Preparation and Investigation of Ni–Co–P Alloy Coatings Using Jet Electrodeposition with Varying Pulse Parameters
Abstract
:1. Introduction
2. Experimental Device and Procedure
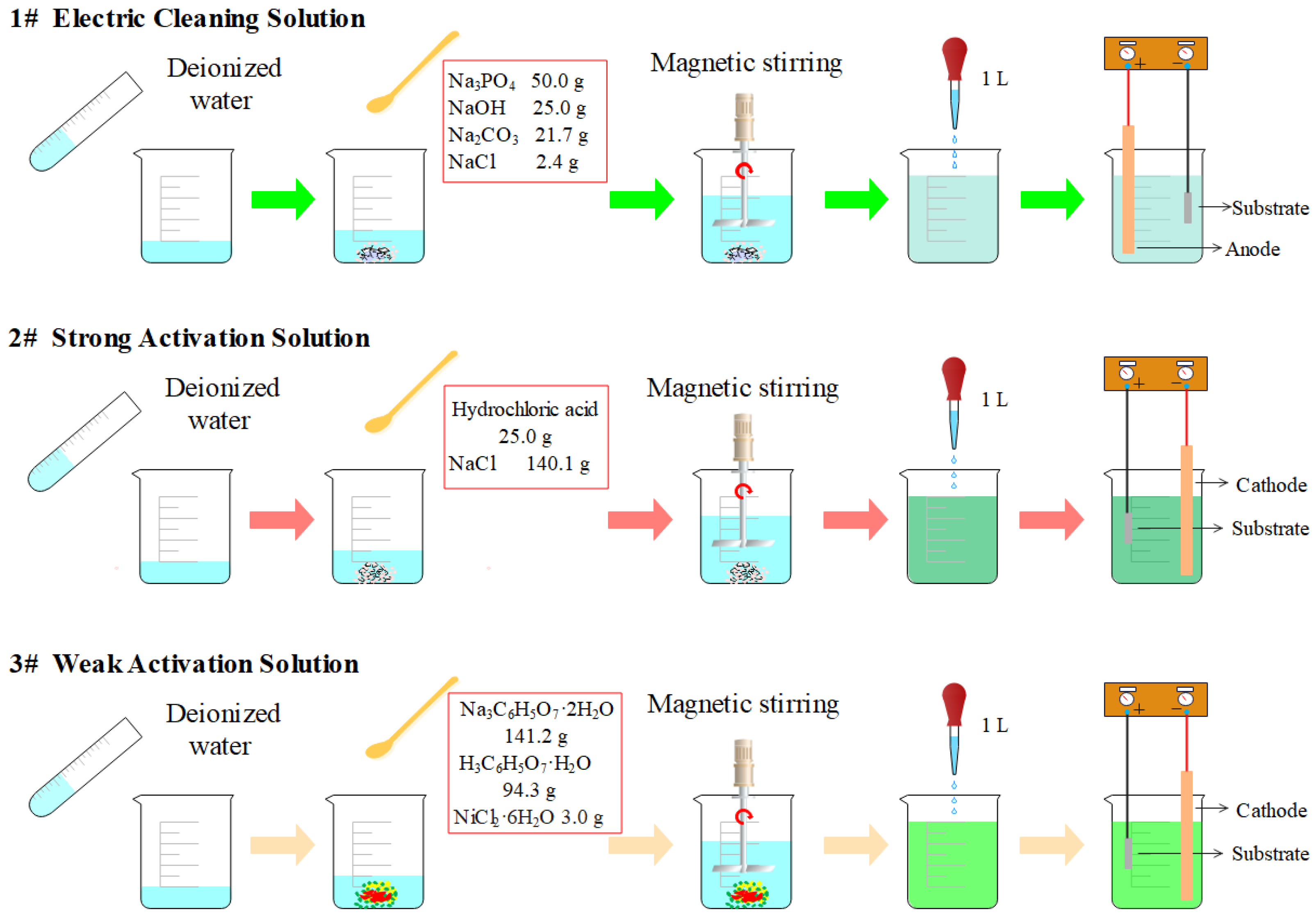
2.1. Materials and Pretreatment
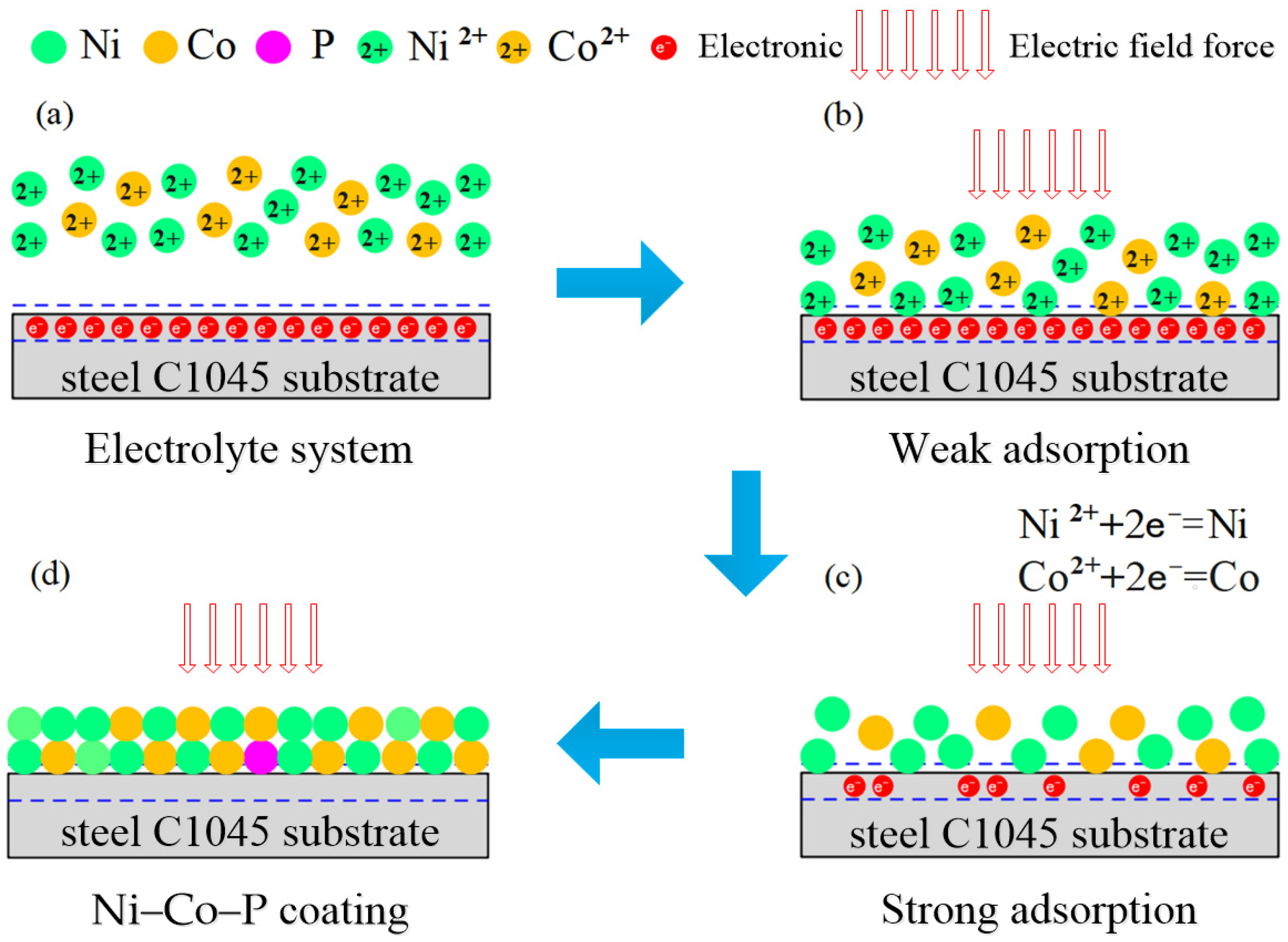
2.2. Preparation of Ni–Co–P Alloy Coatings
2.3. Characterization of Ni–Co–P Alloy Coatings
3. Results and Discussion
3.1. Jet-Electrodeposition of Ni–Co–P Alloy Coatings
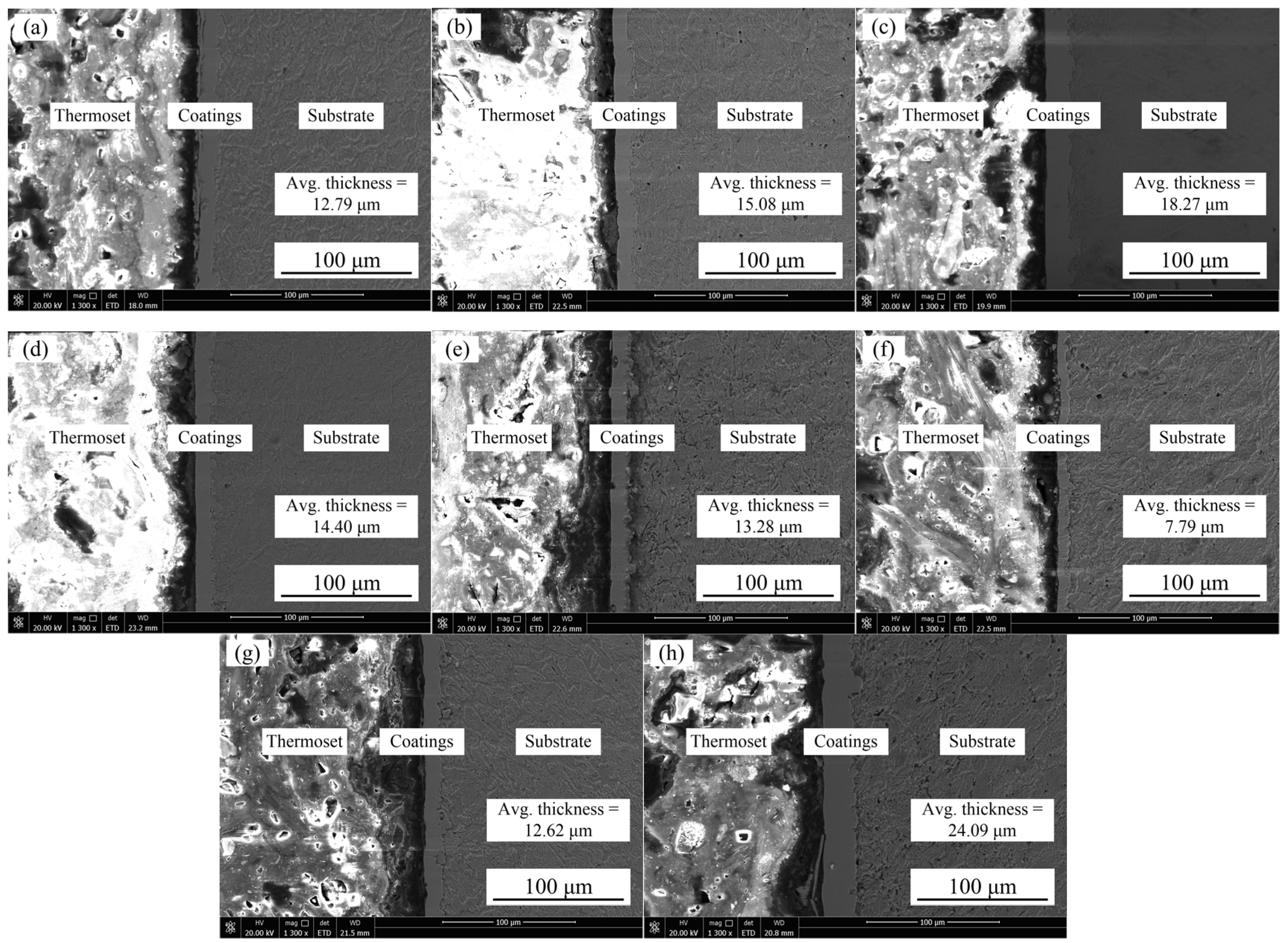
3.2. Cross-Section Morphologies of Samples
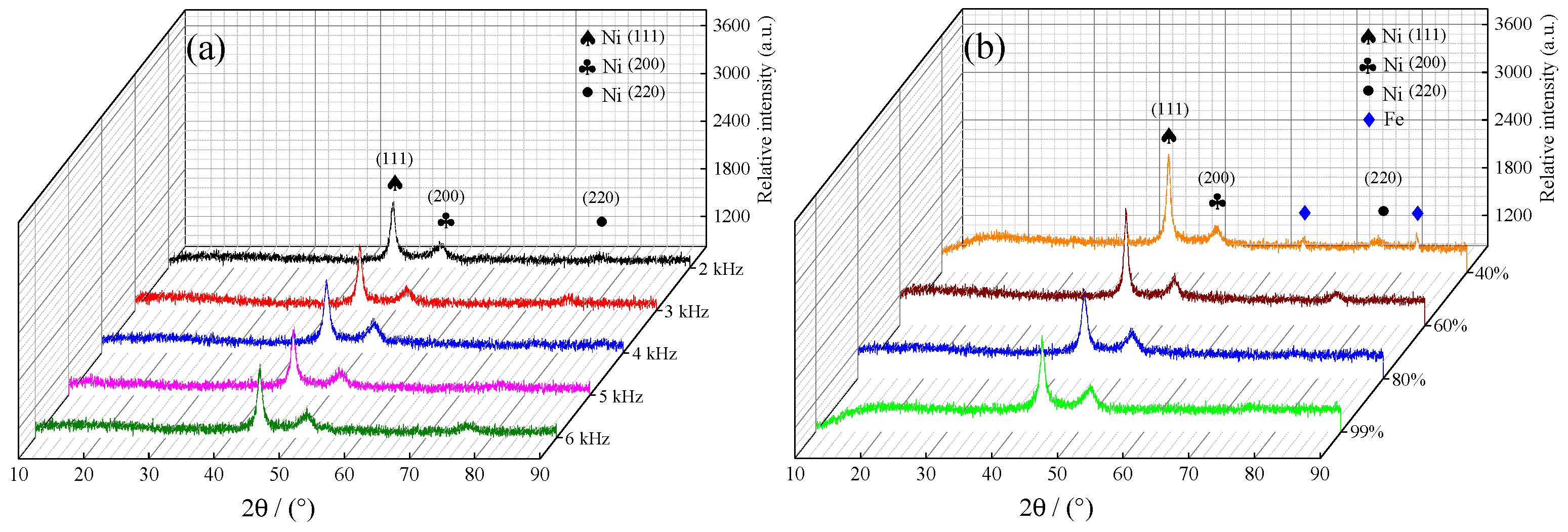
3.3. XRD Patterns of Samples
3.4. EDS Spectra of Samples
3.5. Microhardness of Samples
3.6. Wear Resistance of Samples
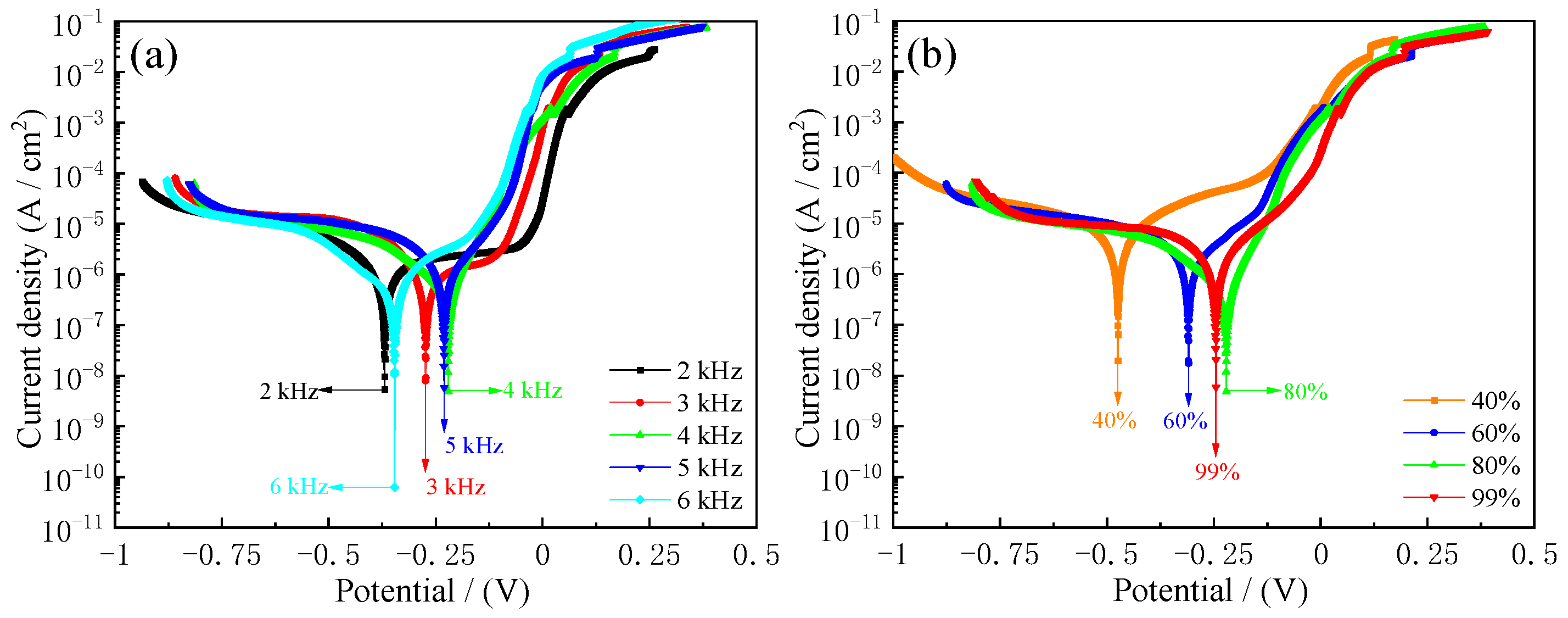
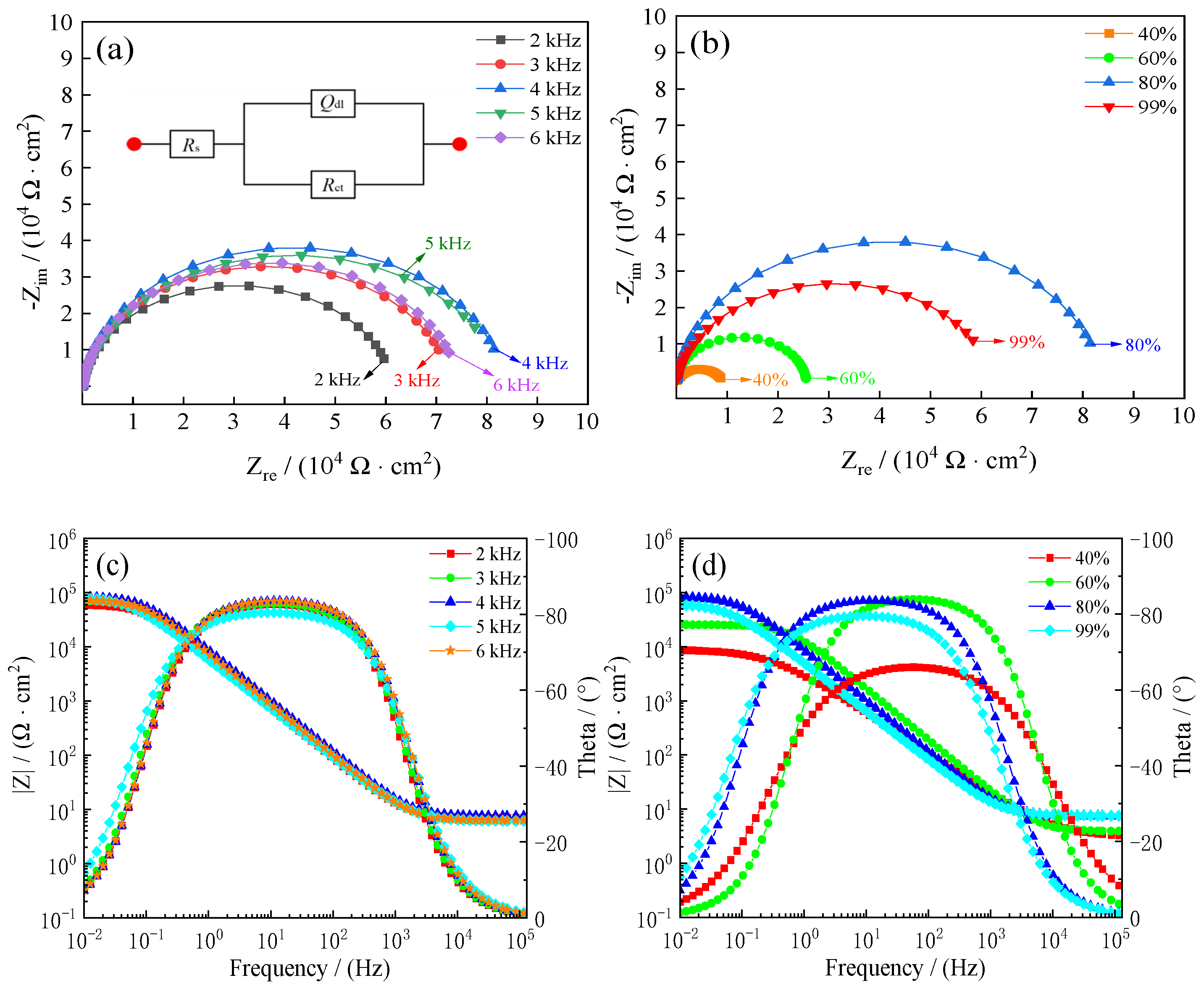
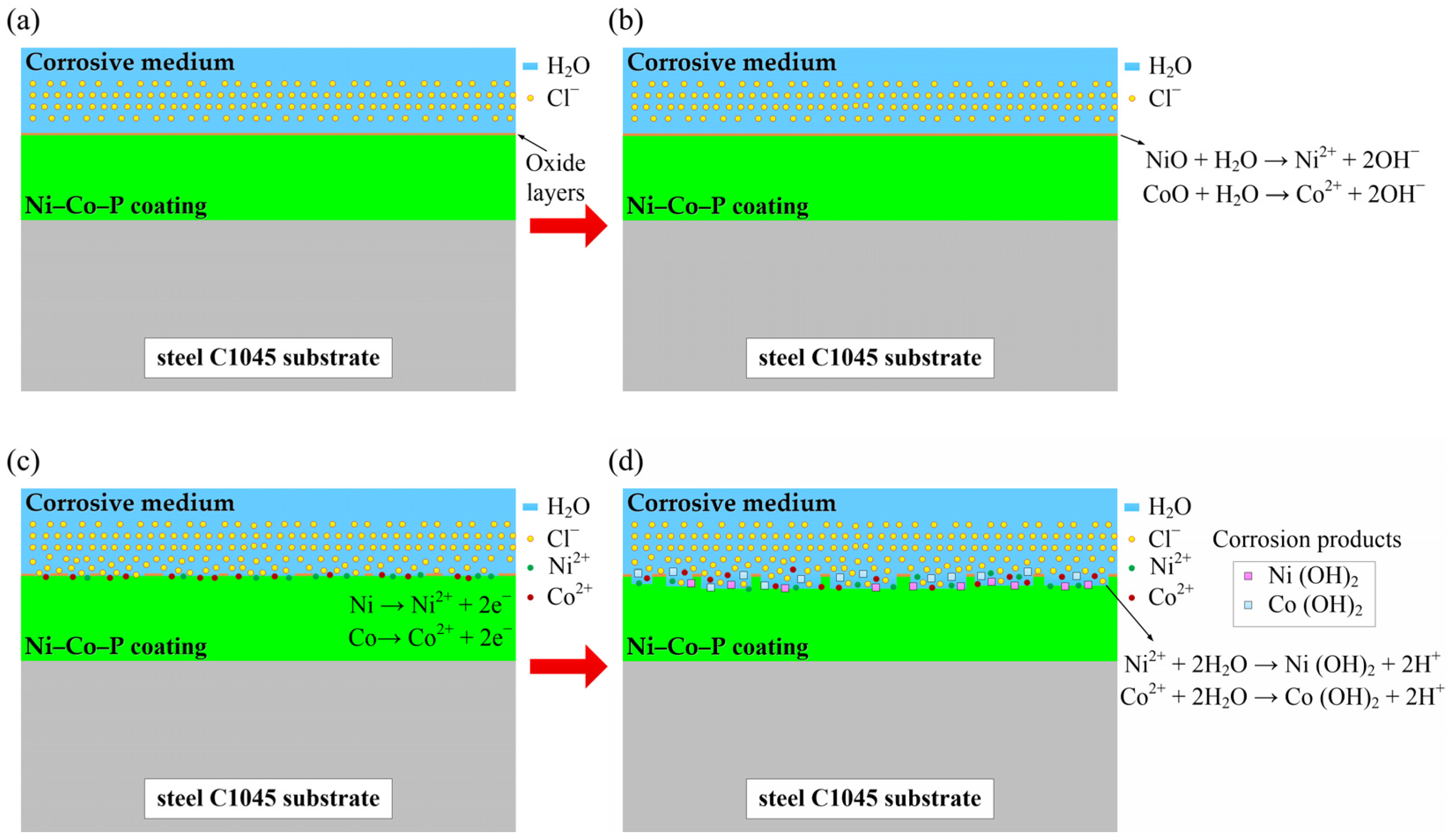
3.7. Seawater Corrosion Resistance of Samples
4. Conclusions
- All the samples fabricated by jet electrodeposition with varying pulse frequencies and duty cycles exhibited an FCC structure, and a distinct preferred orientation in the (111) plane. Under suitable jet electrodeposition process parameters, the content of Co in samples reached 41.09 wt·%. Additionally, the grain size of samples reached 20.6 nm.
- The microhardness of samples first increased, and then decreased, with increases in pulse frequencies and duty cycles, while the wear scar width of samples presented the opposite rule. Additionally, the microhardness of samples reached 656.2 HV0.1 and the wear scar width of samples reached 414.4 µm at 4 kHz pulse frequency and 80% duty cycle.
- Pulse frequencies and duty cycles significantly influenced the seawater corrosion resistance of samples. The corrosion current densities (Icorr) of samples reached 0.74 µA·cm−2, the corrosion rates (Rcorr) reached 8.9 µm·year−1, and the charge transfer resistance (Rct) reached 8.36 × 104 Ω·cm−2 at 4 kHz pulse frequency and 80% duty cycle, which indicated that deposited coatings under these conditions can prolong the longevity of steel C1045 substrates.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Li, B.; Ji, C. Synthesis and characterization of Ni-W/TiN nanocomposite coating with enhanced wear and corrosion resistance deposited by pulse electrodeposition. Ceram. Int. 2019, 45, 14015–14028. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, M.; Mbugua, N.S.; Yao, L.; Jin, M.; Zhu, J. Fabrication of Ni–Co–P alloy coatings using jet electrodeposition with varying reciprocating sweep speed and jet gap to improve wear and seawater corrosion resistance. Coatings 2020, 10, 924. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Luo, P.; Xue, S.; Song, J.; Zhang, Z.; Wang, Y.; He, T.; Fan, Y. Construction of ZrN enhanced Ni-W microcrystalline fflm and exploration of its wear and corrosion resistance. Colloid. Surf. A 2022, 647, 129002. [Google Scholar] [CrossRef]
- Walsh, F.C.; Larson, C. Towards improved electroplating of metal-particle composite coatings. Trans. IMF 2020, 98, 288–299. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Z.; Xu, K.; Lu, J.; Dai, X.; Zhu, H.; Yang, S. Effect of laser single pulse energy on micro-structural, mechanical and corrosion properties of amorphous Ni-Fe-P alloy prepared by laser-assisted electrodeposition. Surf. Interfaces 2021, 22, 100811. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, K.; Zhang, Z.; Dai, X.; Yang, S.; Zhu, H.; Gao, J.; Liu, Y. Effect of laser irradiation on the electrodeposition process, mechanical and corrosion properties of an amorphous Ni–P coating. Surf. Coat. Technol. 2022, 429, 127961. [Google Scholar] [CrossRef]
- Bakonyi, I.; Hansal, W.E.G. Development of pulse-plating technology for the preparation of coatings with varying composition along their thickness: A historical overview. Trans. Inst. Met. Finish 2018, 96, 237–243. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, L.; Yin, Y.; Xu, Z.; Lv, Y.; Liao, Z.; Wei, G.; Zhong, F.; Yuan, M. Electrodeposition and wear behavior of NiCoW ternary alloy coatings reinforced by Al2O3 nanoparticles: Inffuence of current density and electrolyte composition. Surf. Coat. Technol. 2022, 431, 128030. [Google Scholar] [CrossRef]
- Agnieszka, B.; Monika, W.; Marta, J.; Zbigniew, S.; Maciej, J.S.; Rafal, B.; Ewa, B.L. Citrate-based baths for electrodeposition of nanocrystalline nickel coatings with enhanced hardness. J. Alloys Comp. 2021, 850, 156857. [Google Scholar]
- Mohammad, S.; Seyyed, M.; Saleh, H.; Vahid, T.; Nader, S.; Ali, H.; Amirhossein, M.; Saman, H. Wear behavior of alkaline pulsed electrodeposited nickel composite coatings reinforced by ZnO nanoparticles. Wear 2021, 468, 203591. [Google Scholar]
- Tian, S.; Gao, K.; Zhang, H.; Cui, H.; Zhang, G. Corrosion resistance and anti-wear properties: Ni–W–GO nanocomposite coating with lamellar structure. Trans. Indian Inst. Met. 2020, 73, 713–724. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Z.; Wu, H.; Li, L.; Fu, X. Study on preparation of superhydrophobic Ni-Co coating and corrosion resistance by sandblasting–electrodeposition. Coatings 2020, 10, 1164. [Google Scholar] [CrossRef]
- Deo, Y.; Guha, S.; Sarkar, K.; Mohanta, P.; Pradhan, D.; Mondal, A. Electrodeposited Ni-Cu alloy coatings on mild steel for enhanced corrosion properties. Appl. Surf. Sci. 2020, 515, 146078. [Google Scholar] [CrossRef]
- Mohammadpour, Z.; Zare, H.R. The effect of graphene oxide nanosheets (GONSs) and graphene oxide quantum dots (GOQDs) on corrosion resistance enhancement of Ni–Fe nanocomposite coatings. JOM 2020, 72, 4495–4504. [Google Scholar] [CrossRef]
- Meng, X.; Wang, X.; Zhang, D.; Luo, H.; Xu, C.; Liu, S. Electrodeposition of Ni-Mo/TiO2 nanocomposite coatings on low-carbon steels for improving corrosion resistance. Int. J. Electrochem. Sci. 2020, 15, 6198–6206. [Google Scholar] [CrossRef]
- Tozar, A.; Karahan, S.H. Effect of octylphenyl ether group nonionic surfactant on the electrodepositon of the hexagonal boron nitride reinforced Ni-B matrix composite coatings. Surf. Coat. Technol. 2020, 381, 125131. [Google Scholar] [CrossRef]
- Demir, M.; Kanca, E.; Karahan, S.H. Characterization of electrodeposited Ni–Cr/hBN composite coatings. J. Alloys Compd. 2020, 844, 155511. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Hebalkar, N.; Jyothirmayi, A.; Lavakumar, B.; Ramakrishna, M.; Sundararajan, G. Influence of pulse parameters on the mechanical properties and electrochemical corrosion behavior of electrodeposited Ni-W alloy coatings with high tungsten content. Corros. Sci. 2020, 165, 108409. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, Y.; Fan, M.; Lu, X.; Chen, Y.; Zhao, Q. Preparation and investigation of pulse co-deposited duplex nanoparticles reinforced Ni-Mo coatings under different electrodeposition parameters. Ceram. Int. 2022, 48, 29629–29640. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Haridoss, P.; Sundararajan, G. Solid particle Erosion of nanocrystalline nickel coatings: Influence of grain size and adiabatic shear bands. Metall. Mater. Trans. A 2018, 49, 476–489. [Google Scholar] [CrossRef]
- Ramaprakash, M.; Deepika, Y.; Balamurugan, C.; Nagaganapathy, N.; Sekar, R.; Panda Subhendu, K.; Rajasekaran, N. Pulse electrodeposition of nano-crystalline Ni-W alloy and the inffuence of tungsten composition on structure, microhardness and corrosion propertes. J. Alloys Compd. 2021, 866, 158987. [Google Scholar] [CrossRef]
- Chung, C.K.; Chang, W. Effect of pulse frequency and current density on anomalous composition and nanomechanical property of electrodeposited Ni–Co films. Thin Solid Films 2009, 517, 4800–4804. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.F. Fabrication of Ni–Co–SiC composite coatings by pulse electrodeposition—Effects of duty cycle and pulse frequency. Surf. Coat. Technol. 2013, 216, 282–288. [Google Scholar] [CrossRef]
- Xia, F.F.; Jia, W.C.; Ma, C.Y.; Yang, R.; Wang, Y.; Potts, M. Synthesis and characterization of Ni-doped TiN thin films deposited by jet electrodeposition. Appl. Surf. Sci. 2018, 434, 228–233. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, L.; Qiu, M.; Wang, X.; Fan, M.; Tian, Z. Preparation of Ni-SiC composite coatings by magnetic field-enhanced jet electrodeposition. J. Alloys Compd. 2018, 762, 115–124. [Google Scholar] [CrossRef]
- Shen, L.; Wang, Y.; Jiang, W.; Liu, X.; Wang, C.; Tian, Z. Jet electrodeposition multilayer nickel on the surface of sintered NdFeB and corrosion behaviors. Corros. Eng. Sci. Technol. 2017, 52, 311–316. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, M.; Song, H.; Wu, D.; Liu, X.; Tan, J. Effect of ultrasonic power and pulse-on time on the particle content and mechanical property of Co-Cr3C2 composite coatings by jet electrodeposition. Surf. Coat. Technol. 2017, 325, 181–189. [Google Scholar] [CrossRef]
- Li, H.; Kang, M.; Zhang, Y.; Niu, X.; Liu, C.; Jin, M. Influences of jet parameters on structure and wear resistance of Ni-Co-BN (h) nanocomposite coatings. China Surf. Eng. 2018, 2, 103–112. (In Chinese) [Google Scholar]
- Li, H.; Kang, M.; Zhang, Y.; Liu, Y.; Jin, M.; Mbugua, N.S.; Zhu, G.; Liu, C. Fabrication of Ni-Co-BN (h) nanocomposite coatings with jet electrodeposition in different pulse parameters. Coatings 2019, 9, 50. [Google Scholar] [CrossRef]
- Tang, Z.; Ye, X.; Tan, J.; Zhang, Q. Effect of flow of bath on properties of Co-Ni alloy coating prepared by jet electrodeposition. Electroplat. Pollut. Control 2020, 3, 11–14. (In Chinese) [Google Scholar]
- Liu, X.; Shen, L.D.; Qiu, M.B.; Tian, Z.J.; Wang, Y.H.; Zhao, K.L. Jet electrodeposition of nanocrystalline nickel assisted by controllable friction. Surf. Coat. Technol. 2016, 305, 231–240. [Google Scholar] [CrossRef]
- Xia, F.; Jia, W.; Jiang, M.; Cui, W.; Wang, J. Microstructure and corrosion properties of Ni-TiN nanocoatings prepared by jet pulse electrodeposition. Ceram. Int. 2017, 43, 14623–14628. [Google Scholar] [CrossRef]
- Qiao, G.; Jing, T.; Wang, N.; Gao, Y.; Zhao, X.; Zhou, J.; Wang, W. High-speed jet electrodeposition and microstructure of nanocrystalline Ni-Co alloys. Electrochim. Acta 2005, 51, 85–92. [Google Scholar] [CrossRef]
- Pang, J.; Li, Q.; Wang, W.; Xu, X.; Zhai, J. Preparation and characterization of electroless Ni-Co-P ternary alloy on fly ash cenospheres. Surf. Coat. Technol. 2011, 205, 4237–4242. [Google Scholar] [CrossRef]
- Fetohi, A.E.; Hameed, R.M.A.; El-Khatib, K.M.; Souaya, E.R. Ni-P and Ni-Co-P coated aluminum alloy 5251 substrates as metallic bipolar plates for PEM fuel cell applications. Int. J. Hydrogen Energy 2012, 37, 7677–7688. [Google Scholar] [CrossRef]
- Khan, M.R.; Nicholson, E.L. Magnetic properties and microstructure of electroless plated Co-Ni-P and Co-P thin films for longitudinal recording. J. Magn. Magn. Mater. 1986, 54, 1654–1656. [Google Scholar] [CrossRef]
- Behboudi-Khiavi, S.; Javanbakht, M.; Mozaffari, S.A.; Ghaemi, M. Facile pulse elecrodeposition of LixMnO2 nano-structures as high performance cathode materials for lithium ion battery. Electrochim. Acta 2018, 261, 491–502. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, M.; Yao, L.; Mbugua, N.S.; Jin, M.; Zhu, J. Study on the wear and seawater corrosion resistance of Ni-Co-P alloy coatings with jet electrodeposition in different jet voltages and temperatures of plating solution. Coatings 2020, 10, 639. [Google Scholar] [CrossRef]
- Wang, C.; Shen, L.; Qiu, M.; Tian, Z.; Jiang, W. Characterizations of Ni-CeO2 nanocomposite coating by interlaced jet electrodeposition. J. Alloys Compd. 2017, 737, 269–277. [Google Scholar] [CrossRef]
- Yu, Y.D.; Lou, J.W.; Li, W.; Sun, L.X.; Ge, H.L.; Wei, G.Y. Study on preparation of CoPt-W alloy films by electrodeposition. Mater. Sci. Technol. 2013, 28, 448–453. [Google Scholar] [CrossRef]
- Guglielmi, N. Kinetics of the deposition of inert particles from electrolytic baths. J. Electrochem. Soc. 1972, 119, 1009–1012. [Google Scholar] [CrossRef]
- Bahadormanesh, B.; Dolati, A. The kinetics of Ni-Co/SiC composite coatings electrodeposition. J. Alloys Compd. 2010, 504, 514–518. [Google Scholar] [CrossRef]
- Calderon, J.A.; Henao, J.E.; Gomez, M.A. Erosion-corrosion resistance of Ni composite coatings with embedded SiC nanoparticles. Electrochim. Acta 2014, 124, 190–198. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, L.; Xu, M.; Wang, Z.; Tian, Z. Mechanical properties and corrosion resistance of Ni-Co-SiC composite coatings by magnetic field-induced jet electrodeposition. J. Alloys Compd. 2019, 791, 847–855. [Google Scholar] [CrossRef]
- Mei, H.; Ding, J.; Xiao, X.; Luo, Q.; Wang, R.; Zhang, Q.; Gong, W.; Wang, Q. Influence of pulse frequency on microstructure and mechanical properties of Al-Ti-V-Cu-N coatings deposited by HIPIMS. Surf. Coat. Technol. 2021, 405, 126514. [Google Scholar] [CrossRef]
- Karimzadeh, A.; Sabour Rouhaghdam, A.; Aliofkhazraei, M.; Miresmaeili, R. Sliding wear behavior of Ni–Co–P multilayer coatings electrodeposited by pulse reverse method. Tribol. Int. 2020, 141, 105914. [Google Scholar] [CrossRef]
- Shi, X.; Kang, M.; Fu, X.; Feng, H.; Zhang, C.; Liu, Y. Wear resistance of electrodeposited Ni–Mn–SiC composite coatings. Coatings 2021, 11, 72. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and rubbing of flat surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, M.; Fu, X.; Li, H.; Liu, Y. Surface structure and wear resistance of Ni-Co-P-BN(h)-Al2O3 binary nano composite coatings. Mater. Sci. Technol. 2019, 6, 55–66. (In Chinese) [Google Scholar]
- Fan, J.; Liu, Z.; Zhai, H.; Wang, X.; Wang, Y.; Li, Y.; Zhou, X.; Wu, S.; Liu, J. Effect of Co content on the microstructure, spreadability, conductivity and corrosion resistance of Sn-0.7Cu alloy. Microelectron. Reliab. 2020, 107, 113615. [Google Scholar] [CrossRef]
- He, D.; Li, G.; Shen, D.; Guo, C.; Ma, H.; Cai, J. Effect mechanism of ultrasound on growth of micro-arc oxidation coatings on A96061 aluminum alloy. Vacuum 2014, 107, 99–102. [Google Scholar] [CrossRef]
- Kaseem, M.; Zehra, T.; Khan, M.A.; Saffra, A.R.; Cho, H.; Lee, J.; Lee, G.; Yang, H.W.; Park, N. Guar gum-driven high-energy plasma electrolytic oxidation for concurrent improvements in the electrochemical and catalytic properties of Ti-15 Zr alloy. Surf. Interfaces 2022, 34, 102403. [Google Scholar] [CrossRef]
- Kaseem, M.; Choe, H.C. Synchronized Improvements in the Protective and Bioactive Properties of Plasma-Electrolyzed Layers via Cellulose Microcrystalline. ACS Biomater. Sci. Eng. 2023, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, J.; Raj, A.S.; Srinivasan, A.; Ananthakumar, S.; Pillai, U.T.S.; Dhaipule, N.G.K.; Mudali, U.K. Composite magnesium phosphate coatings for improved corrosion resistance of magnesium AZ31 alloy. Corros. Sci. 2016, 113, 104–115. [Google Scholar] [CrossRef]
- Ji, X.J.; Luan, G.F.; Lyu, J.C.; Cui, L.Y.; Li, S.Q.; Zeng, R.C.; Wang, Z.L. Corrosion resistance and tunable release of ciproflfloxacin-loaded multilayers on magnesium alloy: Effects of SiO2 nanoparticles. Appl. Surf. Sci. 2020, 508, 145240. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Li, D. Synthesis and properties of a novel Ni-Co and Ni-Co/ZrO2 composite coating by DC electrodeposition. J. Alloys Compd. 2020, 821, 153258. [Google Scholar] [CrossRef]
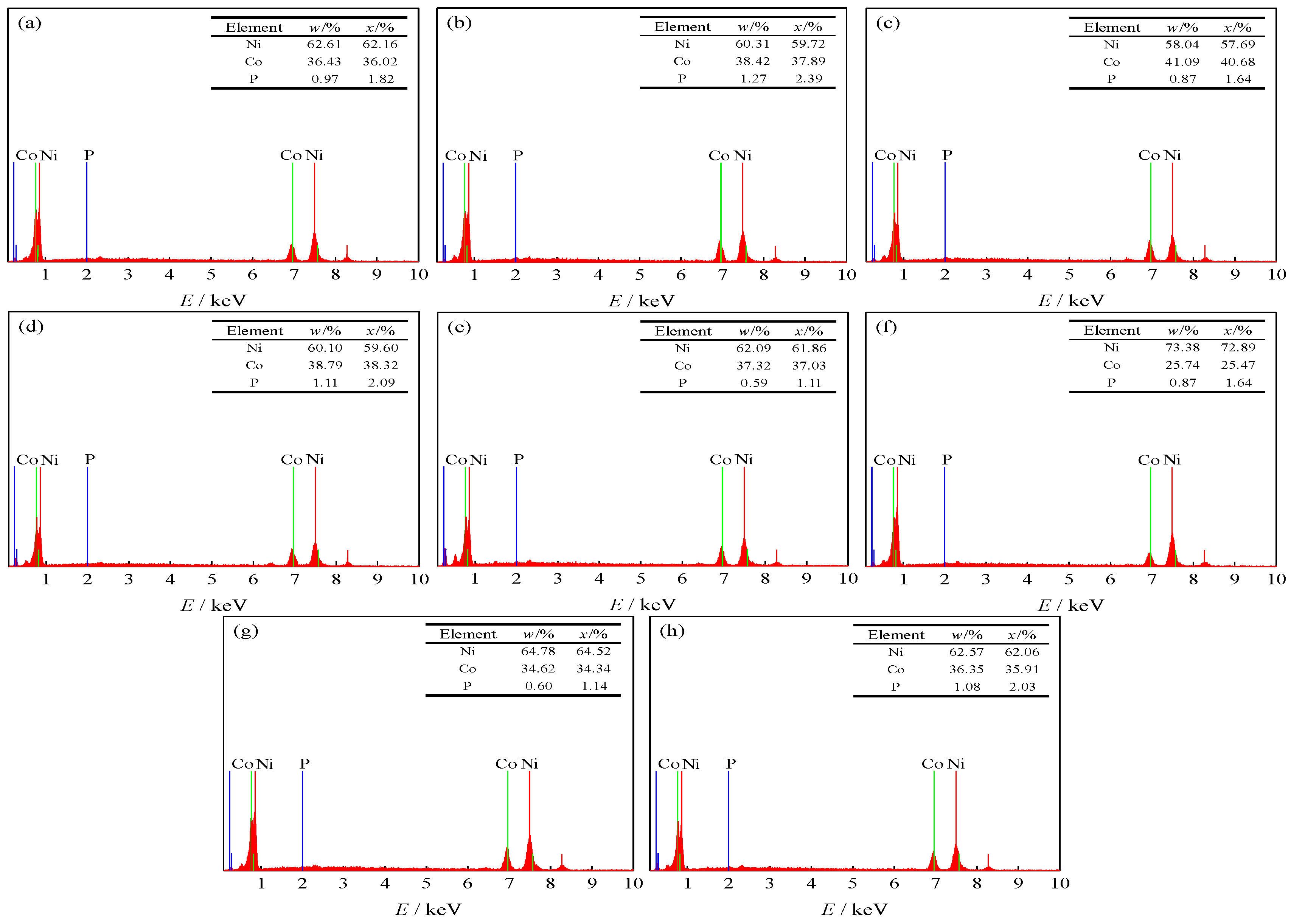
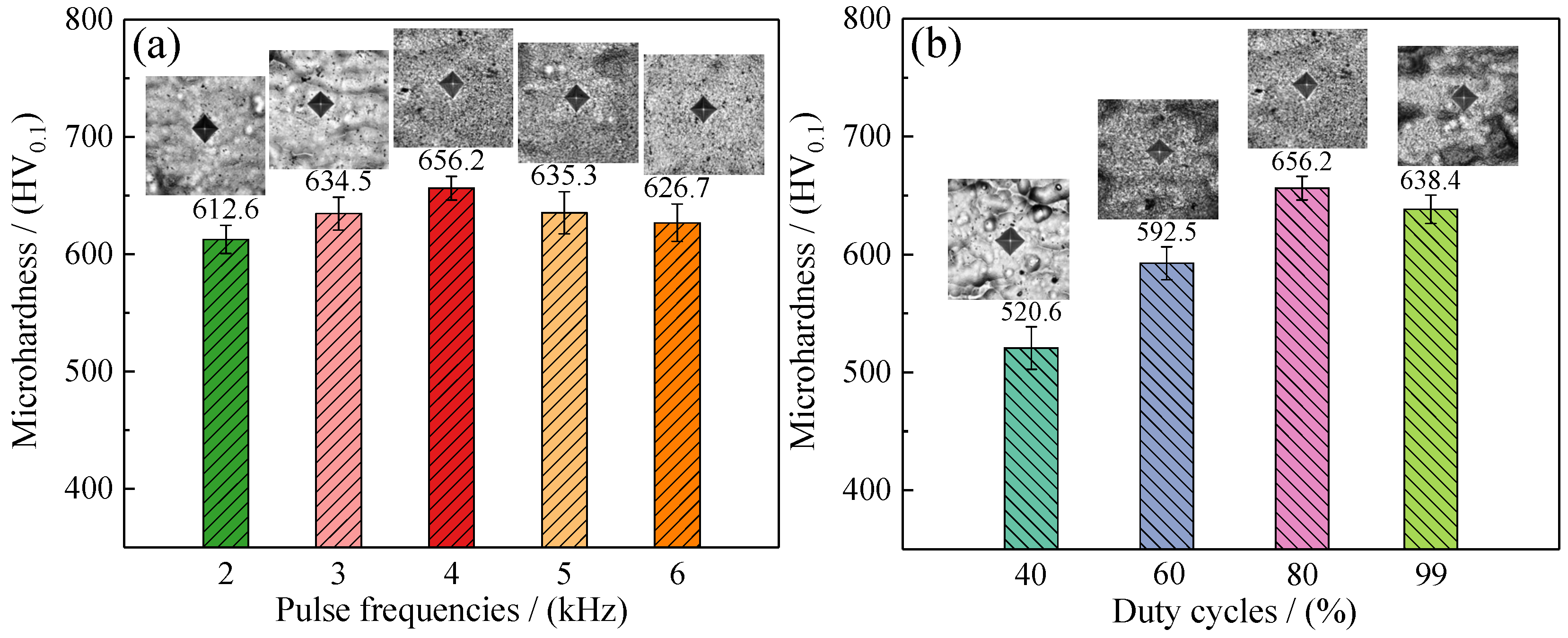

| Pulse Frequencies/(kHz) | Duty Cycles/(%) | Ni/(wt·%) | Co/(wt·%) | Grain Size/(nm) | Microhardness/(HV0.1) |
|---|---|---|---|---|---|
| 2 | 80 | 62.61 | 36.43 | 24.7 | 612.6 |
| 3 | 80 | 60.31 | 38.42 | 22.3 | 634.5 |
| 4 | 80 | 58.04 | 41.09 | 20.6 | 656.2 |
| 5 | 80 | 60.10 | 38.79 | 21.9 | 635.3 |
| 6 | 80 | 62.09 | 37.32 | 23.5 | 626.7 |
| 4 | 40 | 73.38 | 25.74 | 30.7 | 520.6 |
| 4 | 60 | 64.78 | 34.62 | 26.4 | 592.5 |
| 4 | 99 | 62.57 | 36.35 | 22.1 | 638.4 |
| Pulse Frequencies/(kHz) | Duty Cycles/(%) | Ecorr/ (mV) | Icorr/ (µA·cm−2) | βa/ (mV·dec−1) | βc/ (mV·dec−1) | Rcorr/ (µm·year−1) |
|---|---|---|---|---|---|---|
| 2 | 80 | −369 | 2.06 | 494 | −220 | 24.5 |
| 3 | 80 | −273 | 1.65 | 766 | −148 | 19.6 |
| 4 | 80 | −220 | 0.74 | 79 | −251 | 8.9 |
| 5 | 80 | −230 | 0.98 | 121 | −110 | 11.8 |
| 6 | 80 | −346 | 1.12 | 173 | −411 | 13.6 |
| 4 | 40 | −474 | 14.22 | 380 | −629 | 172.3 |
| 4 | 60 | −309 | 5.16 | 403 | −332 | 62.5 |
| 4 | 99 | −245 | 2.59 | 174 | −246 | 31.3 |
| Pulse Frequencies/(kHz) | Duty Cycles/(%) | Rs/ (Ω·cm−2) | CPEdl/ (μF·cm−2) | ndl | Rct/ (104 Ω·cm−2) |
|---|---|---|---|---|---|
| 2 | 80 | 6.98 | 2.78 | 0.93 | 6.14 |
| 3 | 80 | 6.82 | 2.63 | 0.93 | 7.29 |
| 4 | 80 | 7.40 | 2.04 | 0.94 | 8.36 |
| 5 | 80 | 6.09 | 3.18 | 0.90 | 8.32 |
| 6 | 80 | 6.31 | 2.33 | 0.94 | 7.45 |
| 4 | 40 | 3.07 | 7.18 | 0.75 | 0.89 |
| 4 | 60 | 3.88 | 1.17 | 0.94 | 2.55 |
| 4 | 99 | 7.49 | 3.80 | 0.89 | 6.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Mbugua, N.S.; Jin, H.; Chen, L. Preparation and Investigation of Ni–Co–P Alloy Coatings Using Jet Electrodeposition with Varying Pulse Parameters. Crystals 2023, 13, 303. https://doi.org/10.3390/cryst13020303
Zhang Y, Mbugua NS, Jin H, Chen L. Preparation and Investigation of Ni–Co–P Alloy Coatings Using Jet Electrodeposition with Varying Pulse Parameters. Crystals. 2023; 13(2):303. https://doi.org/10.3390/cryst13020303
Chicago/Turabian StyleZhang, Yin, Nyambura Samuel Mbugua, Huawei Jin, and Lingling Chen. 2023. "Preparation and Investigation of Ni–Co–P Alloy Coatings Using Jet Electrodeposition with Varying Pulse Parameters" Crystals 13, no. 2: 303. https://doi.org/10.3390/cryst13020303






