google Scholar] [CrossRef]
- Leineweber, A.; Shang, S.; Liu, Z. C-vacancy concentration in cementite, Fe3C1−, in equilibrium with α-Fe[C] and γ-Fe[C]. Acta Mater. 2015, 86, 374–384. [Google Scholar] [CrossRef]
- Zhai, Q.Y.; Xu, J.F.; Yuan, S. Formation mechanism of the austenite shell around nodular graphite in the hypereutectic nodular iron. Foundry 2001, 1, 18–21. [Google Scholar]


| TFe | TiO2 | SiO2 | CaO | Al2O3 | V2O5 | MgO | MnO | S | P |
|---|---|---|---|---|---|---|---|---|---|
| 47.97 | 19.32 | 6.59 | 2.98 | 2.29 | 1.46 | 0.70 | 0.41 | 0.028 | 0.011 |
| FCad | Vdaf | Mad | St | P | Ad | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Fe2O3 | TiO2 | Na2O | MnO | MgO | Total | |||||
| 81.87 | 6.32 | 1.66 | 0.35 | 0.028 | 6.93 | 2.79 | 0.95 | 0.43 | 0.33 | 0.21 | 0.1 | 0.06 | 11.81 |
| Element (wt.%) | 1# | 2# | 3# | 4# | 5# | 6# |
|---|---|---|---|---|---|---|
| Fe | 85.82 | 86.94 | 91.68 | 88.78 | 91.38 | 92.26 |
| C | 12.00 | 11.16 | 8.00 | 9.54 | 8.11 | 7.44 |
| V | 2.18 | 1.92 | 0.33 | 1.68 | 0.51 | 0.31 |
| Element (wt.%) | 1# | 2# | 3# | 4# |
|---|---|---|---|---|
| Fe | 2.03 | 32.94 | / | 91.26 |
| C | 13.83 | 21 | 100 | 8.04 |
| N | 6.92 | / | / | / |
| V | 35.70 | 43.09 | / | 0.70 |
| Ti | 41.51 | 2.97 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Chen, D.; Sang, X.; Zhao, H.; Zhen, Y.; Wang, L.; Liu, Y.; Meng, F.; Qi, T. Direct Evidence for Phase Transition Process of VC Precipitation from (Fe,V)3C in Low-Temperature V-Bearing Molten Iron. Crystals 2023, 13, 175. https://doi.org/10.3390/cryst13020175
Cao L, Chen D, Sang X, Zhao H, Zhen Y, Wang L, Liu Y, Meng F, Qi T. Direct Evidence for Phase Transition Process of VC Precipitation from (Fe,V)3C in Low-Temperature V-Bearing Molten Iron. Crystals. 2023; 13(2):175. https://doi.org/10.3390/cryst13020175
Chicago/Turabian StyleCao, Lei, Desheng Chen, Xiaomeng Sang, Hongxin Zhao, Yulan Zhen, Lina Wang, Yahui Liu, Fancheng Meng, and Tao Qi. 2023. "Direct Evidence for Phase Transition Process of VC Precipitation from (Fe,V)3C in Low-Temperature V-Bearing Molten Iron" Crystals 13, no. 2: 175. https://doi.org/10.3390/cryst13020175
APA StyleCao, L., Chen, D., Sang, X., Zhao, H., Zhen, Y., Wang, L., Liu, Y., Meng, F., & Qi, T. (2023). Direct Evidence for Phase Transition Process of VC Precipitation from (Fe,V)3C in Low-Temperature V-Bearing Molten Iron. Crystals, 13(2), 175. https://doi.org/10.3390/cryst13020175






