Mn-Ce Oxide Nanoparticles Supported on Nitrogen-Doped Graphene for Low-Temperature Catalytic Reduction of NOx: De-Nitration Characteristics and Kinetics
Abstract
:1. Introduction
2. Experiment Aspect
2.1. Synthesis of Nitrogen Doped Graphene Supporters (NG)
2.2. Synthesis of NG-Supported Manganese and Cerium Oxide Composites
2.3. SCR-Reactor and Reaction Conditions
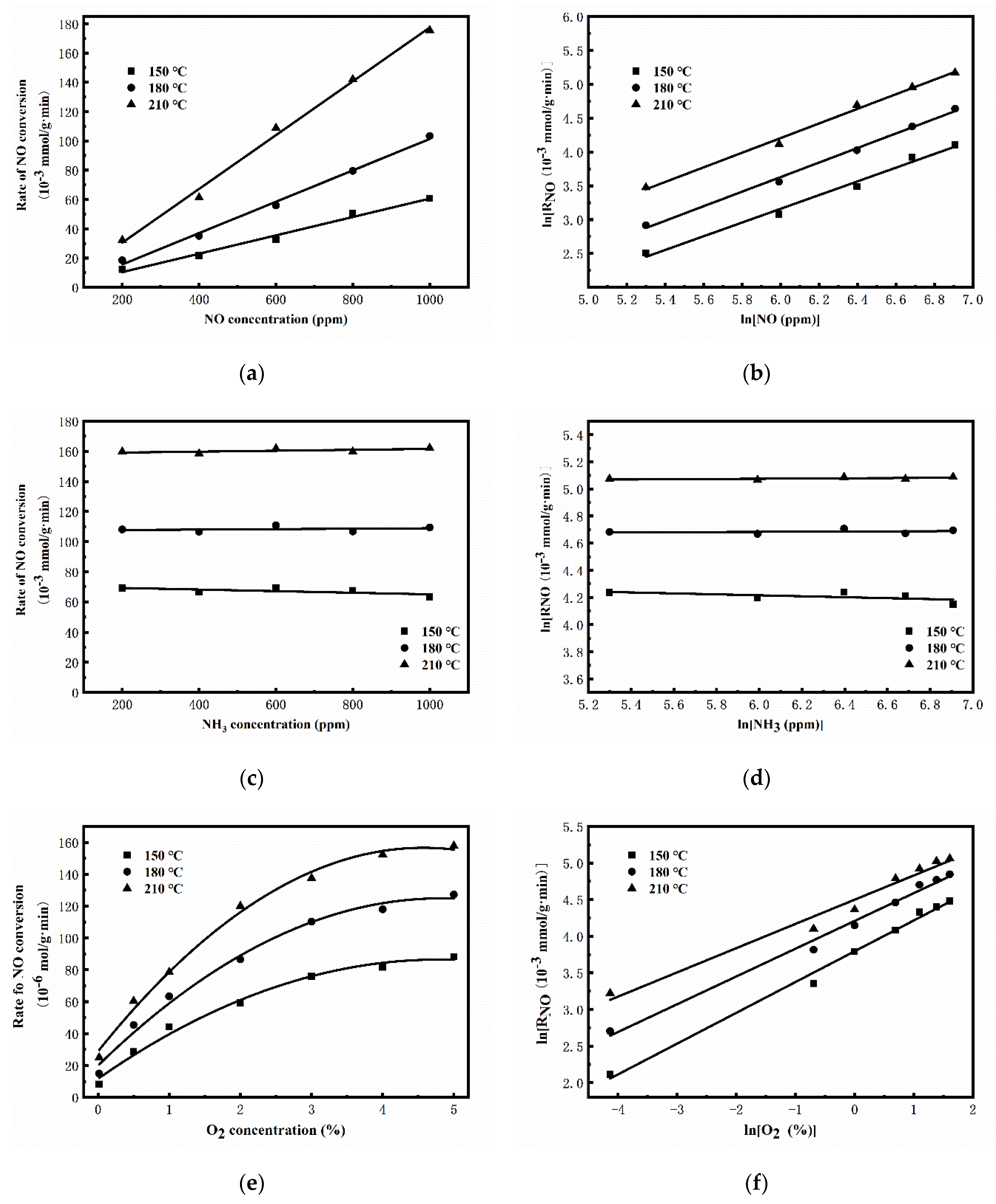
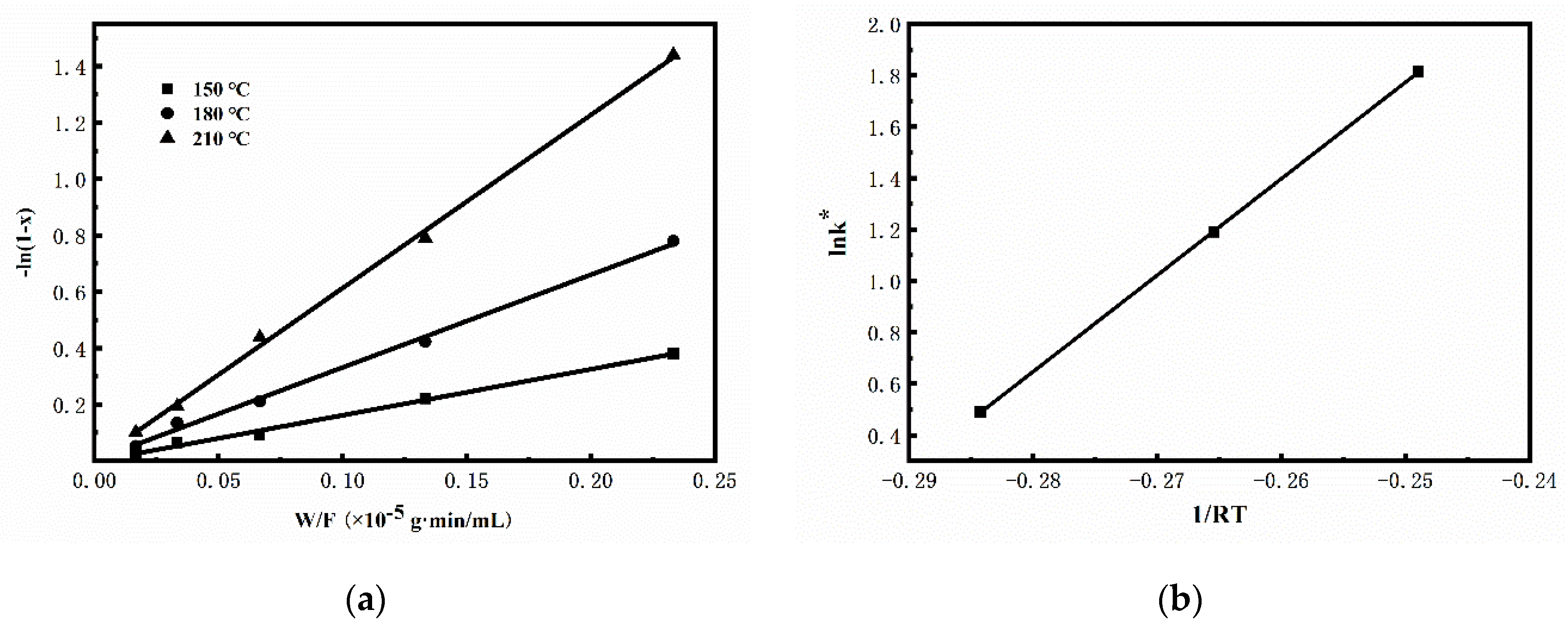
2.3.1. Kinetic Studies
2.3.2. De-Nitration Performance Assessment
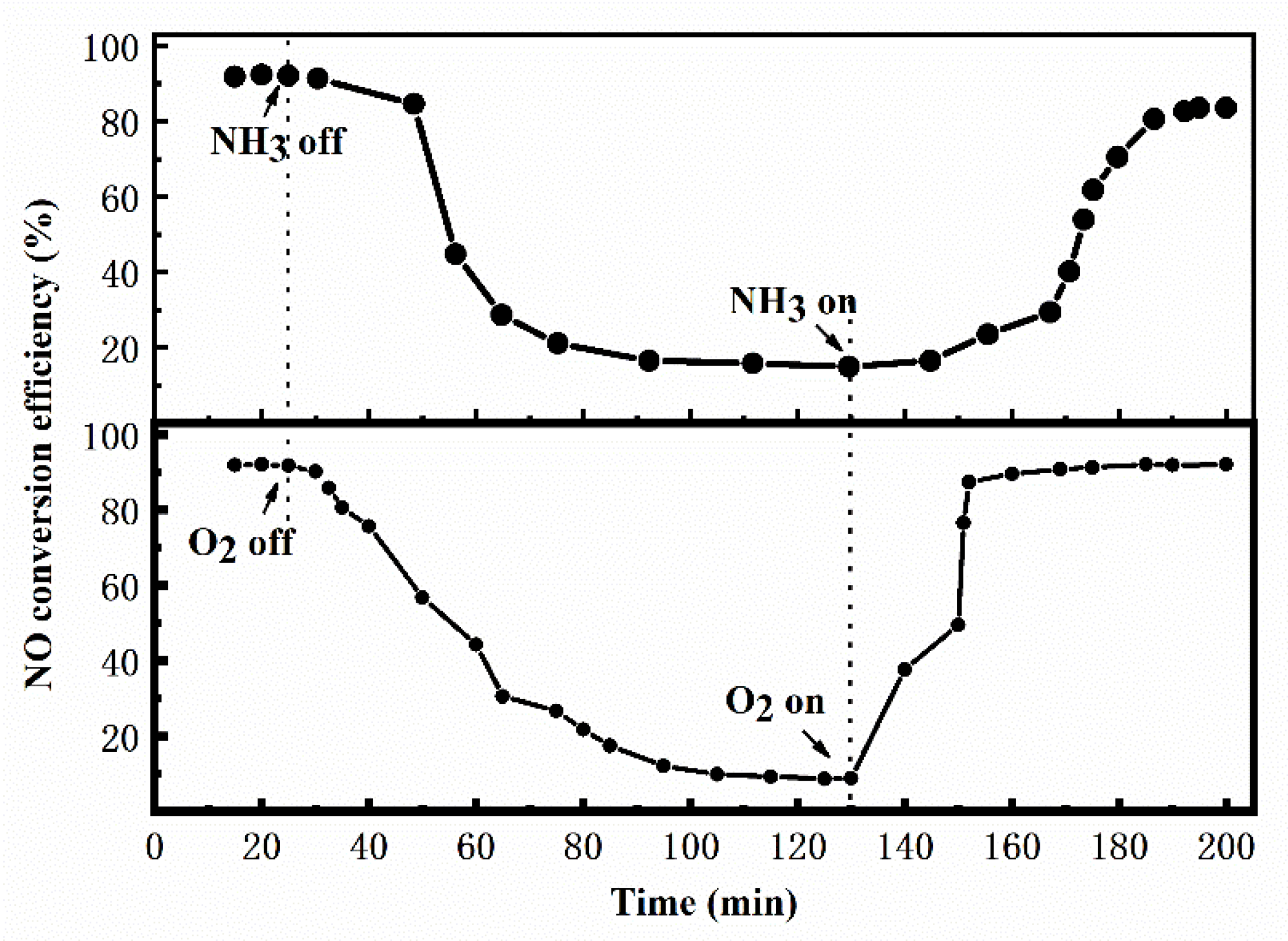
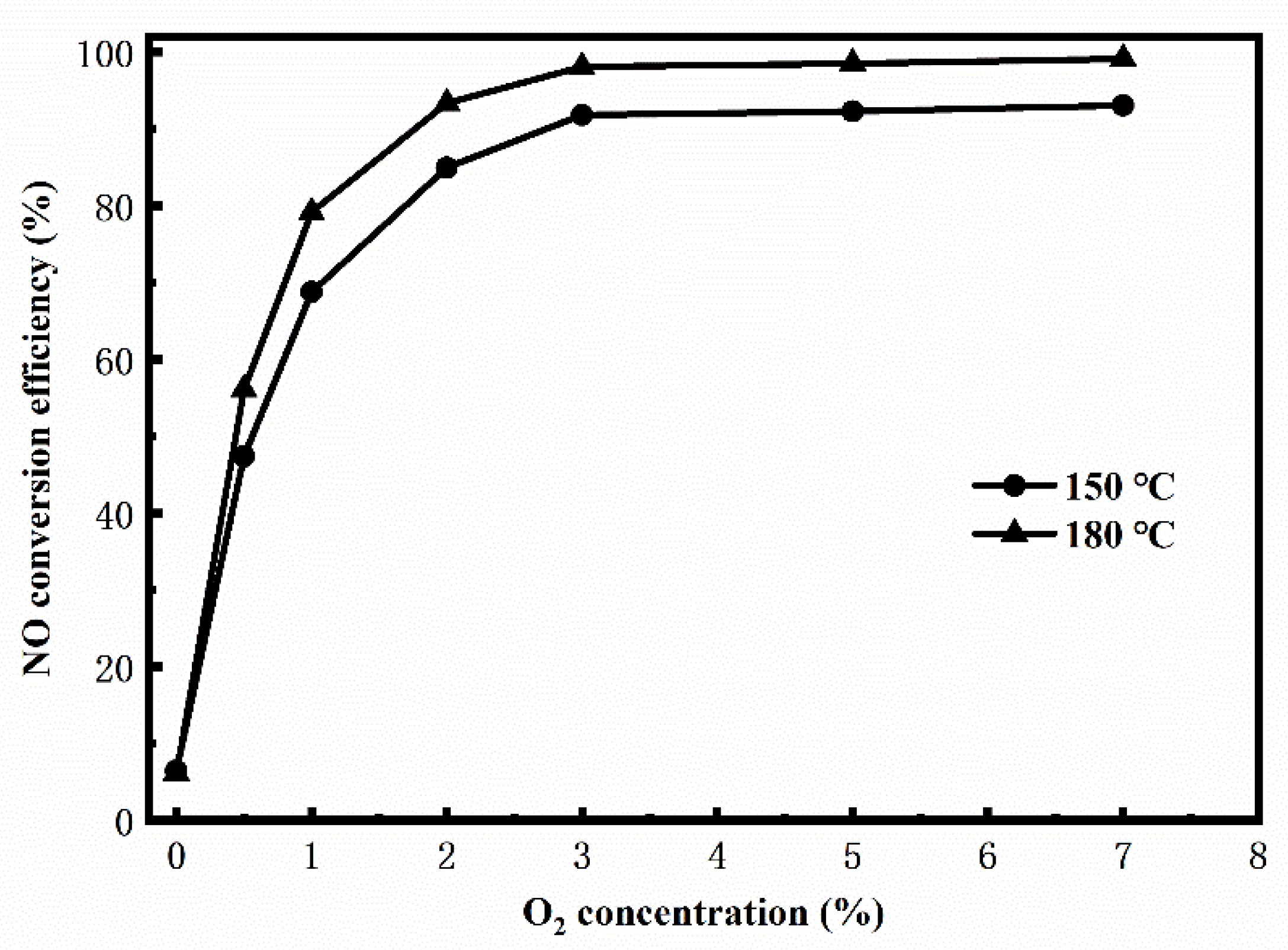
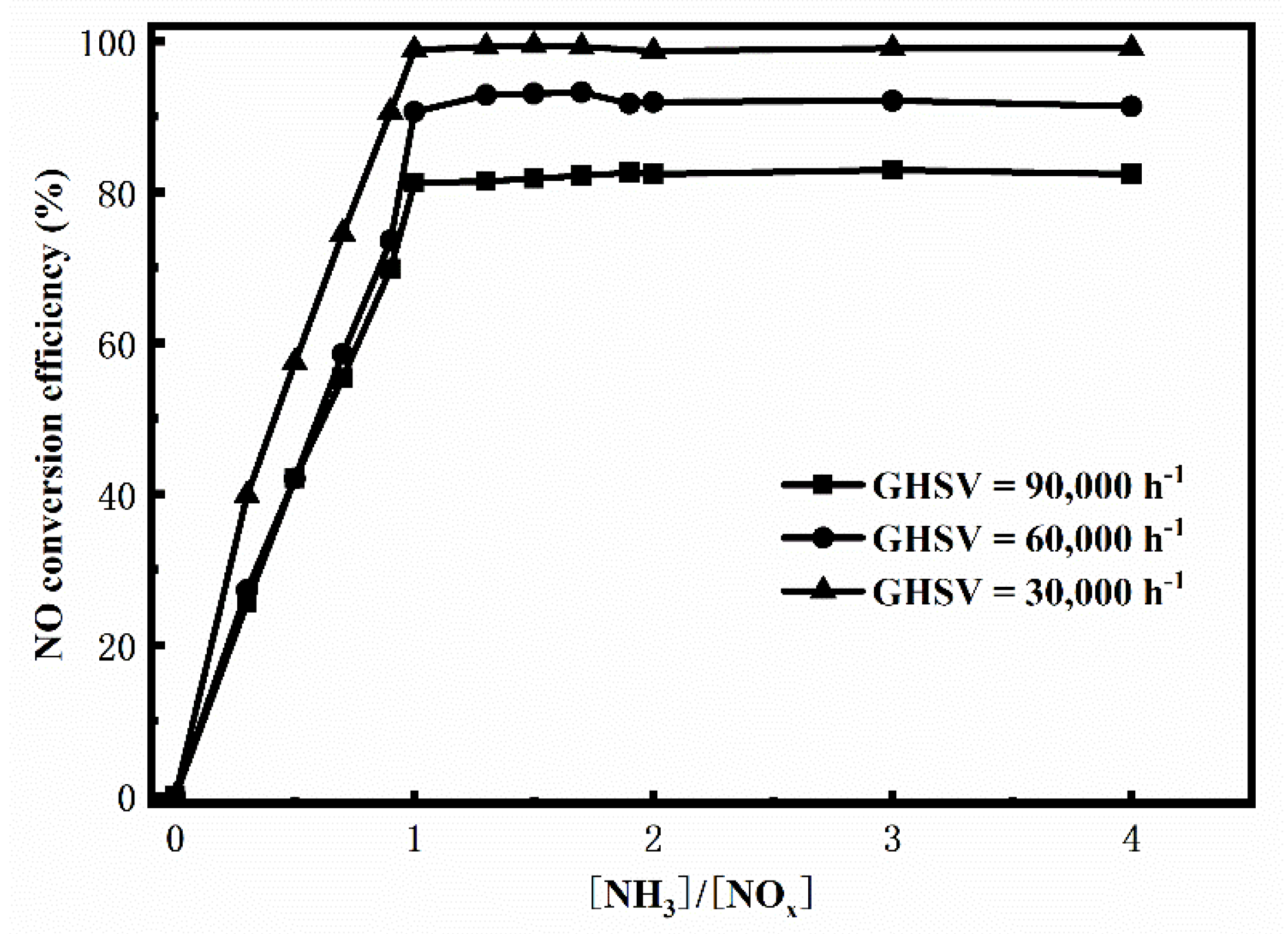
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nova, I.; Lietti, L.; Tronconi, E.; Forzatti, P. Dynamics of SCR reaction over a TiO2-supported vanadia–tungsta commercial catalyst. Catal. Today 2000, 60, 73–82. [Google Scholar] [CrossRef]
- Casanova, M.; Rocchini, E.; Trovarelli, A.; Schermanz, K.; Begsteiger, I. High-temperature stability of V2O5/TiO2-WO3-SiO2 SCR catalysts modified with rare-earths. J. Alloys Compd. 2006, 408, 1108–1112. [Google Scholar] [CrossRef]
- Shang, X.; Hu, G.; He, C.; Zhao, J.; Zhang, F.; Xu, Y.; Zhang, Y.; Li, J.; Chen, J. Regeneration of full-scale commercial honeycomb monolith catalyst (V2O5–WO3/TiO2) used in coal-fired power plant. J. Ind. Eng. Chem. 2012, 18, 513–519. [Google Scholar] [CrossRef]
- Qi, C.; Bao, W.; Wang, L.; Li, H.; Wu, W. Study of the V2O5-WO3/TiO2 Catalyst Synthesized from Waste Catalyst on Selective Catalytic Reduction of NOx by NH3. Catalysts 2017, 7, 110. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.; Zhu, J.; Liu, Y.; Su, F.; Gao, S.; Xu, G. Sulfur poisoning resistant mesoporous Mn-base catalyst for low-temperature SCR of NO with NH3. Appl. Catal. B Environ. 2010, 95, 160–168. [Google Scholar] [CrossRef]
- Maitarad, P.; Zhang, D.; Gao, R.; Shi, L.; Li, H.; Huang, L.; Rungrotmongkol, T.; Zhang, J. Combination of Experimental and Theoretical Investigations of MnOx/Ce0.9Zr0.1O2 Nanorods for Selective Catalytic Reduction of NO with Ammonia. J. Phys. Chem. C 2013, 117, 9999–10006. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, Y.; Wu, Z. Low-temperature selective catalytic reduction of NO on MnOx/TiO2 prepared by different methods. J. Hazard. Mater. 2009, 162, 1249–1254. [Google Scholar] [CrossRef]
- Xie, J.; Fang, D.; He, F.; Chen, J.; Fu, Z.; Chen, X. Performance and mechanism about MnOx species included in MnOx/TiO2 catalysts for SCR at low temperature. Catal. Commun. 2012, 28, 77–81. [Google Scholar] [CrossRef]
- Niu, Y.; Shang, T.; Hui, S.; Zhang, X.; Lei, Y.; Lv, Y.; Wang, S. Synergistic removal of NO and N2O in low-temperature SCR process with MnOx/Ti based catalyst doped with Ce and V. Fuel 2016, 185, 316–322. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T.; Chang, R. MnOx-CeO2 mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2004, 51, 93–106. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wu, Z.; Wang, H.; Gu, T. Relationship between SO2 poisoning effects and reaction temperature for selective catalytic reduction of NO over Mn–Ce/TiO2 catalyst. Catal. Today 2010, 153, 84–89. [Google Scholar] [CrossRef]
- Qiu, L.; Pang, D.; Zhang, C.; Meng, J.; Zhu, R.; Ouyang, F. In situ IR studies of Co and Ce doped Mn/TiO2 catalyst for low-temperature selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2015, 357, 189–196. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R.; Liu, Y.; Wang, H. Ceria modified MnOx/TiO2 as a superior catalyst for NO reduction with NH3 at low-temperature. Catal. Commun. 2008, 9, 2217–2220. [Google Scholar] [CrossRef]
- Boningari, T.; Ettireddy, P.R.; Somogyvari, A.; Liu, Y.; Vorontsov, A.; McDonald, C.A.; Smirniotis, P.G. Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NOx under oxygen-rich conditions. J. Catal. 2015, 325, 145–155. [Google Scholar] [CrossRef]
- Cao, F.; Xiang, J.; Su, S.; Wang, P.; Sun, L.; Hu, S.; Lei, S. The activity and characterization of MnOx–CeO2–ZrO2/γ-Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3. Chem. Eng. J. 2014, 243, 347–354. [Google Scholar] [CrossRef]
- Cao, F.; Xiang, J.; Su, S.; Wang, P.; Hu, S.; Sun, L. Ag modified Mn–Ce/γ-Al2O3 catalyst for selective catalytic reduction of NO with NH3 at low-temperature. Fuel Process. Technol. 2015, 135, 66–72. [Google Scholar] [CrossRef]
- Cao, F.; Su, S.; Xiang, J.; Wang, P.; Hu, S.; Sun, L.; Zhang, A. The activity and mechanism study of Fe–Mn–Ce/γ-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3. Fuel 2015, 139, 232–239. [Google Scholar] [CrossRef]
- Luo, J.Z.; Gao, L.Z.; Leung, Y.L.; Au, C.T. The decomposition of NO on CNTs and 1 wt% Rh/CNTs. Catal. Lett. 2000, 66, 91–97. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Carbon Nanotubes as a Superior Sorbent for Nitrogen Oxides. Ind. Eng. Chem. Res. 2001, 40, 4288–4291. [Google Scholar] [CrossRef]
- Su, Y.; Fan, B.; Wang, L.; Liu, Y.; Huang, B.; Fu, M.; Chen, L.; Ye, D. MnOx supported on carbon nanotubes by different methods for the SCR of NO with NH3. Catal. Today 2013, 201, 115–121. [Google Scholar] [CrossRef]
- You, X.; Sheng, Z.; Yu, D.; Yang, L.; Xiao, X.; Wang, S. Influence of Mn/Ce ratio on the physicochemical properties and catalytic performance of graphene supported MnOx-CeO2 oxides for NH3-SCR at low temperature. Appl. Surf. Sci. 2017, 423, 845–854. [Google Scholar] [CrossRef]
- Yang, L.; You, X.; Sheng, Z.; Ma, D.; Yu, D.; Xiao, X.; Wang, S.J.N.J.o.C. The promoting effect of noble metal (Rh, Ru, Pt, Pd) doping on the performances of MnOx–CeO2/graphene catalysts for the selective catalytic reduction of NO with NH3 at low temperatures. New J. Chem. 2018, 42, 11673–11681. [Google Scholar] [CrossRef]
- Yao, Z.; Guo, Y.; Yang, Y.; Huang, H.; Qu, D.J. MnOx-CeOx nanoparticles supported on graphene aerogel for selective catalytic reduction of nitric oxides. J. Nanomater. 2019, 2019, 4239764. [Google Scholar] [CrossRef]
- Tong, Z.; Lu, X.; Song, C. The CeOx and MnOx nanocrystals supported on TiO2–graphene oxide catalysts and their selective catalytic reduction properties at low temperature. Crystals 2017, 7, 159. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, Y.; Liang, H.; Chen, X.; Tang, J.; Wang, X. N-doped graphene and TiO2 supported manganese and cerium oxides on low-temperature selective catalytic reduction of NOx with NH3. J. Adv. Ceram. 2018, 7, 197–206. [Google Scholar] [CrossRef]
- Li, X.-F.; Lian, K.-Y.; Liu, L.; Wu, Y.; Qiu, Q.; Jiang, J.; Deng, M.; Luo, Y. Unraveling the formation mechanism of graphitic nitrogen-doping in thermally treated graphene with ammonia. Sci. Rep. 2016, 6, 23495. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, K.; Woo, S.I.; Jung, Y. On the mechanism of enhanced oxygen reduction reaction in nitrogen-doped graphene nanoribbons. Phys. Chem. Chem. Phys. 2011, 13, 17505–17510. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Zhang, X.; Han, J.; Wang, Y.; Gu, L.; Zhang, Z.; Wang, X.; Jian, J.; Xu, P.; et al. Direct transformation from graphitic C3N4 to nitrogen-doped graphene: An efficient metal-free electrocatalyst for oxygen reduction reaction. ACS Appl. Mater. Interfaces 2015, 7, 19626–19634. [Google Scholar] [CrossRef] [PubMed]
- Hummers Jr, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Cheekati, S.; Xing, Y.; Zhuang, Y.; Huang, H. Graphene platelets and their manganese composites for lithium-ion batteries. ECS Trans. 2011, 33, 23. [Google Scholar] [CrossRef]
- Huang, H.Y.; Long, R.Q.; Yang, R.T. Kinetics of selective catalytic reduction of NO with NH3 on Fe-ZSM-5 catalyst. Appl. Catal. A Gen. 2002, 235, 241–251. [Google Scholar] [CrossRef]
- Kijlstra, W.S.; Brands, D.S.; Poels, E.K.; Bliek, A. Mechanism of the Selective Catalytic Reduction of NO by NH3 over MnOx/Al2O3. J. Catal. 1997, 171, 208–218. [Google Scholar] [CrossRef]
- Bosch, H.; Janssen, F.J.; van den Kerkhof, F.M.; Oldenziel, J.; van Ommen, J.G.; Ross, J.R. The activity of supported vanadium oxide catalysts for the selective reduction of NO with ammonia. Appl. Catal. 1986, 25, 239–248. [Google Scholar] [CrossRef]
- Høj, M.; Beier, M.J.; Grunwaldt, J.-D.; Dahl, S. The role of monomeric iron during the selective catalytic reduction of NOx by NH3 over Fe-BEA zeolite catalysts. Appl. Catal. B Environ. 2009, 93, 166–176. [Google Scholar] [CrossRef]

| Catalyst Amount (mg) | NO Conversion at Different Temperatures (%) | ||
|---|---|---|---|
| 150 °C | 180 °C | 210 °C | |
| 50 | 2.6 | 5.1 | 9.6 |
| 100 | 6.2 | 12.6 | 17.6 |
| 200 | 8.7 | 19.1 | 35.5 |
| 400 | 19.8 | 34.5 | 54.6 |
| 700 | 31.6 | 54.2 | 76.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.; Yao, Z.; Huang, H.; Liu, F.; Liu, Z.; Wang, X. Mn-Ce Oxide Nanoparticles Supported on Nitrogen-Doped Graphene for Low-Temperature Catalytic Reduction of NOx: De-Nitration Characteristics and Kinetics. Crystals 2023, 13, 313. https://doi.org/10.3390/cryst13020313
Tan S, Yao Z, Huang H, Liu F, Liu Z, Wang X. Mn-Ce Oxide Nanoparticles Supported on Nitrogen-Doped Graphene for Low-Temperature Catalytic Reduction of NOx: De-Nitration Characteristics and Kinetics. Crystals. 2023; 13(2):313. https://doi.org/10.3390/cryst13020313
Chicago/Turabian StyleTan, Shangrong, Zhuo Yao, Hong Huang, Feng Liu, Zechen Liu, and Xuyuan Wang. 2023. "Mn-Ce Oxide Nanoparticles Supported on Nitrogen-Doped Graphene for Low-Temperature Catalytic Reduction of NOx: De-Nitration Characteristics and Kinetics" Crystals 13, no. 2: 313. https://doi.org/10.3390/cryst13020313
APA StyleTan, S., Yao, Z., Huang, H., Liu, F., Liu, Z., & Wang, X. (2023). Mn-Ce Oxide Nanoparticles Supported on Nitrogen-Doped Graphene for Low-Temperature Catalytic Reduction of NOx: De-Nitration Characteristics and Kinetics. Crystals, 13(2), 313. https://doi.org/10.3390/cryst13020313






