Phase Transformation Pathway of DyPO4 to 21.5 GPa
Abstract
:1. Introduction
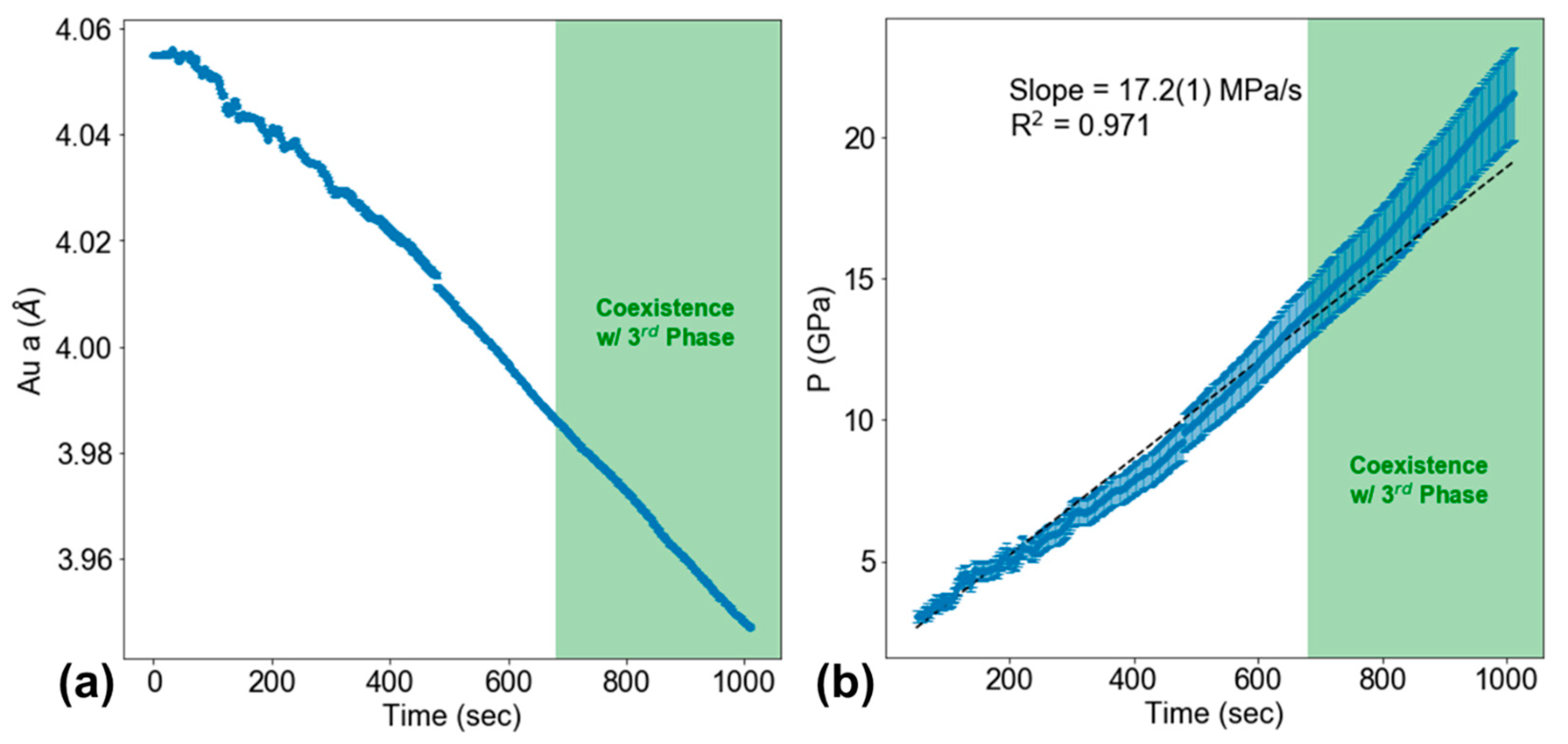
2. Materials and Methods
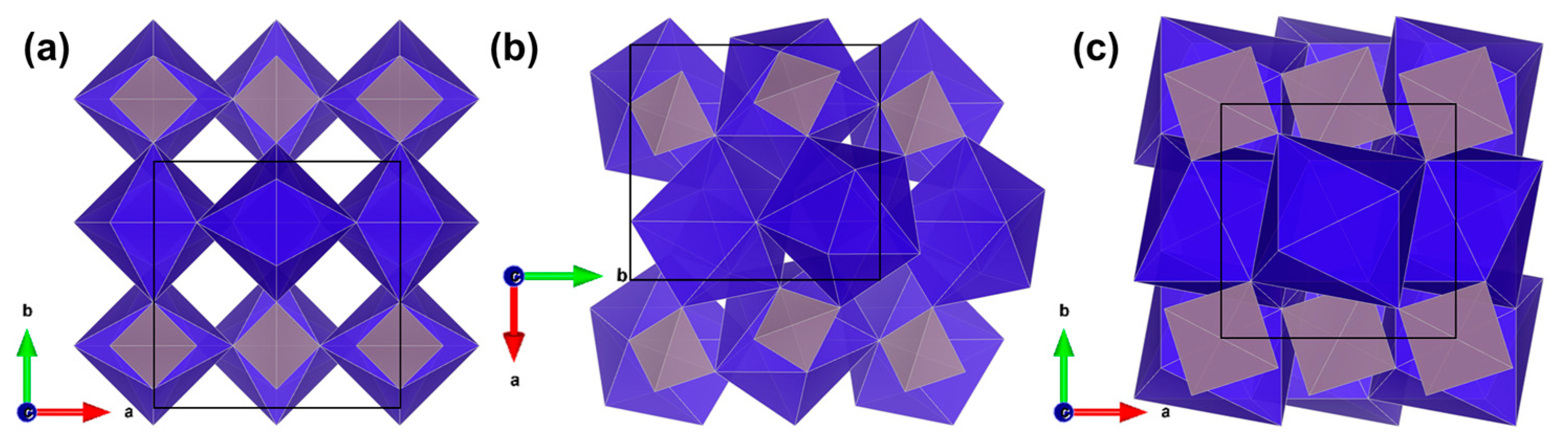
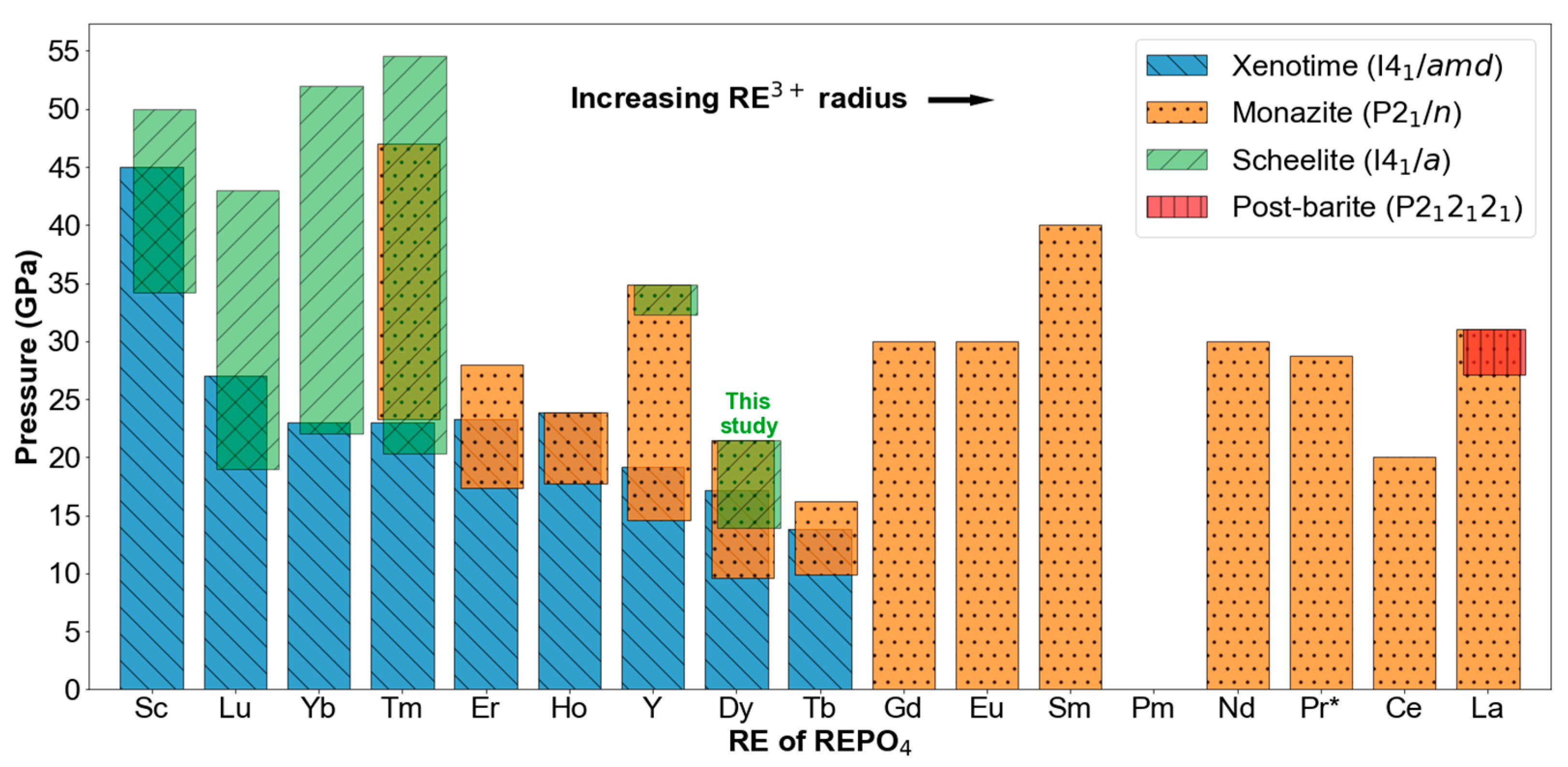
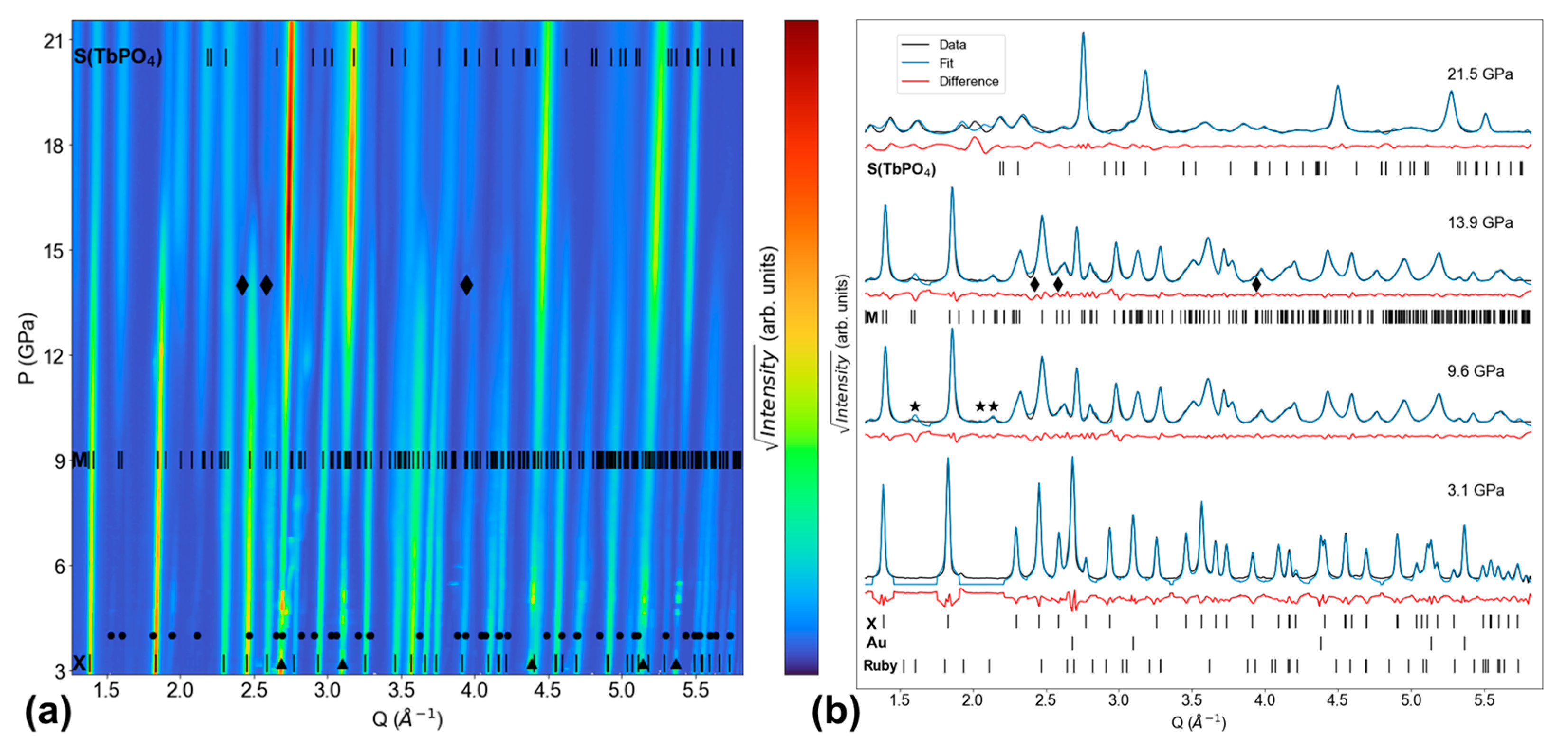
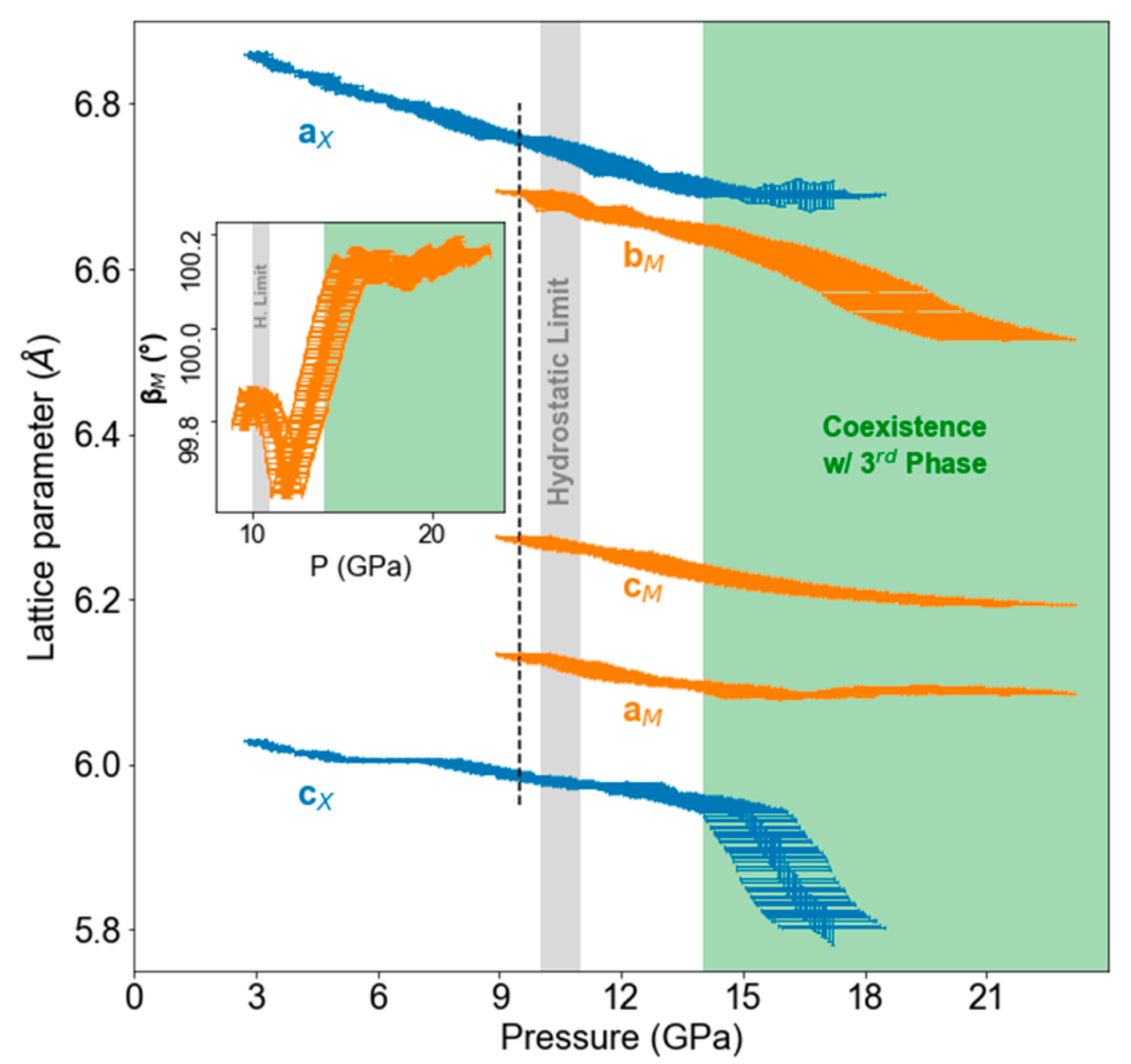
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boatner, L.A. Synthesis, Structure, and Properties of Monazite, Pretulite, and Xenotime. Rev. Mineral. Geochem. 2002, 48, 87–121. [Google Scholar] [CrossRef]
- Vielreicher, N.M.; Groves, D.I.; Fletcher, I.R.; McNaughton, N.J.; Rasmussen, B. Hydrothermal Monazite and Xenotime Geochronology: A New Direction for Precise Dating of Orogenic Gold Mineralization. SEG Discov. 2003, 70, 1–16. [Google Scholar] [CrossRef]
- Cox, M.A.; Cavosie, A.J.; Poelchau, M.; Kenkmann, T.; Bland, P.A.; Miljković, K. Shock Deformation Microstructures in Xenotime from the Spider Impact Structure, Western Australia. In Large Meteorite Impacts and Planetary Evolution VI; Geological Society of America: Boulder, CO, USA, 2021; pp. 449–464. [Google Scholar]
- Morgan, P.E.D.; Marshall, D.B. Ceramic Composites of Monazite and Alumina. J. Am. Ceram. Soc. 1995, 78, 1553–1563. [Google Scholar] [CrossRef]
- Hay, R.S.; Mogilevsky, P.; Boakye, E. Phase Transformations in Xenotime Rare-Earth Orthophosphates. Acta Mater. 2013, 61, 6933–6947. [Google Scholar] [CrossRef]
- Hay, R.S.; Boakye, E.E.; Mogilevsky, P.; Fair, G.E.; Parthasarathy, T.A.; Davis, J.E. Transformation Plasticity in (Gdx Dy1-x) PO 4 Fiber Coatings during Fiber Push Out. J. Am. Ceram. Soc. 2013, 96, 1586–1595. [Google Scholar] [CrossRef]
- Ni, Y.; Hughes, J.M.; Mariano, A.N.; Et, N.I.; Crystal, A.L. Crystal Chemistry of the Monazite and Xenotime Structures. Am. Mineral. 1995, 80, 21–26. [Google Scholar] [CrossRef]
- Errandonea, D.; Manjón, F.J. Pressure Effects on the Structural and Electronic Properties of ABX4 Scintillating Crystals. Prog. Mater. Sci. 2008, 53, 711–773. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Baur, C.; Chable, J.; Barlow, A.J.; Fichtner, M.; Banerjee, A.; Chakraborty, S.; Ahuja, R. Phase Evolution in Calcium Molybdate Nanoparticles as a Function of Synthesis Temperature and Its Electrochemical Effect on Energy Storage. Nanoscale Adv. 2019, 1, 565–580. [Google Scholar] [CrossRef]
- Macey, B.J. The Crystal Chemistry of MTO4 Compounds with the Zicron, Scheelite, and Monazite Structure Types; Virginia Tech: Blackburg, VA, USA, 2009. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Zhang, F.X.; Wang, J.W.; Lang, M.; Zhang, J.M.; Ewing, R.C.; Boatner, L.A. High-Pressure Phase Transitions of ScPO4 and YPO4. Phys. Rev. B Cover. Condens. Matter Mater. Phys. 2009, 80, 184114. [Google Scholar] [CrossRef]
- Lösch, H.; Hirsch, A.; Holthausen, J.; Peters, L.; Xiao, B.; Neumeier, S.; Schmidt, M.; Huittinen, N. A Spectroscopic Investigation of Eu3+ Incorporation in LnPO4 (Ln = Tb, Gd1-XLux, X = 0.3, 0.5, 0.7, 1) Ceramics. Front. Chem. 2019, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Tschauner, O.; Ushakov, S.V.; Navrotsky, A.; Boatner, L.A. Phase Transformations and Indications for Acoustic Mode Softening in Tb-Gd Orthophosphate. J. Phys. Condens. Matter 2016, 28, 035403. [Google Scholar] [CrossRef] [PubMed]
- Kolitsch, U.; Holtstam, D. Crystal Chemistry of REEXO4 Compounds (X = P,As, V). II. Review of REEXO4 Compounds and Their Stability Fields. Eur. J. Mineral. 2004, 16, 117–126. [Google Scholar] [CrossRef]
- Finch, R.J. Structure and Chemistry of Zircon and Zircon-Group Minerals. Rev. Mineral. Geochem. 2003, 53, 1–25. [Google Scholar] [CrossRef]
- Bose, P.P.; Mittal, R.; Chaplot, S.L.; Loong, C.K.; Boatner, L.A. Inelastic Neutron Scattering, Lattice Dynamics, and High-Pressure Phase Stability of Zircon-Structured Lanthanide Orthophosphates. Phys. Rev. B Cover. Condens. Matter Mater. Phys. 2010, 82, 094309. [Google Scholar] [CrossRef]
- Stavrou, E.; Tatsi, A.; Raptis, C.; Efthimiopoulos, I.; Syassen, K.; Muñoz, A.; Rodríguez-Hernández, P.; López-Solano, J.; Hanfland, M.; Muñoz, A.; et al. Effects of Pressure on the Structure and Lattice Dynamics of TmPO4: Experiments and Calculations. Phys. Rev. B Condens. Matter Mater. Phys. 2012, 85, 24117. [Google Scholar] [CrossRef]
- Zhang, F.X.; Lang, M.; Ewing, R.C.; Lian, J.; Wang, Z.W.; Hu, J.; Boatner, L.A. Pressure-Induced Zircon-Type to Scheelite-Type Phase Transitions in YbPO4 and LuPO4. J. Solid State Chem. 2008, 181, 2633–2638. [Google Scholar] [CrossRef]
- Lacomba-Perales, R.; Errandonea, D.; Meng, Y.; Bettinelli, M. High-Pressure Stability and Compressibility of A PO4 (A=La, Nd, Eu, Gd, Er, and Y) Orthophosphates: An X-ray Diffraction Study Using Synchrotron Radiation. Phys. Rev. B Cover. Condens. Matter Mater. Phys. 2010, 81, 064113. [Google Scholar] [CrossRef]
- Gomis, O.; Lavina, B.; Rodríguez-Hernández, P.; Muǹoz, A.; Errandonea, R.; Errandonea, D.; Bettinelli, M. High-Pressure Structural, Elastic, and Thermodynamic Properties of Zircon-Type HoPO4 and TmPO4. J. Phys. Condens. Matter 2017, 29, 095401. [Google Scholar] [CrossRef]
- Sharma, J.; Musselman, M.; Haberl, B.; Packard, C.E. In Situ Synchrotron Diffraction of Pressure-Induced Phase Transition in DyPO4 under Variable Hydrostaticity. Phys. Rev. B 2021, 103, 184105. [Google Scholar] [CrossRef]
- López-Solano, J.; Rodríguez-Hernández, P.; Muñoz, A.; Gomis, O.; Santamaría-Perez, D.; Errandonea, D.; Manjón, F.J.; Kumar, R.S.; Stavrou, E.; Raptis, C. Theoretical and Experimental Study of the Structural Stability of TbPO4 at High Pressures. Phys. Rev. B Cover. Condens. Matter Mater. Phys. 2010, 81, 144126. [Google Scholar] [CrossRef]
- Heuser, J.M.; Palomares, R.I.; Bauer, J.D.; Rodriguez, M.J.L.; Cooper, J.; Lang, M.; Scheinost, A.C.; Schlenz, H.; Winkler, B.; Bosbach, D.; et al. Journal of the European Ceramic Society Structural Characterization of (Sm, Tb) PO4 Solid Solutions and Pressure-Induced Phase Transitions. J. Eur. Ceram. Soc. 2018, 38, 4070–4081. [Google Scholar] [CrossRef]
- Huang, T.; Lee, J.S.; Kung, J.; Lin, C.M. Study of Monazite under High Pressure. Solid State Commun. 2010, 150, 1845–1850. [Google Scholar] [CrossRef]
- Errandonea, D.; Gomis, O.; Rodriguez-Hernández, P.; Munz, A.; Ruiz-Fuertes, J.; Gupta, M.; Achary, S.N.; Hirsch, A.; Manjon, F.J.; Peters, L.; et al. High-Pressure Structural and Vibrational Properties of Monazite-Type BiPO4, LaPO4, CePO4, and PrPO4. J. Phys. Condens. Matter 2018, 30, 065401. [Google Scholar] [CrossRef] [PubMed]
- Musselman, M.A.; Wilkinson, T.M.; Haberl, B.; Packard, C.E. In Situ Raman Spectroscopy of Pressure-Induced Phase Transformations in Polycrystalline TbPO4, DyPO4, and GdxDy(1−x)PO4. J. Am. Ceram. Soc. 2018, 101, 2562–2570. [Google Scholar] [CrossRef]
- Rustad, J.R. Density Functional Calculations of the Enthalpies of Formation of Rare-Earth Orthophosphates. Am. Mineral. 2012, 97, 791–799. [Google Scholar] [CrossRef]
- Kowalski, P.M.; Li, Y. Relationship between the Thermodynamic Excess Properties of Mixing and the Elastic Moduli in the Monazite-Type Ceramics. J. Eur. Ceram. Soc. 2016, 36, 2093–2096. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Helean, K.B.; Navrotsky, A.; Boatner, L.A. Thermochemistry of Rare-Earth Orthophosphates. J. Mater. Res. 2001, 16, 2623–2633. [Google Scholar] [CrossRef]
- Blanca Romero, A.; Kowalski, P.M.; Beridze, G.; Schlenz, H.; Bosbach, D. Performance of DFT+U Method for Prediction of Structural and Thermodynamic Parameters of Monazite-Type Ceramics. J. Comput. Chem. 2014, 35, 1339–1346. [Google Scholar] [CrossRef]
- Ruiz-Fuertes, J.; Hirsch, A.; Friedrich, A.; Winkler, B.; Bayarjargal, L.; Morgenroth, W.; Peters, L.; Roth, G.; Milman, V. High-Pressure Phase of LaPO4 Studied by X-ray Diffraction and Second Harmonic Generation. Phys. Rev. B Cover. Condens. Matter Mater. Phys. 2016, 94, 134109. [Google Scholar] [CrossRef]
- Musselman, M.A. In Situ Raman Spectroscopy of Pressure-Induced Phase Transformations in DyPO4 and GdxDy(1-x)PO4. Master’s Thesis, Colorado School of Mines, Golden, CO, USA, 2017. [Google Scholar]
- Sinogeikin, S.V.; Smith, J.S.; Rod, E.; Lin, C.; Kenney-Benson, C.; Shen, G. Online Remote Control Systems for Static and Dynamic Compression and Decompression Using Diamond Anvil Cells. Rev. Sci. Instrum. 2015, 86, 072209. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Wang, Y.; Dewaele, A.; Wu, C.; Fratanduono, D.E.; Eggert, J.; Klotz, S.; Dziubek, K.F.; Loubeyre, P.; Fat’yanov, O.V.; et al. Toward an International Practical Pressure Scale: A Proposal for an IPPS Ruby Gauge (IPPS-Ruby2020). High Press. Res. 2020, 40, 299–314. [Google Scholar] [CrossRef]
- Anderson, O.L.; Isaak, D.G.; Yamamoto, S. Anharmonicity and the Equation of State for Gold. J. Appl. Phys. 1989, 65, 1534–1543. [Google Scholar] [CrossRef]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A Program for Reduction of Two-Dimensional X-ray Diffraction Data and Data Exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The High Score Suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- Bail, A. Le Whole Powder Pattern Decomposition Methods and Applications: A Retrospection. Powder Diffr. 2005, 20, 316–326. [Google Scholar] [CrossRef]
- Milligan, W.O.; Mullica, D.F.; Beall, G.W.; Boatner, L.A. The Structures of Three Lanthanide Orthophosphates. Inorganica Chim. Acta 1983, 70, 133–136. [Google Scholar] [CrossRef]
- Heuser, J.M.; Neumeier, S.; Peters, L.; Schlenz, H.; Bosbach, D.; Deissmann, G. Structural Characterisation of Metastable Tb- and Dy-Monazites. J. Solid State Chem. 2019, 273, 45–52. [Google Scholar] [CrossRef]
- Couderc, J.J.; Garigue, G.; Lafourcade, L.; Nguyen, Q.T. Standard X-ray Diffraction Powder Patterns. Z. Met. 1959, 50, 708–716. [Google Scholar]
- Jephcoat, A.P.; Hemley, R.J.; Mao, H.K. X-ray Diffraction of Ruby (Al2O3:Cr3+) to 175 GPa. Phys. B+C 1988, 150, 115–121. [Google Scholar] [CrossRef]
- Klotz, S.; Chervin, J.-C.C.; Munsch, P.; Le Marchand, G. Hydrostatic Limits of 11 Pressure Transmitting Media. J. Phys. D Appl. Phys. 2009, 42, 075413. [Google Scholar] [CrossRef]
- Garg, A.B.; Shanavas, K.V.; Wani, B.N.; Sharma, S.M. Phase Transition and Possible Metallization in CeVO4 under Pressure. J. Solid State Chem. 2013, 203, 273–280. [Google Scholar] [CrossRef]

| Lattice Parameter | Axial Compressibility (Å,°/GPa) × 103 | Intercept at 0 GPa (Å,°) | R2 of Linear Fit |
|---|---|---|---|
| ax | −16.09 (12) | 6.907 (1) | 0.9871 |
| cx | −6.238 (127) | 6.045 (1) | 0.8876 |
| am | −10.67 (22) | 6.236 (2) | 0.9921 |
| bm | −11.64 (72) | 6.807 (7) | 0.9326 |
| cm | −11.10 (21) | 6.382 (2) | 0.9934 |
| βm | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, J.; Afful, H.Q.; Packard, C.E. Phase Transformation Pathway of DyPO4 to 21.5 GPa. Crystals 2023, 13, 249. https://doi.org/10.3390/cryst13020249
Sharma J, Afful HQ, Packard CE. Phase Transformation Pathway of DyPO4 to 21.5 GPa. Crystals. 2023; 13(2):249. https://doi.org/10.3390/cryst13020249
Chicago/Turabian StyleSharma, Jai, Henry Q. Afful, and Corinne E. Packard. 2023. "Phase Transformation Pathway of DyPO4 to 21.5 GPa" Crystals 13, no. 2: 249. https://doi.org/10.3390/cryst13020249
APA StyleSharma, J., Afful, H. Q., & Packard, C. E. (2023). Phase Transformation Pathway of DyPO4 to 21.5 GPa. Crystals, 13(2), 249. https://doi.org/10.3390/cryst13020249





