Photoalignment and Photofixation of Chromonic Mesophase in Ionic Linear Polysiloxanes Using a Dual Irradiation System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
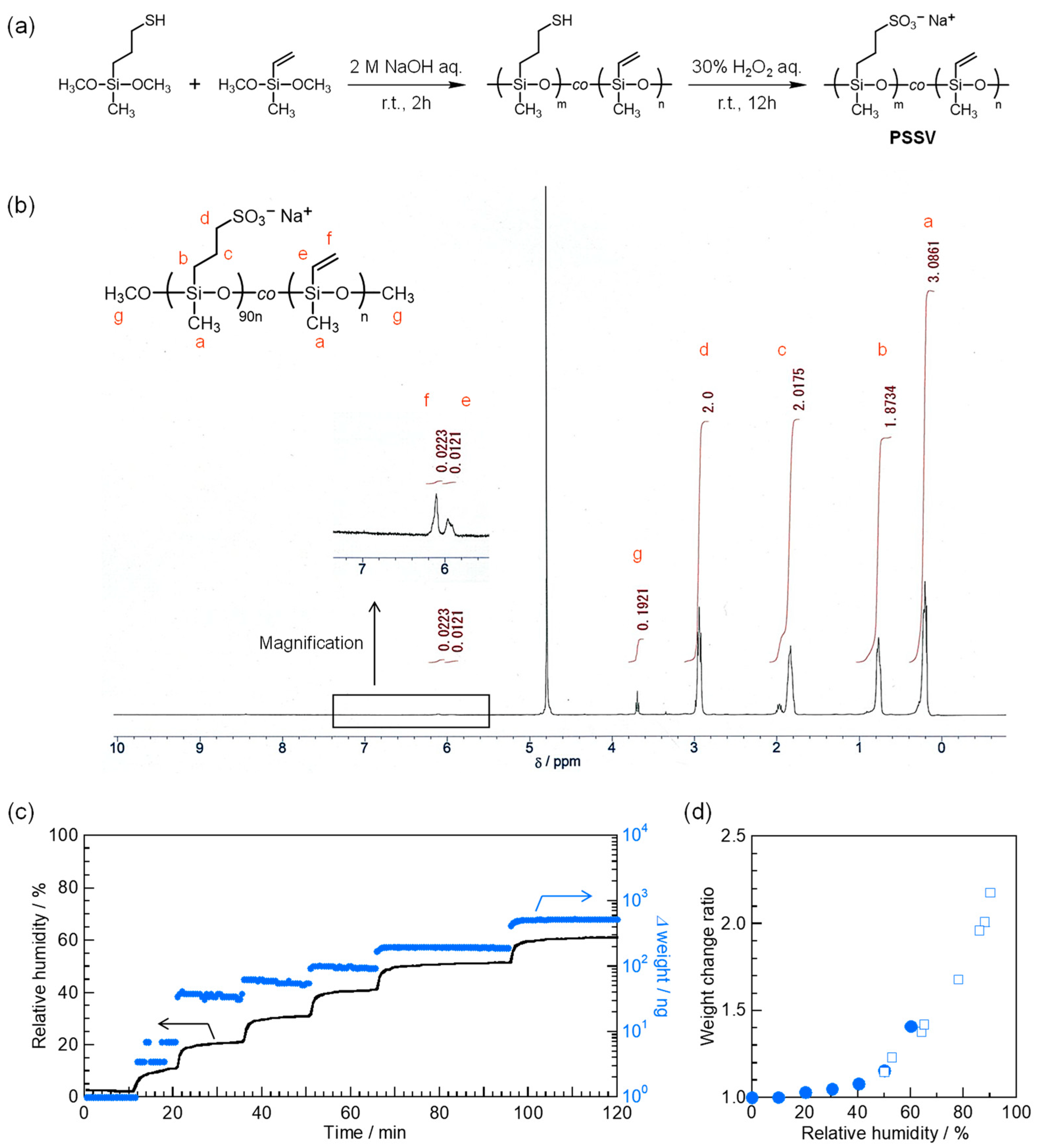
2.2. Synthesis of PSSV
2.3. Preparation of Pure BY Spin-Coated Films
2.4. Preparation of BY-PSSV Spin-Coated Films
2.5. Film Thickness Measurement
2.6. Water Absorption Measurements
2.7. Photoalignment of the BY Columnar Phase
2.8. Evaluation of the Photoaligned BY Columnar Phase by X-ray Scattering Measurements
2.9. UV-Curing of BY Columnar Phase
3. Results and Discussion
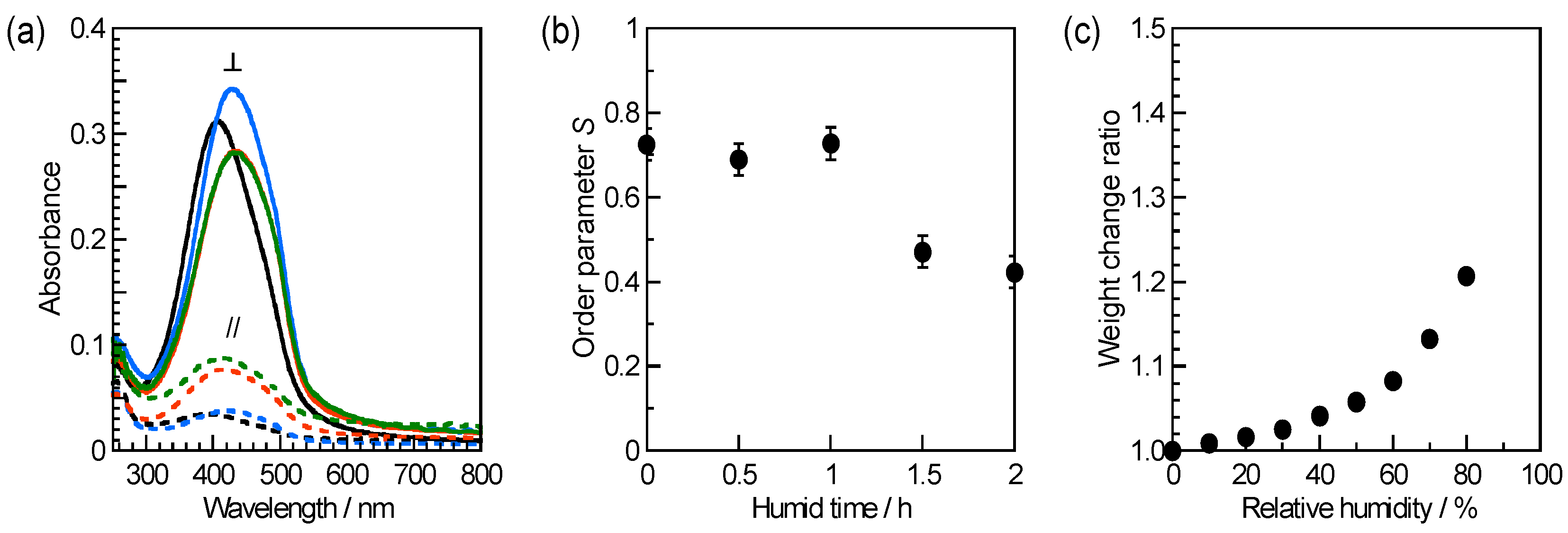
3.1. Photoalignment and Humidity Resistance of Pure BY Film
3.2. Hygroscopicity of Ionic Linear Polysiloxane Containing Sodium Sulfonate Groups
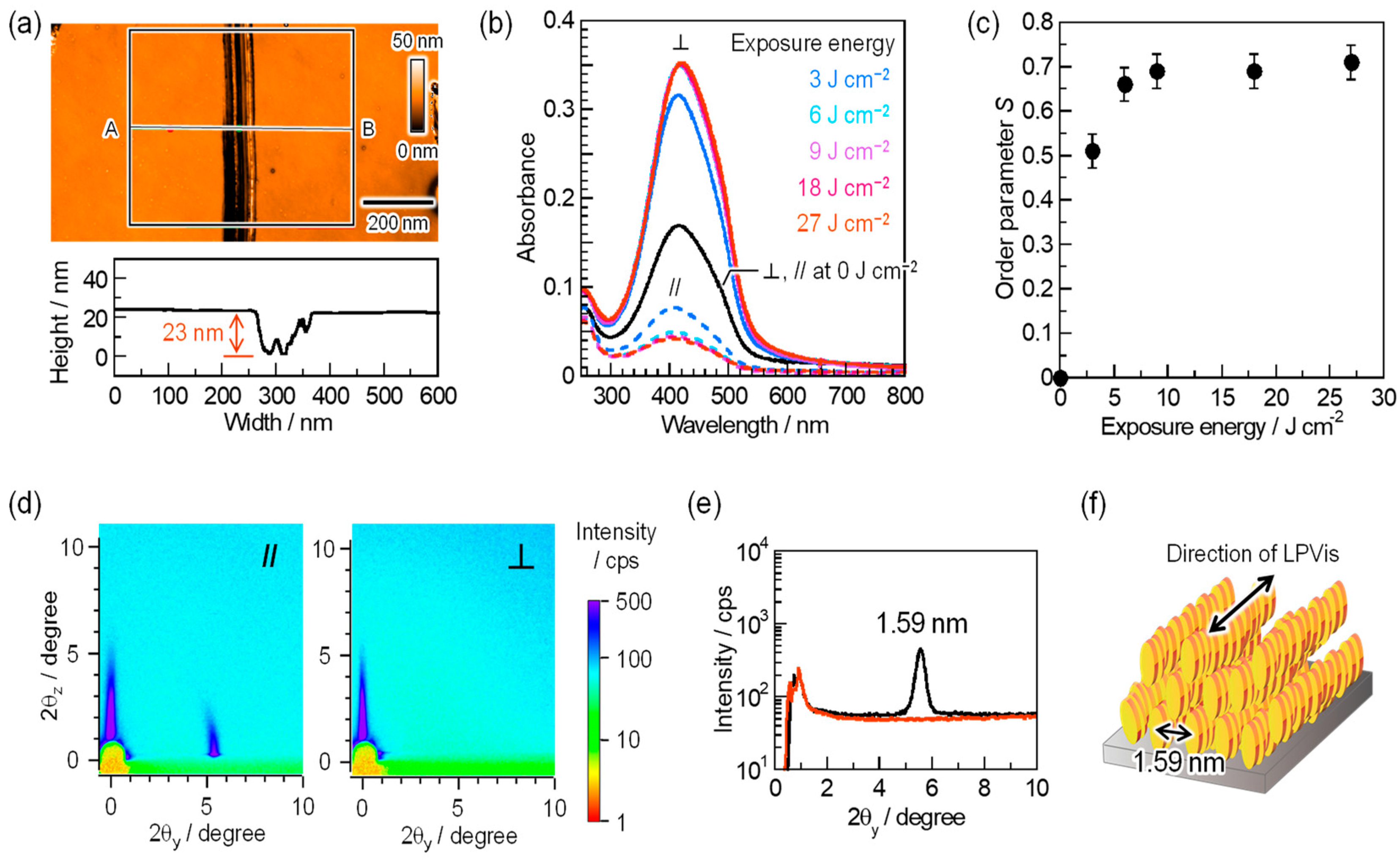
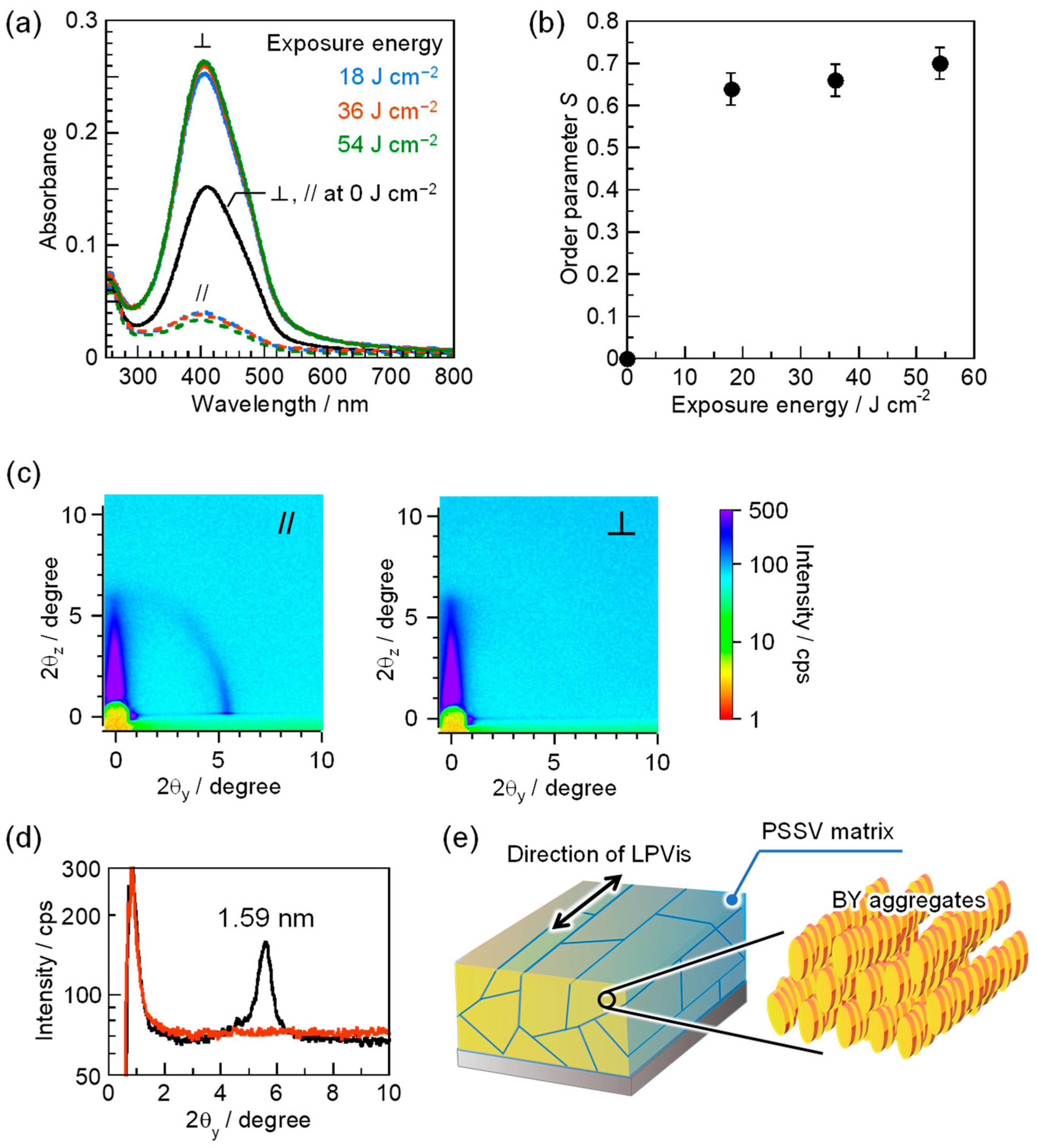
3.3. Photoalignment of the BY Columnar Phase in PSSV Matrix Using LPVis Light
3.4. UV Curing of the BY Columnar Phase in the PSSV Matrix
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional Liquid-Crystalline Assemblies: Self-Organized Soft Materials. Angew. Chem. Int. Ed. 2006, 45, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Bukusoglu, E.; Bedolla Pantoja, M.; Mushenheim, P.C.; Wang, X.; Abbott, N.L. Design of Responsive and Active (Soft) Materials Using Liquid Crystals. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 163–196. [Google Scholar] [CrossRef] [PubMed]
- Kloos, J.; Joosten, N.; Schenning, A.; Nijmeijer, K. Self-assembling liquid crystals as building blocks to design nanoporous membranes suitable for molecular separations. J. Membr. Sci. 2021, 620, 118849. [Google Scholar] [CrossRef]
- Uchida, J.; Soberats, B.; Gupta, M.; Kato, T. Advanced Functional Liquid Crystals. Adv. Mater. 2022, 34, 2109063. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Liquid Crystals: Versatile Self-Organized Smart Soft Materials. Chem. Rev. 2022, 122, 4887–4926. [Google Scholar] [CrossRef]
- Tam-Chang, S.-W.; Huang, L. Chromonic liquid crystals: Properties and applications as functional materials. Chem. Commun. 2008, 17, 1957–1967. [Google Scholar] [CrossRef]
- Lydon, J. Chromonic review. J. Mater. Chem. 2010, 20, 10071–10099. [Google Scholar] [CrossRef]
- Collings, P.J.; Goldstein, J.N.; Hamilton, E.J.; Mercado, B.R.; Nieser, K.J.; Regan, M.H. The nature of the assembly process in chromonic liquid crystals. Liq. Cryst. Rev. 2015, 3, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Dierking, I.; Martins Figueiredo Neto, A. Novel Trends in Lyotropic Liquid Crystals. Crystals 2020, 10, 604. [Google Scholar] [CrossRef]
- Bosire, R.; Ndaya, D.; Kasi, R.M. Recent progress in functional materials from lyotropic chromonic liquid crystals. Polym. Int. 2021, 70, 938–943. [Google Scholar] [CrossRef]
- Ichikawa, T.; Kuwana, M.; Suda, K. Chromonic Ionic Liquid Crystals Forming Nematic and Hexagonal Columnar Phases. Crystals 2022, 12, 1548. [Google Scholar] [CrossRef]
- Schneider, T.; Lavrentovich, O.D. Self-Assembled Monolayers and Multilayered Stacks of Lyotropic Chromonic Liquid Crystalline Dyes with In-Plane Orientational Order. Langmuir 2000, 16, 5227–5230. [Google Scholar] [CrossRef]
- Iverson, I.K.; Casey, S.M.; Seo, W.; Tam-Chang, S.-W.; Pindzola, B.A. Controlling Molecular Orientation in Solid Films via Self-Organization in the Liquid-Crystalline Phase. Langmuir 2002, 18, 3510–3516. [Google Scholar] [CrossRef]
- Hara, M.; Nagano, S.; Mizoshita, N.; Seki, T. Chromonic/Silica Nanohybrids: Synthesis and Macroscopic Alignment. Langmuir 2007, 23, 12350–12355. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-K.; Kim, S.-E.; Kim, D.-Y.; Kang, S.-W.; Shin, S.; Kuo, S.-W.; Hwang, S.-H.; Lee, S.H.; Lee, M.-H.; Jeong, K.-U. Polymer-Stabilized Chromonic Liquid-Crystalline Polarizer. Adv. Funct. Mater. 2011, 21, 2129–2139. [Google Scholar] [CrossRef]
- Ichimura, K. Photoalignment of Liquid-Crystal Systems. Chem. Rev. 2000, 100, 1847–1873. [Google Scholar] [CrossRef]
- Seki, T.; Nagano, S.; Hara, M. Versatility of photoalignment techniques: From nematics to a wide range of functional materials. Polymer 2013, 54, 6053–6072. [Google Scholar] [CrossRef] [Green Version]
- Priimagi, A.; Barrett, C.J.; Shishido, A. Recent twists in photoactuation and photoalignment control. J. Mater. Chem. C 2014, 2, 7155–7162. [Google Scholar] [CrossRef] [Green Version]
- Ichimura, K.; Fujiwara, T.; Momose, M.; Matsunaga, D. Surface-assisted photoalignment control of lyotropic liquid crystals. Part 1. Characterization and photoalignment of aqueous solutions of a water-soluble dye as lyotropic liquid crystals. J. Mater. Chem. 2002, 12, 3380–3386. [Google Scholar] [CrossRef]
- Fujiwara, T.; Ichimura, K. Surface-assisted photoalignment control of lyotropic liquid crystals. Part 2. Photopatterning of aqueous solutions of a water-soluble anti-asthmatic drug as lyotropic liquid crystals. J. Mater. Chem. 2002, 12, 3387–3391. [Google Scholar] [CrossRef]
- Hara, M.; Nagano, S.; Kawatsuki, N.; Seki, T. Photoalignment and patterning of a chromonic-silica nanohybrid on photocrosslinkable polymer thin films. J. Mater. Chem. 2008, 18, 3259–3263. [Google Scholar] [CrossRef]
- Van der Asdonk, P.; Hendrikse, H.C.; Fernandez-Castano Romera, M.; Voerman, D.; Ramakers, B.E.I.; Löwik, D.W.P.M.; Sijbesma, R.P.; Kouwer, P.H.J. Patterning of Soft Matter across Multiple Length Scales. Adv. Funct. Mater. 2016, 26, 2609–2616. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, M.; Peng, C.; Sun, K.; Yaroshchuk, O.; Lavrentovich, O.D.; Wei, Q.-H. Designs of Plasmonic Metamasks for Photopatterning Molecular Orientations in Liquid Crystals. Crystals 2017, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Wani, O.M.; Zeng, H.; Wasylczyk, P.; Priimagi, A. Programming Photoresponse in Liquid Crystal Polymer Actuators with Laser Projector. Adv. Opt. Mater. 2018, 6, 1700949. [Google Scholar] [CrossRef]
- Wang, J.; McGinty, C.; Reich, R.; Finnemeyer, V.; Clark, H.; Berry, S.; Bos, P. Process for a Reactive Monomer Alignment Layer for Liquid Crystals Formed on an Azodye Sublayer. Materials 2018, 11, 1195. [Google Scholar] [CrossRef] [Green Version]
- Berteloot, B.; Nys, I.; Poy, G.; Beeckman, J.; Neyts, K. Ring-shaped liquid crystal structures through patterned planar photo-alignment. Soft Matter 2020, 16, 4999–5008. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Xiong, J.; He, Z.; Wu, S.-T. Patterning Liquid-Crystal Alignment for Ultrathin Flat Optics. ACS Omega 2020, 5, 31485–31489. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.-U.; Choi, S.; Heo, K.; Ahn, S.-K.; Na, J.-H. High-Definition Optophysical Image Construction Using Mosaics of Pixelated Wrinkles. Adv. Sci. 2020, 7, 2002134. [Google Scholar] [CrossRef]
- Nys, I.; Berteloot, B.; Poy, G. Surface Stabilized Topological Solitons in Nematic Liquid Crystals. Crystals 2020, 10, 840. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, S.-T. Planar liquid crystal polarization optics for augmented reality and virtual reality: From fundamentals to applications. eLight 2021, 1, 3. [Google Scholar] [CrossRef]
- Kim, H.; Abdelrahman, M.K.; Choi, J.; Kim, H.; Maeng, J.; Wang, S.; Javed, M.; Rivera-Tarazona, L.K.; Lee, H.; Ko, S.H.; et al. From Chaos to Control: Programmable Crack Patterning with Molecular Order in Polymer Substrates. Adv. Mater. 2021, 33, 2008434. [Google Scholar] [CrossRef] [PubMed]
- Padmini, H.N.; Rajabi, M.; Shiyanovskii, S.V.; Lavrentovich, O.D. Azimuthal Anchoring Strength in Photopatterned Alignment of a Nematic. Crystals 2021, 11, 675. [Google Scholar] [CrossRef]
- Folwill, Y.; Zeitouny, Z.; Lall, J.; Zappe, H. A practical guide to versatile photoalignment of azobenzenes. Liq. Cryst. 2021, 48, 862–872. [Google Scholar] [CrossRef]
- McGinty, C.P.; Kołacz, J.; Spillmann, C.M. Large rewritable liquid crystal pretilt angle by in situ photoalignment of brilliant yellow films. Appl. Phys. Lett. 2021, 119, 141111. [Google Scholar] [CrossRef]
- Liu, S.; Nys, I.; Neyts, K. Two-Step Photoalignment with High Resolution for the Alignment of Blue Phase Liquid Crystal. Adv. Opt. Mater. 2022, 10, 2200711. [Google Scholar] [CrossRef]
- Pinchin, N.P.; Lin, C.-H.; Kinane, C.A.; Yamada, N.; Pena-Francesch, A.; Shahsavan, H. Plasticized liquid crystal networks and chemical motors for the active control of power transmission in mechanical devices. Soft Matter 2022, 18, 8063–8070. [Google Scholar] [CrossRef] [PubMed]
- Lall, J.; Zappe, H. In situ, spatially variable photoalignment of liquid crystals inside a glass cell using brilliant yellow. In Proceedings of the Photosensitive Materials and their Applications II, Strasbourg, France, 3 April–23 May 2022; Volume 12151. [Google Scholar]
- Li, Y.; Luo, Z.; Wu, S.-T. High-Precision Beam Angle Expander Based on Polymeric Liquid Crystal Polarization Lenses for LiDAR Applications. Crystals 2022, 12, 349. [Google Scholar] [CrossRef]
- Matsumori, M.; Takahashi, A.; Tomioka, Y.; Hikima, T.; Takata, M.; Kajitani, T.; Fukushima, T. Photoalignment of an Azobenzene-Based Chromonic Liquid Crystal Dispersed in Triacetyl Cellulose: Single-Layer Alignment Films with an Exceptionally High Order Parameter. ACS Appl. Mater. Interfaces 2015, 7, 11074–11078. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; McGinty, C.; West, J.; Bryant, D.; Finnemeyer, V.; Reich, R.; Berry, S.; Clark, H.; Yaroshchuk, O.; Bos, P. Effect of humidity and surface on photoalignment of brilliant yellow. Liq. Cryst. 2016, 44, 863–872. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, C.; Ho, J.Y.-L.; Vashchenko, V.V.; Srivastava, A.K.; Chigrinov, V.G.; Kwok, H.-S.; Song, F.; Luo, D. Exotic Property of Azobenzensulfonic Photoalignment Material Based on Relative Humidity. Langmuir 2017, 33, 3968–3974. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, C.; Ho, J.Y.-L.; Song, F.; Chigrinov, V.G.; Luo, D.; Kwok, H.-S.; Sun, X.W. High Photoinduced Ordering and Controllable Photostability of Hydrophilic Azobenzene Material Based on Relative Humidity. Langmuir 2018, 34, 4465–4472. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhao, D. On the Controllable Soft-Templating Approach to Mesoporous Silicates. Chem. Rev. 2007, 107, 2821–2860. [Google Scholar] [CrossRef] [PubMed]
- Hara, M. Mesostructure and orientation control of lyotropic liquid crystals in a polysiloxane matrix. Polym. J. 2019, 51, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Huo, Q.; Margolese, D.I.; Ciesla, U.; Feng, P.; Gier, T.E.; Sieger, P.; Leon, R.; Petroff, P.M.; Schüth, F.; Stucky, G.D. Generalized synthesis of periodic surfactant/inorganic composite materials. Nature 1994, 368, 317–321. [Google Scholar] [CrossRef]
- Hara, M.; Orito, T.; Nagano, S.; Seki, T. Humidity-responsive phase transition and on-demand UV-curing in a hygroscopic polysiloxane-surfactant nanohybrid film. Chem. Commun. 2018, 54, 1457–1460. [Google Scholar] [CrossRef]
- Hara, M.; Wakitani, N.; Kodama, A.; Nagano, S.; Seki, T. Hierarchical Photocomposition of Heteronanostructures in a Surfactant-Polysiloxane Hybrid Film toward Next-Generation Nanolithography. ACS Appl. Polym. Mater. 2020, 2, 2284–2290. [Google Scholar] [CrossRef]
- Kaneko, Y. Ionic silsesquioxanes: Preparation, structure control, characterization, and applixations. Polymer 2018, 144, 205–224. [Google Scholar] [CrossRef]
- Kaneko, Y.; Toyodome, H.; Mizumo, T.; Shikinaka, K.; Iyi, N. Preparation of a Sulfo-Group-Containing Rod-Like Polysilsesquioxane with a Hexagonally Stacked Structure and Its Proton Conductivity. Chem. Eur. J. 2014, 20, 9394–9399. [Google Scholar] [CrossRef]
- Hara, M.; Ueno, Y.; Nagano, S.; Seki, T. Water-Retentive/Lipophilic Amphiphilic Surface Properties Attained by Hygroscopic Polysiloxane Ultrathin Films. J. Fiber Sci. Technol. 2022, 78, 169–177. [Google Scholar] [CrossRef]
- Hara, M.; Iijima, Y.; Nagano, S.; Seki, T. Simple linear ionic polysiloxane showing unexpected nanostructure and mechanical properties. Sci. Rep. 2021, 11, 17683. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, M.; Masuda, A.; Nagano, S.; Seki, T. Photoalignment and Photofixation of Chromonic Mesophase in Ionic Linear Polysiloxanes Using a Dual Irradiation System. Crystals 2023, 13, 326. https://doi.org/10.3390/cryst13020326
Hara M, Masuda A, Nagano S, Seki T. Photoalignment and Photofixation of Chromonic Mesophase in Ionic Linear Polysiloxanes Using a Dual Irradiation System. Crystals. 2023; 13(2):326. https://doi.org/10.3390/cryst13020326
Chicago/Turabian StyleHara, Mitsuo, Ayaka Masuda, Shusaku Nagano, and Takahiro Seki. 2023. "Photoalignment and Photofixation of Chromonic Mesophase in Ionic Linear Polysiloxanes Using a Dual Irradiation System" Crystals 13, no. 2: 326. https://doi.org/10.3390/cryst13020326




