Transverse Microradiography Evidence on the Effect of Phosphoryl Oligosaccharides of Calcium (POs-Ca) in Toothpaste on Decalcified Enamel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Study Design
2.2. Specimen Preparation
2.3. pH-Cycling
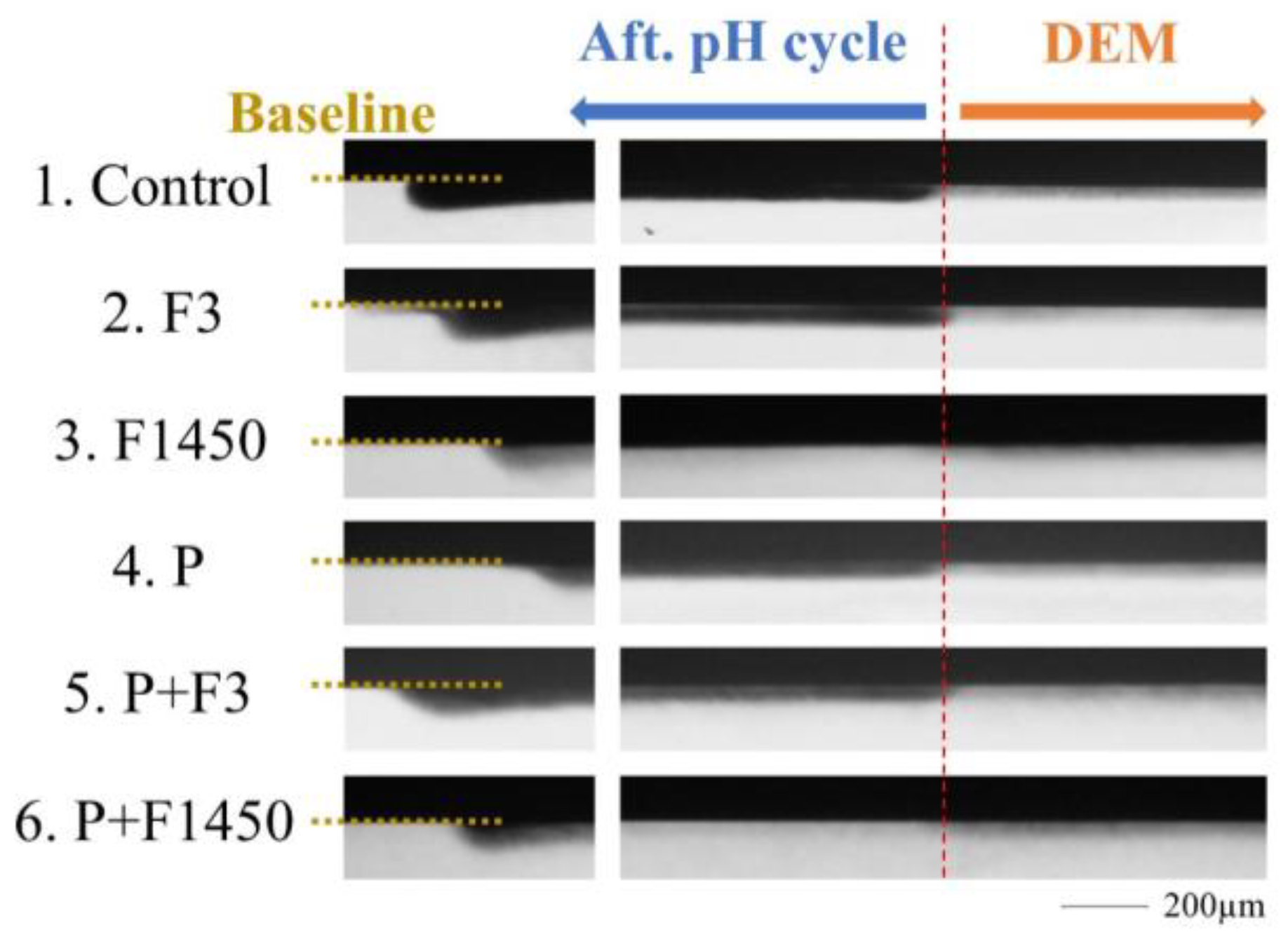
2.4. TMR Analysis
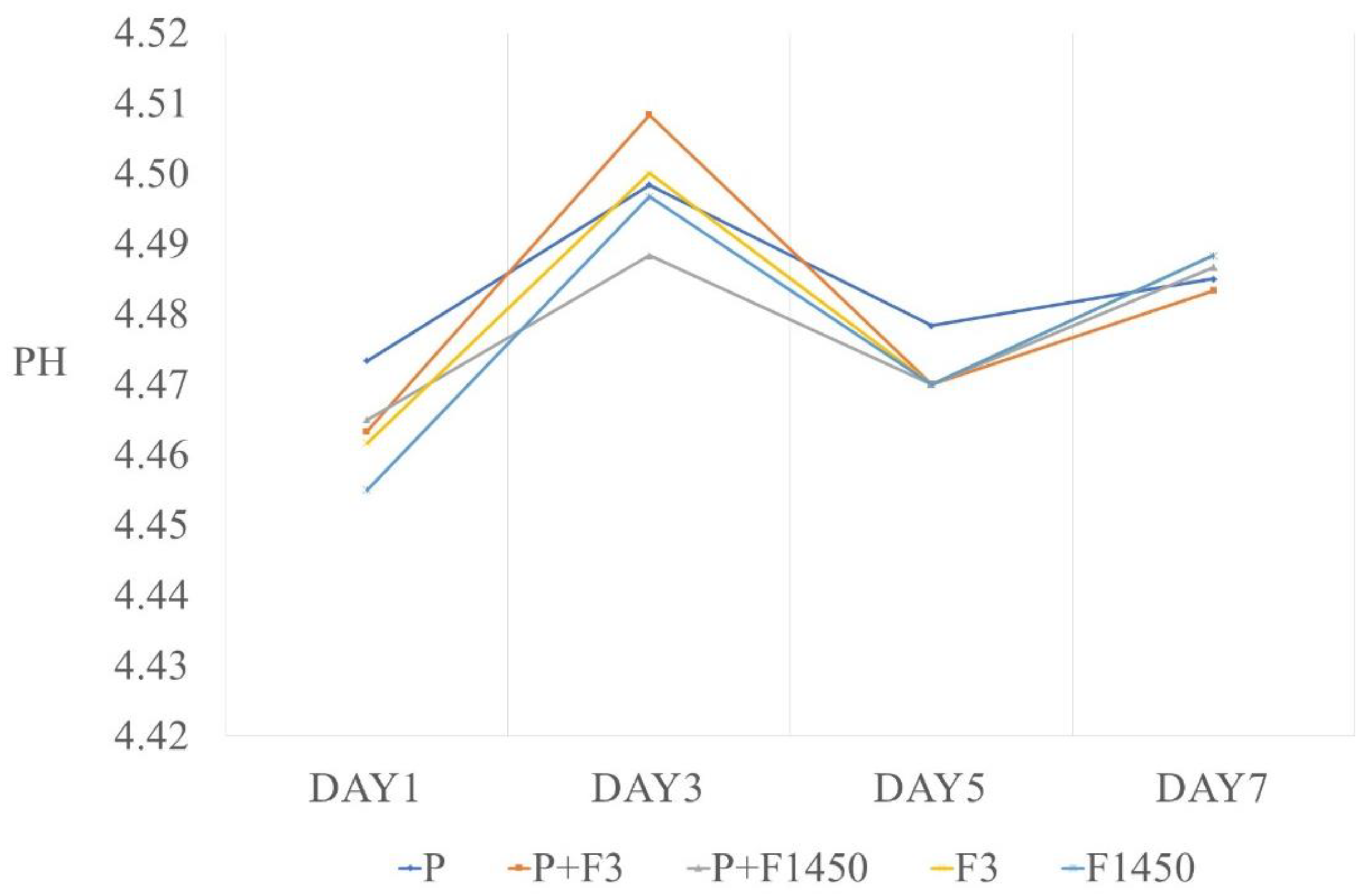
2.5. pH Measurement
2.6. Data Analysis
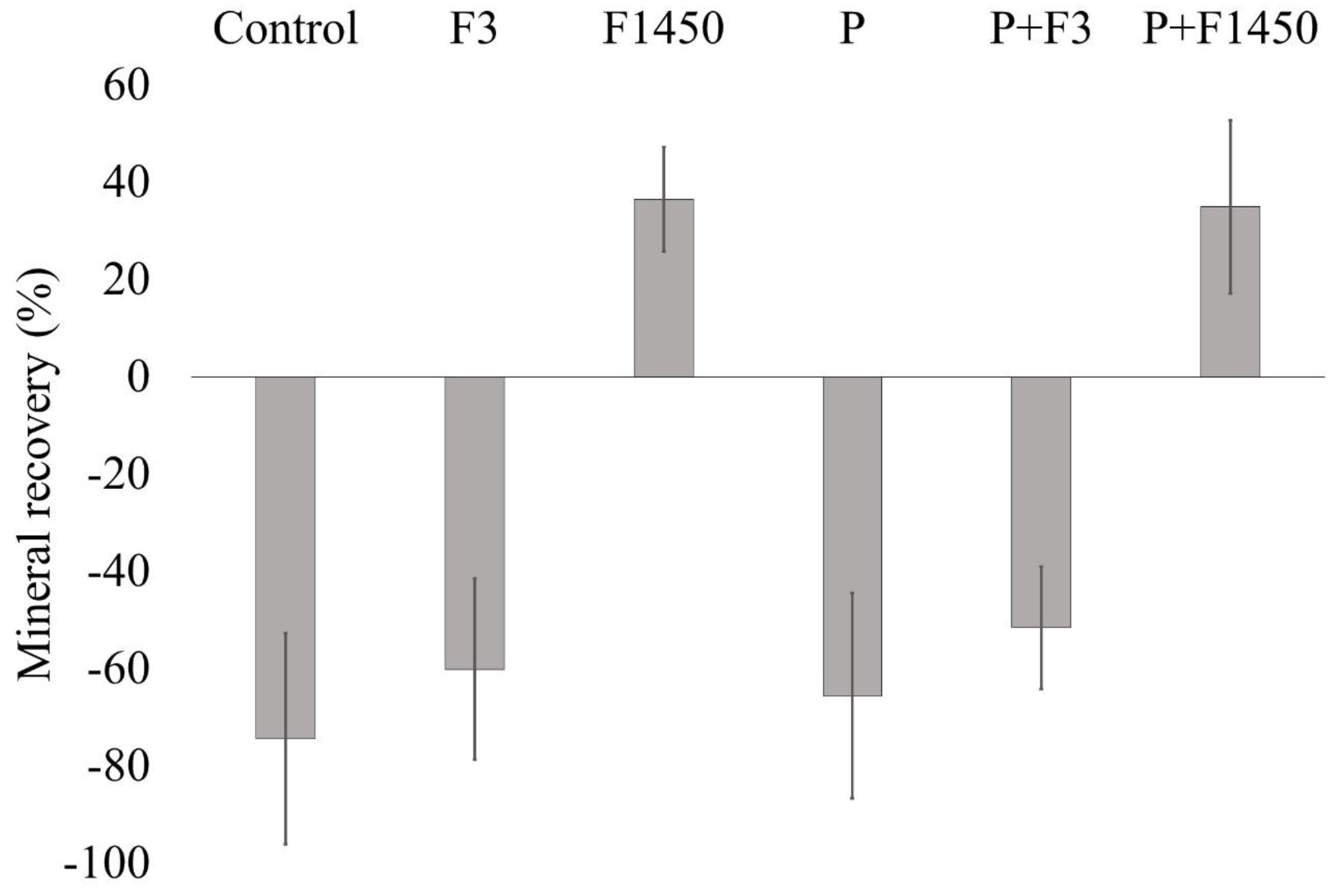
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanji, F.; Komiyama, T.; Ohi, T.; Hattori, Y.; Watanabe, M.; Lu, Y.; Tsuji, I. The Association between Number of Remaining Teeth and Maintenance of Successful Aging in Japanese Older People: A 9-Year Longitudinal Study. Tohoku J. Exp. Med. 2020, 252, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Dibello, V.; Lobbezoo, F.; Lozupone, M.; Sardone, R.; Ballini, A.; Berardino, G.; Mollica, A.; Coe-lho-Júnior, H.J.; de Pergola, G.; Stallone, R.; et al. Oral Frailty Indicators to Target Major Adverse Health-Related Outcomes in Older Age: A Systematic Review; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Singh, K.A.; Brennan, D.S. Chewing disability in older adults attributable to tooth loss and other oral conditions. Gerodontology 2012, 29, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Konta, T.; Susa, S.; Ishizawa, K.; Togashi, H.; Ueno, Y.; Yamashita, H.; Kayama, T.; Iino, M. Association between presence of 20 or more natural teeth and all-cause, cancer-related, and cardiovascular disease-related mortality: Yamagata (Takahata) prospective observational study. BMC Oral Health 2020, 20, 353. [Google Scholar] [CrossRef] [PubMed]
- Deari, S.; Wegehaupt, F.J.; Tauböck, T.T.; Attin, T. Influence of Different Pretreatments on the Microtensile Bond Strength to Eroded Dentin. J. Adhes. Dent. 2017, 19, 147–155. [Google Scholar]
- Wegehaupt, F.J.; Tauböck, T.T.; Attin, T. Durability of the Anti-Erosive Effect of Surfaces Sealants under Erosive Abrasive Conditions. Acta Odontol. Scand. 2013, 71, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Attin, T.; Becker, K.; Wiegand, A.; Tauböck, T.T.; Wegehaupt, F.J. Impact of Laminar Flow Velocity of Different Acids on Enamel Calcium Loss. Clin. Oral Investig. 2013, 17, 595–600. [Google Scholar] [CrossRef]
- Yamato, M.; Matsuyama, S.; Murakami, Y.; Aida, J.; Lu, Y.; Sugawara, Y.; Tsuji, I. Association between the number of remaining teeth and disability-free life expectancy, and the impact of oral self-care in older Japanese adults: A prospective cohort study. BMC Geriatr. 2022, 22, 820. [Google Scholar] [CrossRef]
- Ekstrand, K.R. High Fluoride Dentifrices for Elderly and Vulnerable Adults: Does It Work and if So, Then Why? Caries Res. 2016, 50, 15–21. [Google Scholar] [CrossRef]
- Marinho, V.; Higgins, J.; Logan, S.; Sheiham, A. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003, 2003, CD002278. [Google Scholar] [CrossRef]
- Jesen, M.K.; Kohout, F. The effect of a fluoridated dentifrice on root and coronal caries in an older adult population. J. Am. Dent. Assoc. 1988, 117, 829–832. [Google Scholar] [CrossRef]
- Gluzman, R.; Katz, R.V.; Frey, B.J.; McGowan, R. Prevention of Root Caries: A Literature Review of Primary and Secondary Preventive Agents. Spec. Care Dent. 2013, 33, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Pretty, I.R. High Fluoride Concentration Toothpastes for Children and Adolescents. Caries Res. 2016, 50, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Worthington, H.V.; Glenny, A.M.; Marinho, V.C.C.; Jeroncic, A. Fluoride toothpastes of different concentrations for preventing dental caries. Cochrane Database Syst. Rev. 2019, 3, CD007868. [Google Scholar] [CrossRef]
- Featherstone, J.D. Prevention and reversal of dental caries: Role of low level fluoride. Community Dent. Oral Epidemiol. 1999, 27, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Shellies, R.; Duckworth, R. Studies on the cariostatic mechanisms of fluoride. Int. Dent. J. 1994, 44, 263–273. [Google Scholar]
- Reddy, D.; Selvan, A.; Paul, S.T.; Azher, U. Antimicrobial Efficacy of Commercially Available Low-Fluoride and Fluoride-Free Dentifrices for Children. Int. J. Clin. Pediatr. Dent. 2021, 14, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Kantrong, N.; Khongkhaphet, K.; Sitornsud, N.; Lo-Apirukkul, P.; Phanprom, W.; Rojviriya, C.; Amonpat-taratkit, P.; Ariyakriangkai, W. Synchrotron Radiation Analysis of Root Dentin: The Roles of Fluoride and Calcium Ions in Hydroxyapatite Remineralization. J. Synchrotron Rad. 2022, 29, 496–504. [Google Scholar] [CrossRef]
- da Silva Spinola, M.; Tenuta, L.M.A. Calcium pretreatment enhances fluoride reactivity with enamel and dentine. Arch. Oral Biol. 2022, 134, 105338. [Google Scholar] [CrossRef]
- Cochrane, N.J.; Cai, F.; Huq, N.L.; Burrow, M.F.; Reynold, E.C. New approaches to enhanced remineralization of tooth enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef]
- Kitasasko, Y.; Tanaka, M.; Sadr, A.; Hamba, H.; Ikeda, M.; Tagami, J. Effects of a chewing gum containing phosphoryl oligosaccharides of calcium (POs-Ca) and fluoride on remineralization and crystallization of enamel subsurface lesions in situ. J. Dent. 2011, 39, 771–779. [Google Scholar] [CrossRef]
- Reynolds, E.C.; Cai, F.; Shen, P.; Walker, G.D. Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouthrinse or sugar-free chewing gum. J. Dent. Res. 2003, 82, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Manton, D.J.; Cochrane, N.J.; Walker, G.D.; Yuan, Y.; Reynolds, C.; Reynolds, E.C. Effect of added calcium phosphate on enamel remineralization by fluoride in a randomized controlled in situ trial. J. Dent. 2011, 39, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kobayashi, T.; Takii, H.; Kamasaka, H.; Ohta, N.; Matsuo, T.; Yagi, N.; Kuriki, T. Optimization of calcium concentration of saliva with phosphoryl oligosaccharides of calcium (POs-Ca) for enamel remineralization in vitro. Arch. Oral Biol. 2013, 58, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Kamasaka, H.; Imai, S.; Nishimura, T.; Kuriki, T.; Nishizawa, T. Effect of phosphoryl oligosaccharides from potato starch on acid fermentation by mutans streptococci. J. Dent. Health 2002, 52, 66–71. [Google Scholar]
- Inaba, D.; Minami, K.; Kamasaka, H.; Yonemitsu, M. Remineralization of enamel and dentin by a chewing gum containing Phosphryl-Oligosaccharide calcium (POs-Ca) in situ. Dent. J. Iwate Med. Univ. 2002, 27, 203–209. [Google Scholar]
- To-o, K.; Kamasaka, K.; Nishimura, T.; Kuriki, T.; Saeki, S.; Nakabou, Y. Bioavailability of calcium-bound phosphoryl oligosaccharides in rats. J. Appl. Glicosci. 2002, 49, 159–165. [Google Scholar] [CrossRef]
- ten Cate, J.M.; Dundon, K.A.; Vernon, P.G.; Damato, F.A.; Huntington, E.; Exterkate, R.A.M.; Wefel, J.S.; Jordan, T.; Stephen, K.W.; Roberts, A.J. Preparation and measurement of artificial enamel lesions, a four-laboratory ring test. Caries Res. 1996, 30, 400–407. [Google Scholar] [CrossRef]
- ten Cate, J.M.; Exterkate, R.A.M.; Buijs, M.J. The relative efficacy of fluoride toothpastes assessed with pH cycling. Caries Res. 2006, 40, 136–141. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Im-plantology-a Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef]
- Twentman, S.; Ekstrand, K.R. Caries management by influencing mineralization. In Caries Management—Science and Clinical Practice, 1st ed.; Meyer-Lueckel, H., Paris, S., Ekstrand, K.R., Eds.; Thieme: Stuttgart, Germany, 2013; pp. 177–190. [Google Scholar]
- Bruun, C.; Givskov, H. Formation of CaF2 on sound enamel and caries like lesions after different forms of fluoride application in vitro. Caries Res. 1991, 25, 96–100. [Google Scholar] [CrossRef]
- Tanaka, T.; Yagi, N.; Ohta, T.; Matsuo, Y.; Terada, H.; Kamasaka, K.; To-o, K.; Kometani, T.; Kuriki, T. Evaluation of the distribution and orientation of remineralized enamel crystallites in subsurface lesions by X-ray diffraction. Caries Res. 2010, 44, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kobayashi, T.; Tamenori, Y.; Sakanaka, A.; Kuriki, T.; Amano, A. Phosphoryl oligosaccha-rides of calcium enhance mineral availability and fluorapatite formation. Arch. Oral Biol. 2019, 10, 135–141. [Google Scholar] [CrossRef]
- Inaba, D.; Minami, K.; Kamasaka, H.; Yonemitsu, M. Effects of phosphoryl-oligosaccharides(POs) on remineralization of enamel lesions in vitro. Dent. J. Iwate Med. Univ. 2002, 27, 197–202. [Google Scholar]
- ten Cate, J.M.; Jongebloed, W.L.; Arends, J. Remineralization of artificial enamel lesions in vitro—IV. Influence of fluorides and diphosphonates on short-and long-term remineralization. Caries Res. 1981, 15, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Boddé, H.E.; Koops, P.G.; Arends, J. Effect of an APF pretreatment on in vitro remineralization of initial enamel lesions. Caries Res. 1984, 18, 344–347. [Google Scholar] [CrossRef]
- Buzalaf, M.A.R.; Hannas, A.R.; Magalhaes, A.C.; Rios, D.; Honorio, H.M.; Delbem, A.C.B. PH-cycling models for in vitro evaluation of the efficacy of fluoridated dentifrices for caries control: Strengths and limitations. J. Appl. Oral Sci. 2010, 18, 316–334. [Google Scholar] [CrossRef]


| Group | F in Formulation (ppm) | F in Solution (ppm) | Ca from POs-Ca in Formulation (mM) | Ca from POs-Ca in Solution (mM) |
|---|---|---|---|---|
| 1. Control | 0 | 0 | 0 | 0 |
| 2. F3 | 3 | 1 | 0 | 0 |
| 3. F1450 | 1450 | 480 | 0 | 0 |
| 4. P | 0 | 0 | 13.2 | 4.3 |
| 5. P + F3 | 3 | 1 | 13.2 | 4.3 |
| 6. P + F1450 | 1450 | 480 | 13.2 | 4.3 |
| Group | MLDEM | MLREM | MLDEM − MLREM | %R |
|---|---|---|---|---|
| 1. Control | 19.56 ± 4.47 | 33.67 ± 6.80 | −14.11 ± 3.43 | −74.39 ± 21.68 a |
| 2. F3 | 20.75 ± 2.90 | 32.85 ± 3.51 | −12. 10 ± 3.37 | −60.09 ± 18.64 a |
| 3. F1450 | 21.95 ± 4.80 | 13.71 ± 2.78 | 8.24 ± 3.69 | 36.54 ± 10.76 b |
| 4. P | 18.95 ± 3.28 | 31.03 ± 4.99 | −12.07 ± 4.06 | −65.56 ± 21.05 a |
| 5. P + F3 | 22.36 ± 3.85 | 33.62 ± 5.17 | −11.27 ± 2.76 | −51.55 ± 12.59 a |
| 6. P + F1450 | 18.26 ± 6.43 | 11.80 ± 5.24 | 6.46 ± 3.69 | 34.93 ± 17.81 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, G.; Chen, X.; Shimada, Y. Transverse Microradiography Evidence on the Effect of Phosphoryl Oligosaccharides of Calcium (POs-Ca) in Toothpaste on Decalcified Enamel. Crystals 2023, 13, 206. https://doi.org/10.3390/cryst13020206
Inoue G, Chen X, Shimada Y. Transverse Microradiography Evidence on the Effect of Phosphoryl Oligosaccharides of Calcium (POs-Ca) in Toothpaste on Decalcified Enamel. Crystals. 2023; 13(2):206. https://doi.org/10.3390/cryst13020206
Chicago/Turabian StyleInoue, Go, Xuefei Chen, and Yasushi Shimada. 2023. "Transverse Microradiography Evidence on the Effect of Phosphoryl Oligosaccharides of Calcium (POs-Ca) in Toothpaste on Decalcified Enamel" Crystals 13, no. 2: 206. https://doi.org/10.3390/cryst13020206
APA StyleInoue, G., Chen, X., & Shimada, Y. (2023). Transverse Microradiography Evidence on the Effect of Phosphoryl Oligosaccharides of Calcium (POs-Ca) in Toothpaste on Decalcified Enamel. Crystals, 13(2), 206. https://doi.org/10.3390/cryst13020206








