Monte Carlo Simulation for Investigating the Sintering Temperatures Effects on Radiation Shielding Performances of Lead-Free ABO3 Perovskite Ceramic
Abstract
:1. Introduction
2. Experimental Details
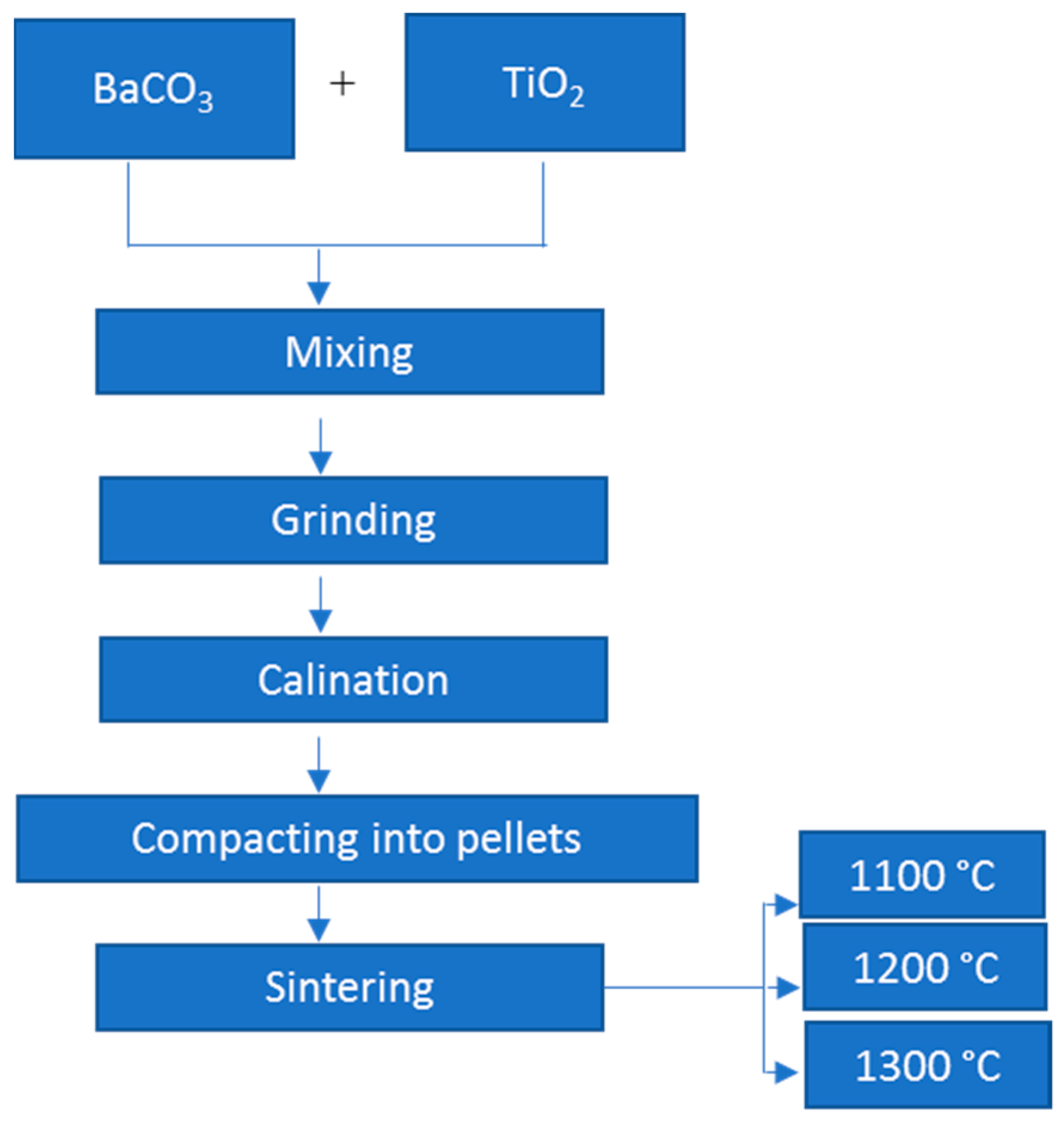
2.1. Chemicals and Method
2.2. Ceramics Characterizations
2.3. Radiation Protection Qualifications
3. Results and Discussion
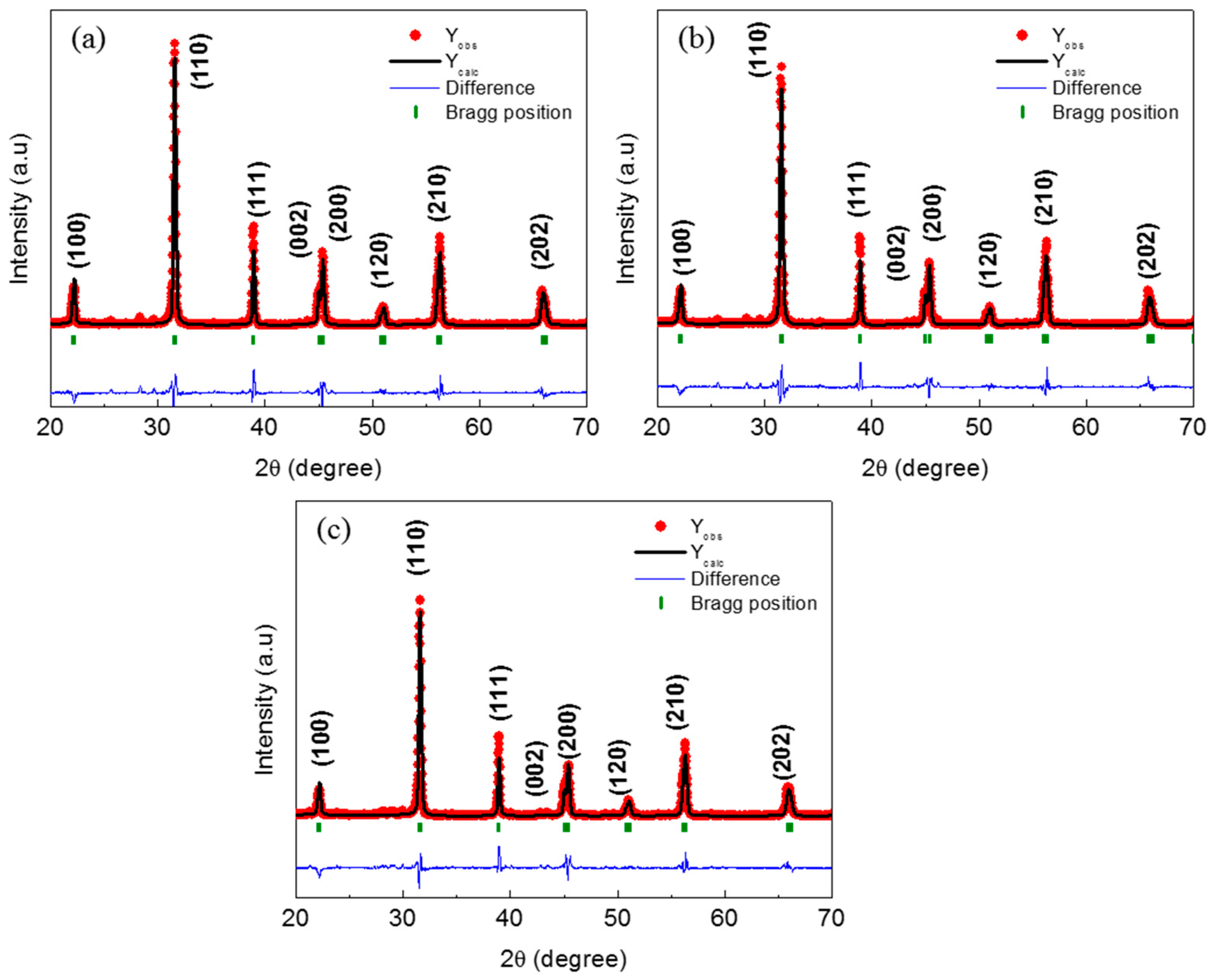
3.1. Structural Analyses
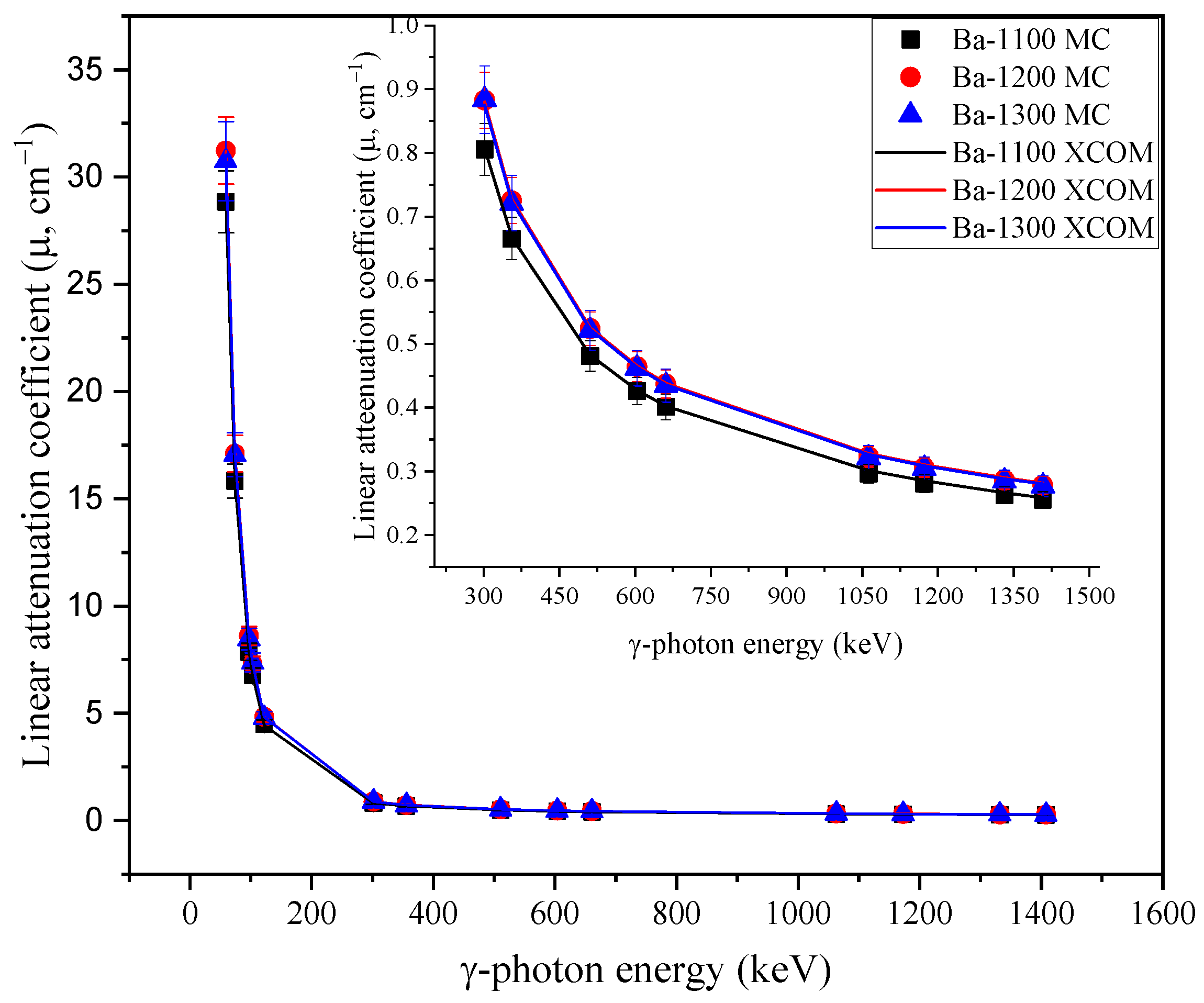
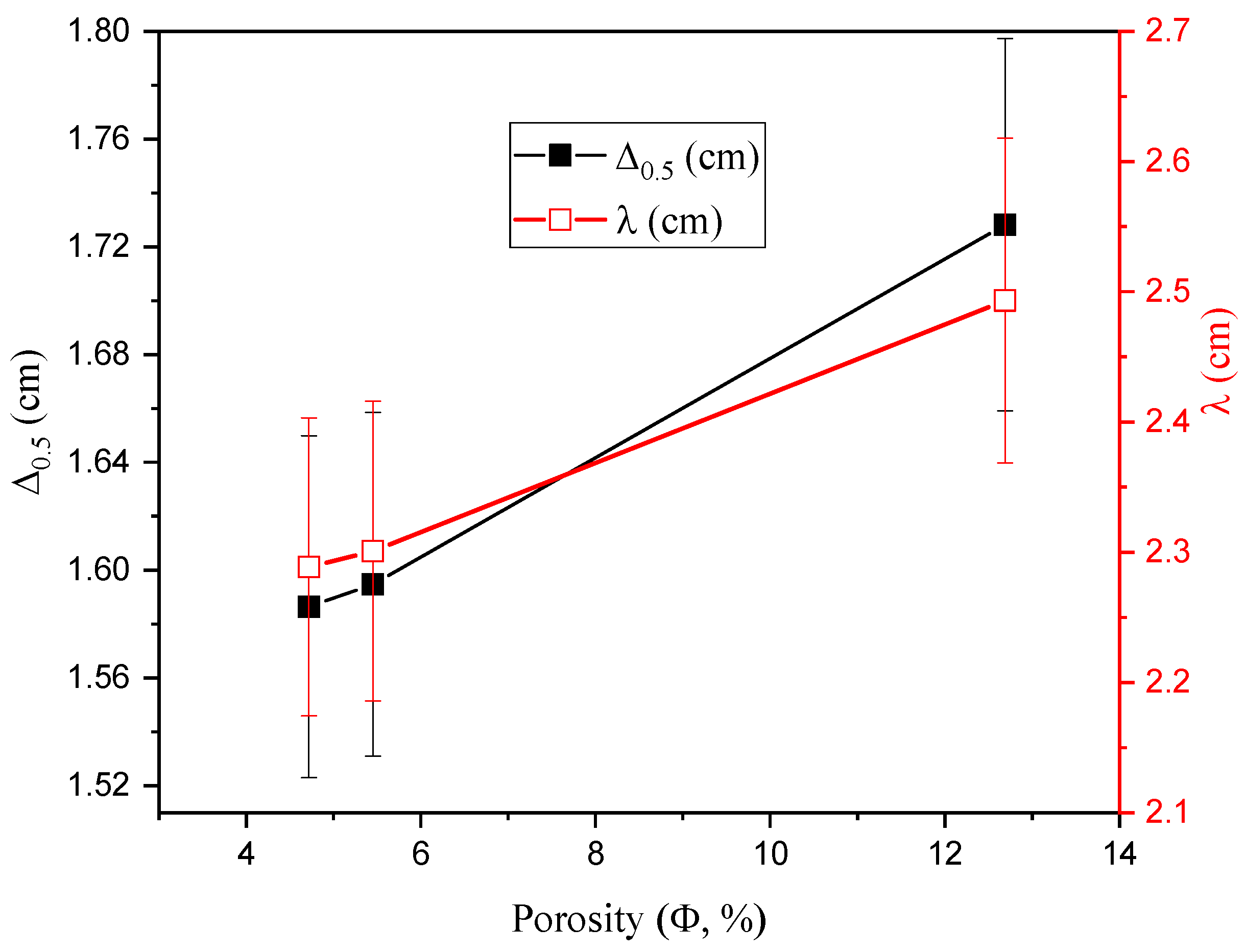
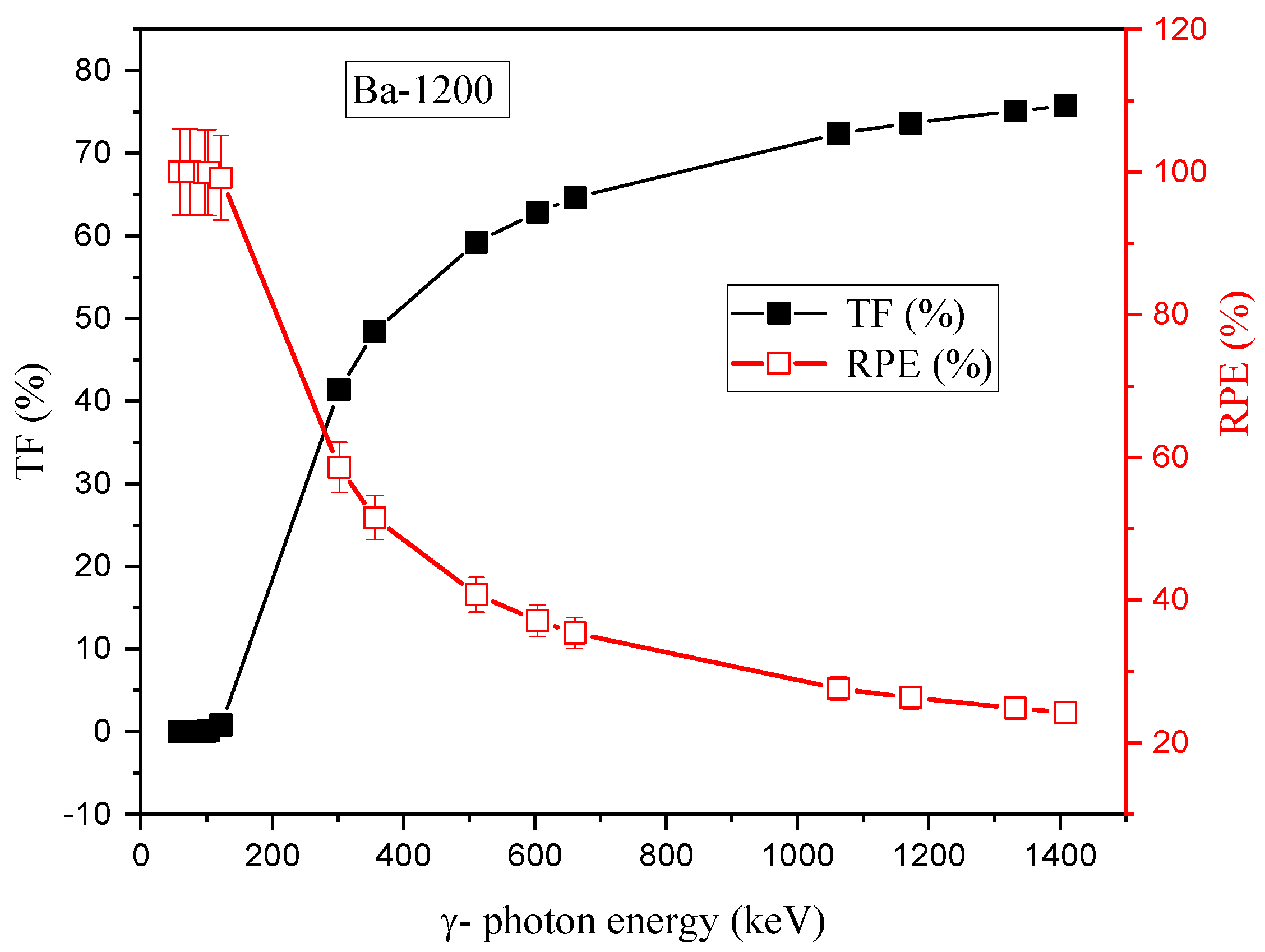
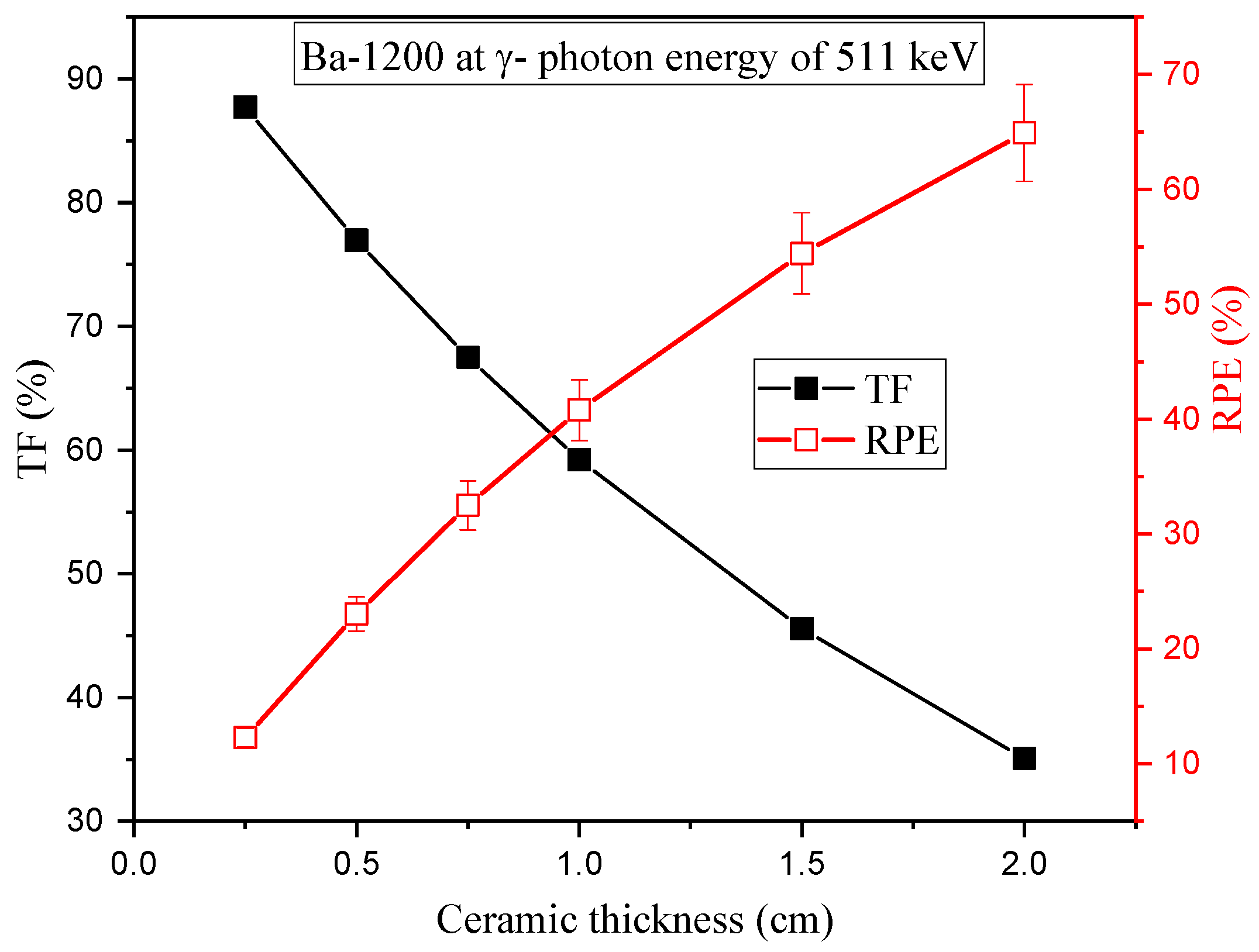
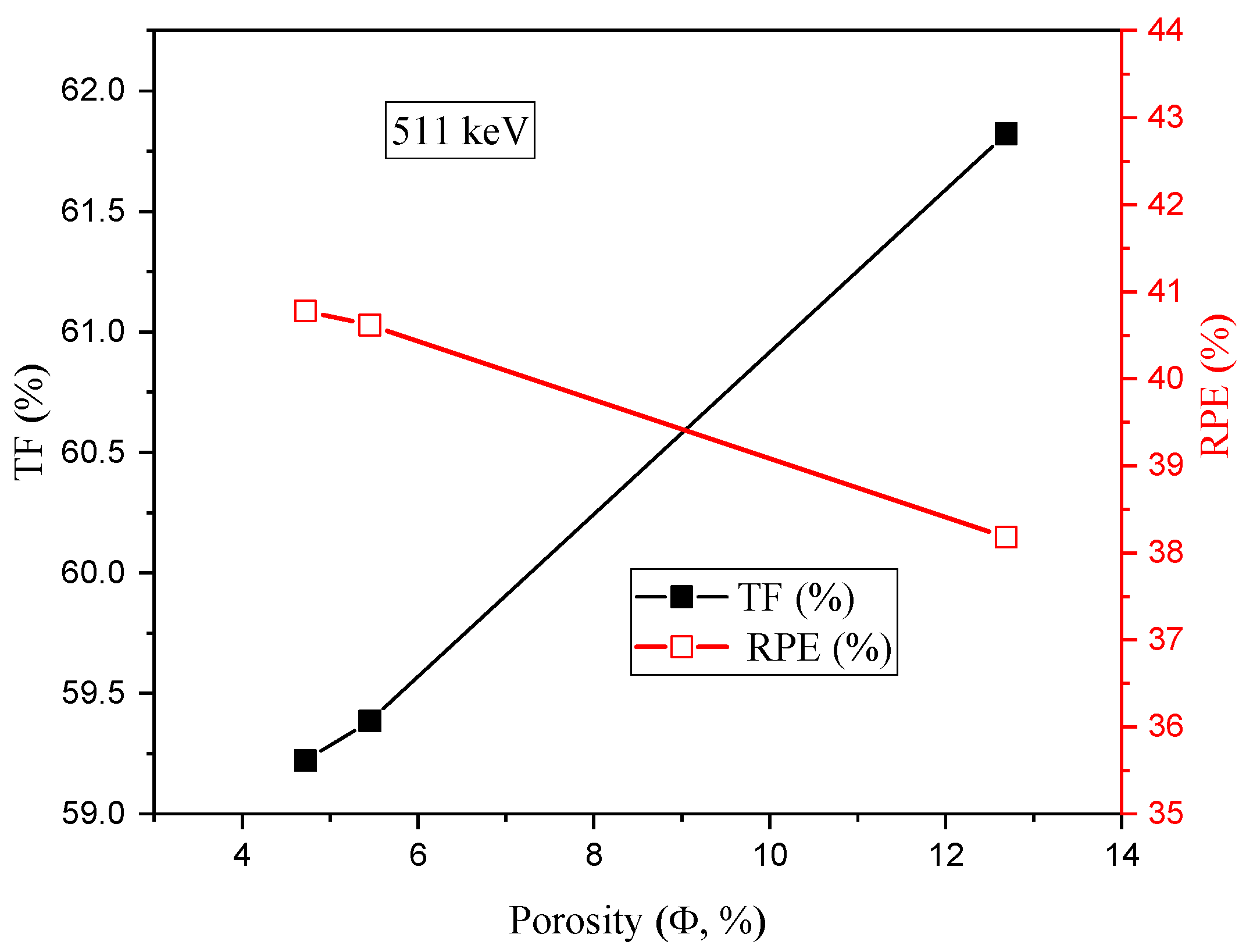
3.2. Radiation-protecting Parameters
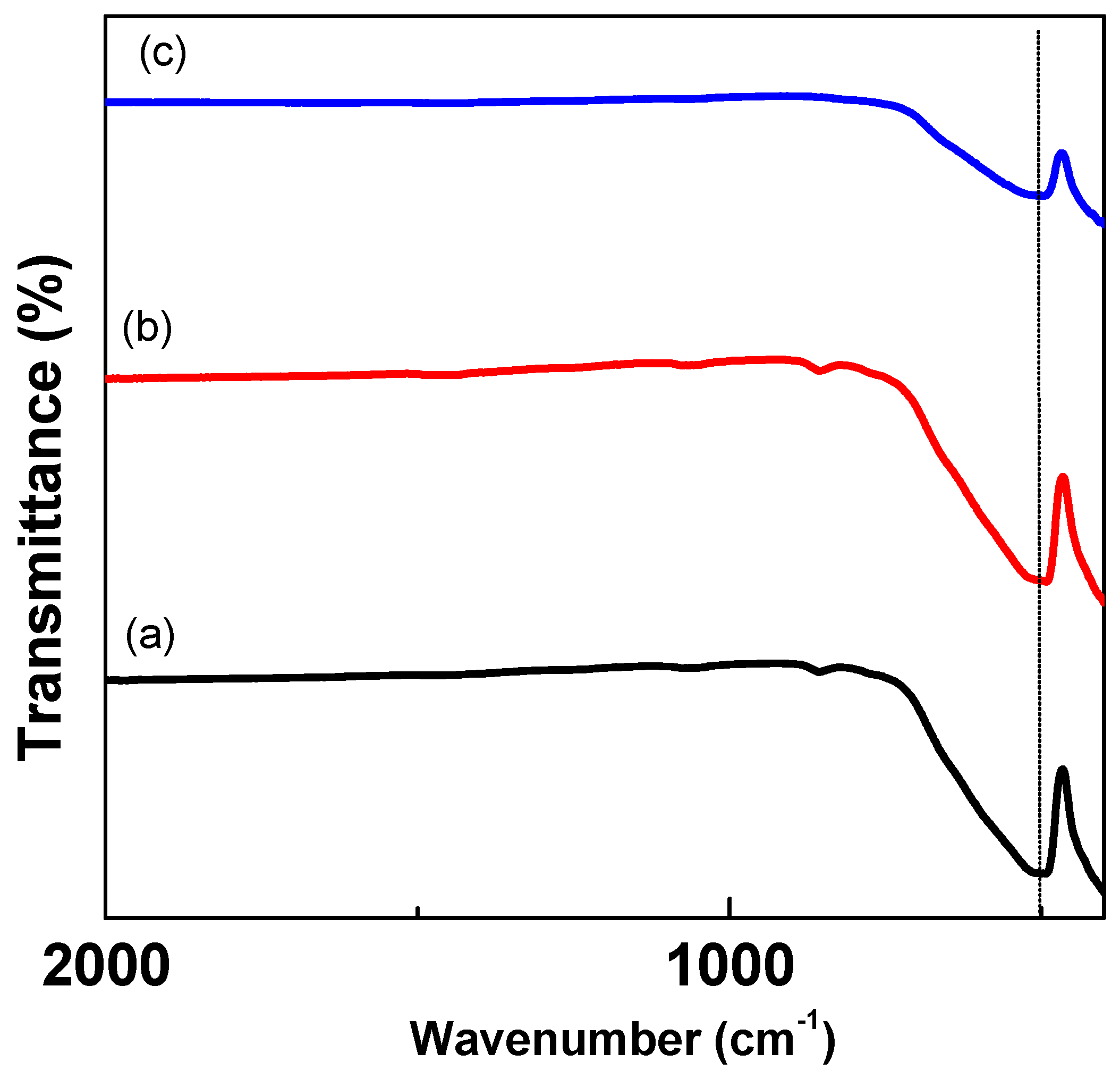
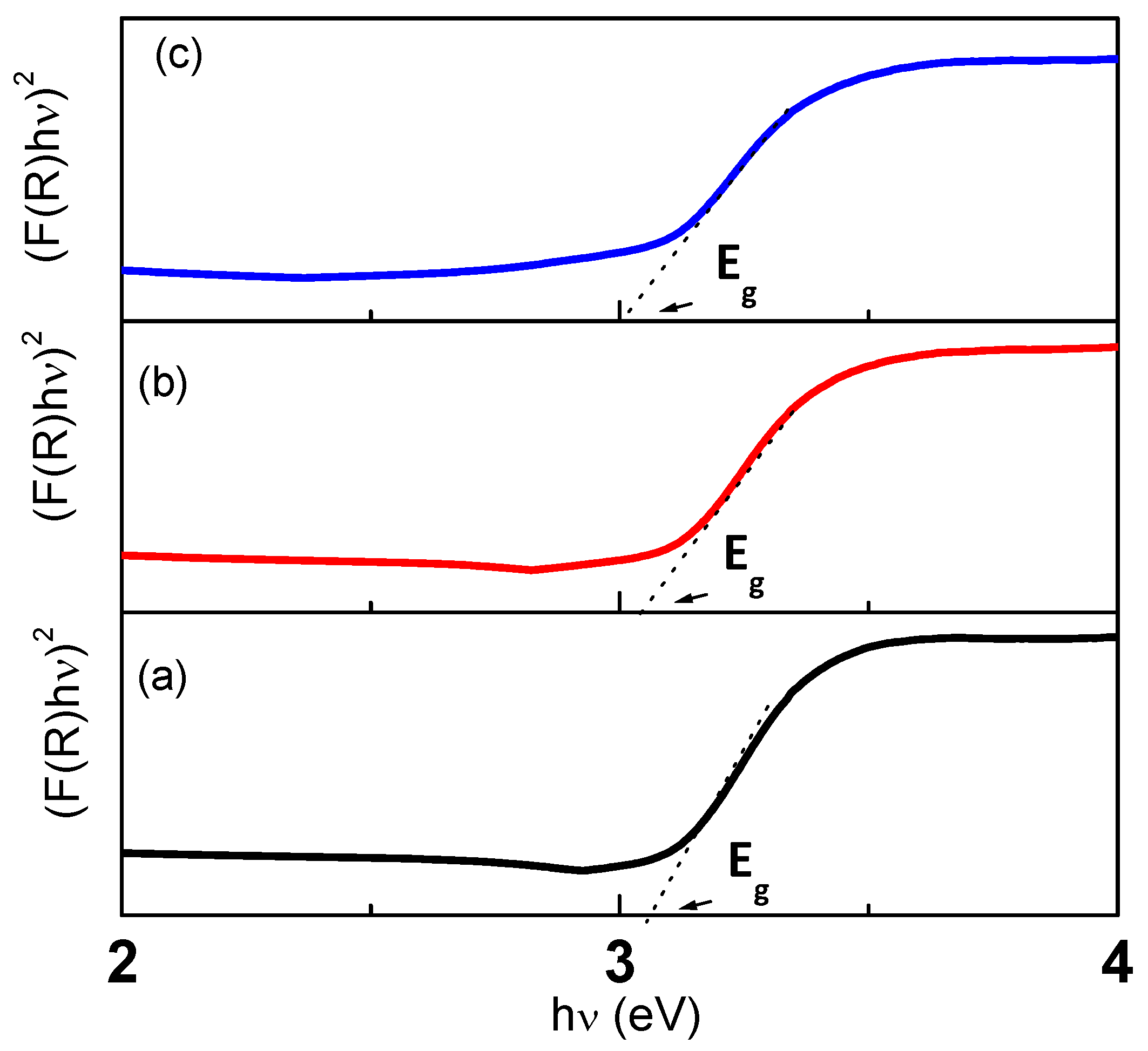
3.3. Optical Bandgap Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, W.; Zhang, L.; Li, X.; Ling, G.; Zhang, P. Nuclear Targeting Subcellular-delivery Nanosystems for Precise Cancer Treatment. Int. J. Pharm. 2022, 619, 121735. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Hosokawa, M.; Tatsumi, A.; Asaumi, S.; Imai, R.; Ogawara, K.-I. Improvement of resistance to oxaliplatin by vorinostat in human colorectal cancer cells through inhibition of Nrf2 nuclear translocation. Biochem. Biophys. Res. Commun. 2022, 607, 9–14. [Google Scholar] [CrossRef]
- Pinsky, R.; Sabharwall, P.; Hartvigsen, J.; O’Brien, J. Comparative review of hydrogen production technologies for nuclear hybrid energy systems. Prog. Nucl. Energy 2020, 123, 103317. [Google Scholar] [CrossRef]
- Parker, H.M.O.; Joyce, M.J. The use of ionising radiation to image nuclear fuel: A review. Prog. Nucl. Energy 2015, 85, 297–318. [Google Scholar] [CrossRef] [Green Version]
- Golden, A.P.; Cohen, S.S.; Chen, H.; Ellis, E.D.; Boice, J.D., Jr. Evaluation of statistical modeling approaches for epidemiologic studies of low-dose radiation health effects. Int. J. Radiat. Biol. 2022, 98, 572–579. [Google Scholar] [CrossRef]
- DiCarlo, A.L.; Bandremer, A.C.; Hollingsworth, B.A.; Kasim, S.; Laniyonu, A.; Todd, N.F.; Wang, S.-J.; Wertheimer, E.R.; Rios, C.I. Cutaneous radiation injuries: Models, assessment and treatments. Radiat. Res. 2020, 194, 315–344. [Google Scholar] [CrossRef]
- Kaewjaeng, S.; Kothan, S.; Chaiphaksa, W.; Chanthima, N.; Rajaramakrishna, R.; Kim, H.J.; Kaewkhao, J. High transparency La2O3-CaO-B2O3-SiO2 glass for diagnosis X-rays shielding material application. Radiat. Phys. Chem. 2019, 160, 41–47. [Google Scholar] [CrossRef]
- Ahmad, I.; Shahzada, K.; Ahmad, M.I.; Khan, F.; Badrashi, Y.I.; Khan, S.W.; Muhammad, N.; Ahmad, H. Densification of Concrete using Barite as Fine Aggregate and its Effect on Concrete Mechanical and Radiation Shielding Properties. J. Eng. Res. 2019, 7, 81–95. [Google Scholar]
- Amin, M.N.; Ahmad, I.; Iqbal, M.; Abbas, A.; Khan, K.; Faraz, M.I.; Alabdullah, A.A.; Ullah, S. Computational AI Models for Investigating the Radiation Shielding Potential of High-Density Concrete. Materials 2022, 15, 4573. [Google Scholar] [CrossRef]
- Khalaf, M.A.; Ban, C.C.; Ramli, M.; Ahmed, N.M.; Sern, L.J.; Khaleel, H.A. Physicomechanical and gamma-ray shielding properties of high-strength heavyweight concrete containing steel furnace slag aggregate. J. Build. Eng. 2020, 30, 101306. [Google Scholar] [CrossRef]
- Mostofinejad, D.; Reisi, M.; Shirani, A. Mix design effective parameters on γ-ray attenuation coefficient and strength of normal and heavyweight concrete. Constr. Build. Mater. 2012, 28, 224–229. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Sayyed, M.; Al-Hadeethi, Y.; Kumar, A.; Elsafi, M.; Mahmoud, K.; Khandaker, M.U.; Bradley, D. A novel CaO–K2O–Na2O–P2O5 glass systems for radiation shielding applications. Radiat. Phys. Chem. 2021, 188, 109645. [Google Scholar] [CrossRef]
- Kamislioglu, M. Research on the effects of bismuth borate glass system on nuclear radiation shielding parameters. Results Phys. 2021, 22, 103844. [Google Scholar] [CrossRef]
- Katubi, K.M.; Ibraheem, A.A.; Alwadai, N.; Alrowaili, Z.; Olarinoye, I.; Sriwunkum, C.; Al-Buriahi, M. Enhancement on radiation shielding performance of B2O3 + Li2O + ZnO + Na2O glass system. Radiat. Phys. Chem. 2022, 201, 110457. [Google Scholar] [CrossRef]
- Sayyed, M.; Kumar, A.; Albarzan, B.; Jecong, J.; Kurtulus, R.; Almuqrin, A.H.; Kavas, T. Investigation of the optical, mechanical, and radiation shielding features for strontium-borotellurite glass system: Fabrication, characterization, and EPICS2017 computations. Optik 2021, 243, 167468. [Google Scholar] [CrossRef]
- Acikgoz, A.; Demircan, G.; Yılmaz, D.; Aktas, B.; Yalcin, S.; Yorulmaz, N. Structural, mechanical, radiation shielding properties and albedo parameters of alumina borate glasses: Role of CeO2 and Er2O3. Mater. Sci. Eng. B 2022, 276, 115519. [Google Scholar] [CrossRef]
- Madbouly, A.; Sallam, O.; Issa, S.A.; Rashad, M.; Hamdy, A.; Tekin, H.; Zakaly, H.M. Experimental and FLUKA evaluation on structure and optical properties and γ-radiation shielding capacity of bismuth borophosphate glasses. Prog. Nucl. Energy 2022, 148, 104219. [Google Scholar] [CrossRef]
- Alzahrani, J.S.; Eke, C.; Alrowaili, Z.A.; Boukhris, I.; Mutuwong, C.; Bourham, M.A.; Al-Buriahi, M.S. A theoretical study on the radiation shielding performance of borate and tellurite glasses. Solid State Sci. 2022, 129, 106902. [Google Scholar] [CrossRef]
- Mhareb, M.; Slimani, Y.; Alajerami, Y.; Sayyed, M.; Lacomme, E.; Almessiere, M. Structural and radiation shielding properties of BaTiO3 ceramic with different concentrations of Bismuth and Ytterbium. Ceram. Int. 2020, 46, 28877–28886. [Google Scholar] [CrossRef]
- Slimani, Y.; Selmi, A.; Hannachi, E.; Almessiere, M.A.; Baykal, A.; Ercan, I. Impact of ZnO addition on structural, morphological, optical, dielectric and electrical performances of BaTiO3 ceramics. J. Mater. Sci. Mater. Electron. 2019, 30, 9520–9530. [Google Scholar] [CrossRef]
- Slimani, Y.; Selmi, A.; Hannachi, E.; Almessiere, M.A.; Mumtaz, M.; Baykal, A.; Ercan, I. Study of tungsten oxide effect on the performance of BaTiO3 ceramics. J. Mater. Sci. Mater. Electron. 2019, 30, 13509–13518. [Google Scholar] [CrossRef]
- Hannachi, E.; Sayyed, M.; Slimani, Y.; Elsafi, M. Experimental investigation on the physical properties and radiation shielding efficiency of YBa2Cu3Oy/M@ M3O4 (M= Co, Mn) ceramic composites. J. Alloys Compd. 2022, 904, 164056. [Google Scholar] [CrossRef]
- Rashid, R.S.M.; Salem, S.M.; Azreen, N.M.; Voo, Y.L.; Haniza, M.; Shukri, A.A.; Yahya, M.-S. Effect of elevated temperature to radiation shielding of ultra-high performance concrete with silica sand or magnetite. Constr. Build. Mater. 2020, 262, 120567. [Google Scholar] [CrossRef]
- Hannachi, E.; Mahmoud, K.; Sayyed, M.; Slimani, Y. Effect of sintering conditions on the radiation shielding characteristics of YBCO superconducting ceramics. J. Phys. Chem. Solids 2022, 164, 110627. [Google Scholar] [CrossRef]
- Slimani, Y.; Hamad, M.K.; Olarinoye, I.O.; Alajerami, Y.S.; Sayyed, M.I.; Almessiere, M.A.; Mhareb, M.H.A. Determination of structural features of different Perovskite ceramics and investigation of ionizing radiation shielding properties. J. Mater. Sci. Mater. Electron. 2021, 32, 20867–20881. [Google Scholar] [CrossRef]
- Hannachi, E.; Mahmoud, K.; Sayyed, M.; Slimani, Y. Structure, optical properties, and ionizing radiation shielding performance using Monte Carlo simulation for lead-free BTO perovskite ceramics doped with ZnO, SiO2, and WO3 oxides. Mater. Sci. Semicond. Process. 2022, 145, 106629. [Google Scholar] [CrossRef]
- Şakar, E.; Alim, B.; Özpolat, Ö.F.; Şakar, B.C.; Baltakesmez, A.; Akbaba, U. A surveying of photon and particle radiation interaction characteristics of some perovskite materials. Radiat. Phys. Chem. 2021, 189, 109719. [Google Scholar] [CrossRef]
- Kacal, M.R.; Akman, F.; Sayyed, M.I. Investigation of radiation shielding properties for some ceramics. Radiochim. Acta 2019, 107, 179–191. [Google Scholar] [CrossRef]
- Hannachi, E.; Sayyed, M.; Slimani, Y.; Almessiere, M.; Baykal, A.; Elsafi, M. Synthesis, characterization, and performance assessment of new composite ceramics towards radiation shielding applications. J. Alloys Compd. 2022, 899, 163173. [Google Scholar] [CrossRef]
- Sayyed, M.; Hannachi, E.; Slimani, Y.; Khandaker, M.U.; Elsafi, M. Radiation shielding properties of bi-ferroic ceramics added with CNTs. Radiat. Phys. Chem. 2022, 200, 110096. [Google Scholar] [CrossRef]
- Hannachi, E.; Sayyed, M.I.; Mahmoud, K.A.; Slimani, Y.; Akhtar, S.; Albarzan, B.; Almuqrin, A.H. Almuqrin. Impact of tin oxide on the structural features and radiation shielding response of some ABO3 perovskites ceramics (A = Ca, Sr, Ba; B = Ti). Appl. Phys. A 2021, 127, 970. [Google Scholar] [CrossRef]
- X-5 Monte Carlo Team, MCNP—A General Monte Carlo N-Particle Transport Code; Version 5, La-Ur-03-1987. II; Los Alamos National Laboratory: Los Alamos, NM, USA, 2003.
- Hannachi, E.; Sayyed, M.I.; Almuqrin, A.H.; Mahmoud, K.G. Study of the structure and radiation-protective properties of yttrium barium copper oxide ceramic doped with different oxides. J. Alloys Compd. 2021, 885, 161142. [Google Scholar] [CrossRef]
- Naseer, K.; Marimuthu, K.; Mahmoud, K.; Sayyed, M. The concentration impact of Yb3+ on the bismuth boro-phosphate glasses: Physical, structural, optical, elastic, and radiation-shielding properties. Radiat. Phys. Chem. 2021, 188, 109617. [Google Scholar] [CrossRef]
- Sayyed, M.; Zaid, M.H.M.; Effendy, N.; Matori, K.A.; Sidek, H.A.A.; Lacomme, E.; Mahmoud, K.; Al Shammari, M.M. The influence of PbO and Bi2O3 on the radiation shielding and elastic features for different glasses. J. Mater. Res. Technol. 2020, 9, 8429–8438. [Google Scholar] [CrossRef]
- Abouhaswa, A.; I Sayyed, M.; Altowyan, A.S.; Al-Hadeethi, Y.; Mahmoud, K.A. Synthesis, structural, optical and radiation shielding features of tungsten trioxides doped borate glasses using Monte Carlo simulation and phy-X program. J. Non-Cryst. Solids 2020, 543, 120134. [Google Scholar] [CrossRef]
- Varshney, D.; Dar, M.A. Structural and magneto-transport properties of (1 − x) La0. 67Sr0. 33MnO3 (LSMO)+(x) BaTiO3 (BTO) composites. J. Alloys Compd. 2015, 619, 122–130. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Z.; Lu, Z.; Wang, X. Effects of sintering temperature and Bi2O3, Y2O3 and MgO co-doping on the dielectric properties of X8R BaTiO3-based ceramics. Ceram. Int. 2022, 48, 2377–2384. [Google Scholar] [CrossRef]
- Rani, A.; Kolte, J.; Gopalan, P. Influence of sintering temperature on structural, electrical, and magnetoelectric properties of multiferroic Fe-substituted BaTiO3 ceramics. Appl. Phys. A 2022, 128, 442. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.; Chu, R.; Fu, P.; Hao, J. Structure and electrical properties of BaTiO3 prepared by sol–gel process. J. Alloys Compd. 2009, 482, 137–140. [Google Scholar] [CrossRef]
- Ying, K.-L.; Hsieh, T.-E. Sintering behaviors and dielectric properties of nanocrystalline barium titanate. Mater. Sci. Eng. B 2007, 138, 241–245. [Google Scholar] [CrossRef]
- Singh, M.; Yadav, B.; Ranjan, A.; Kaur, M.; Gupta, S. Synthesis and characterization of perovskite barium titanate thin film and its application as LPG sensor. Sens. Actuators B Chem. 2017, 241, 1170–1178. [Google Scholar] [CrossRef]
- Ashiri, R. Detailed FT-IR spectroscopy characterization and thermal analysis of synthesis of barium titanate nanoscale particles through a newly developed process. Vib. Spectrosc. 2013, 66, 24–29. [Google Scholar] [CrossRef]
- Kumari, A.; Kumari, K.; Ahmed, F.; Alshoaibi, A.; Alvi, P.; Dalela, S.; Ahmad, M.M.; Aljawfi, R.N.; Dua, P.; Vij, A.; et al. Influence of Sm doping on structural, ferroelectric, electrical, optical and magnetic properties of BaTiO3. Vacuum 2021, 184, 109872. [Google Scholar] [CrossRef]
- Rawat, M.; Yadav, K.L. Study of structural, electrical, magnetic and optical properties of 0.65 BaTiO3–0.35 Bi0.5Na0.5TiO3–BiFeO3 multiferroic composite. J. Alloys Compd. 2014, 597, 188–199. [Google Scholar] [CrossRef]
- Sahana, M.; Sudakar, C.; Dixit, A.; Thakur, J.; Naik, R.; Naik, V. Quantum confinement effects and band gap engineering of SnO2 nanocrystals in a MgO matrix. Acta Mater. 2012, 60, 1072–1078. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannachi, E.; Mahmoud, K.G.; Slimani, Y.; Sayyed, M.I.; Arayro, J.; Maghrbi, Y. Monte Carlo Simulation for Investigating the Sintering Temperatures Effects on Radiation Shielding Performances of Lead-Free ABO3 Perovskite Ceramic. Crystals 2023, 13, 230. https://doi.org/10.3390/cryst13020230
Hannachi E, Mahmoud KG, Slimani Y, Sayyed MI, Arayro J, Maghrbi Y. Monte Carlo Simulation for Investigating the Sintering Temperatures Effects on Radiation Shielding Performances of Lead-Free ABO3 Perovskite Ceramic. Crystals. 2023; 13(2):230. https://doi.org/10.3390/cryst13020230
Chicago/Turabian StyleHannachi, Essia, Karem G. Mahmoud, Yassine Slimani, M. I. Sayyed, Jack Arayro, and Yasser Maghrbi. 2023. "Monte Carlo Simulation for Investigating the Sintering Temperatures Effects on Radiation Shielding Performances of Lead-Free ABO3 Perovskite Ceramic" Crystals 13, no. 2: 230. https://doi.org/10.3390/cryst13020230






