Nanomechanical and Electrochemical Corrosion Testing of Nanocomposite Coating Obtained on AZ31 via Plasma Electrolytic Oxidation Containing TiN and SiC Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Electrolytic Oxidation
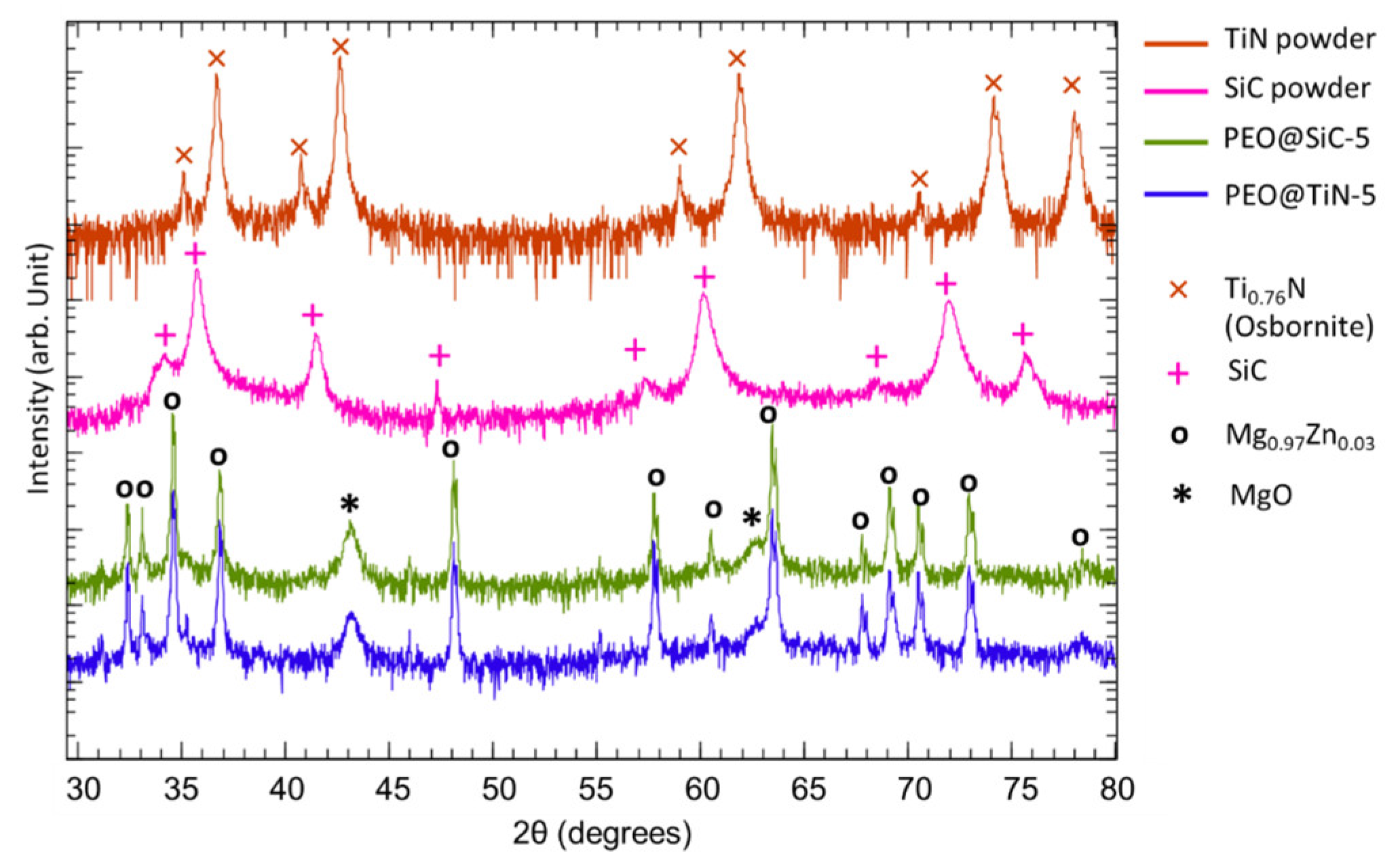
2.3. Chemical, Phase, and Morphological Characterization
2.4. Electrochemical Corrosion Testing
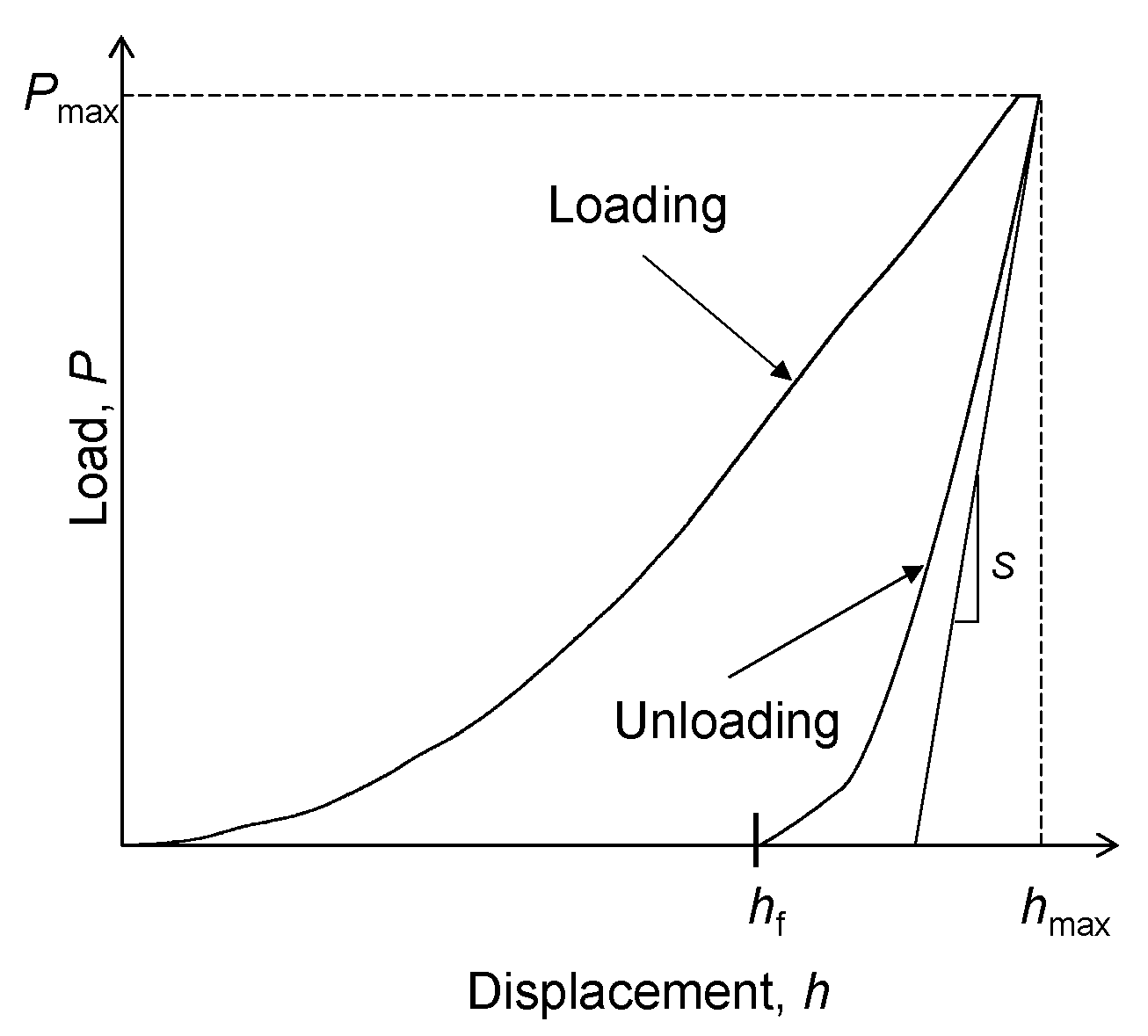
2.5. Mechanical Characterization
3. Results and Discussion
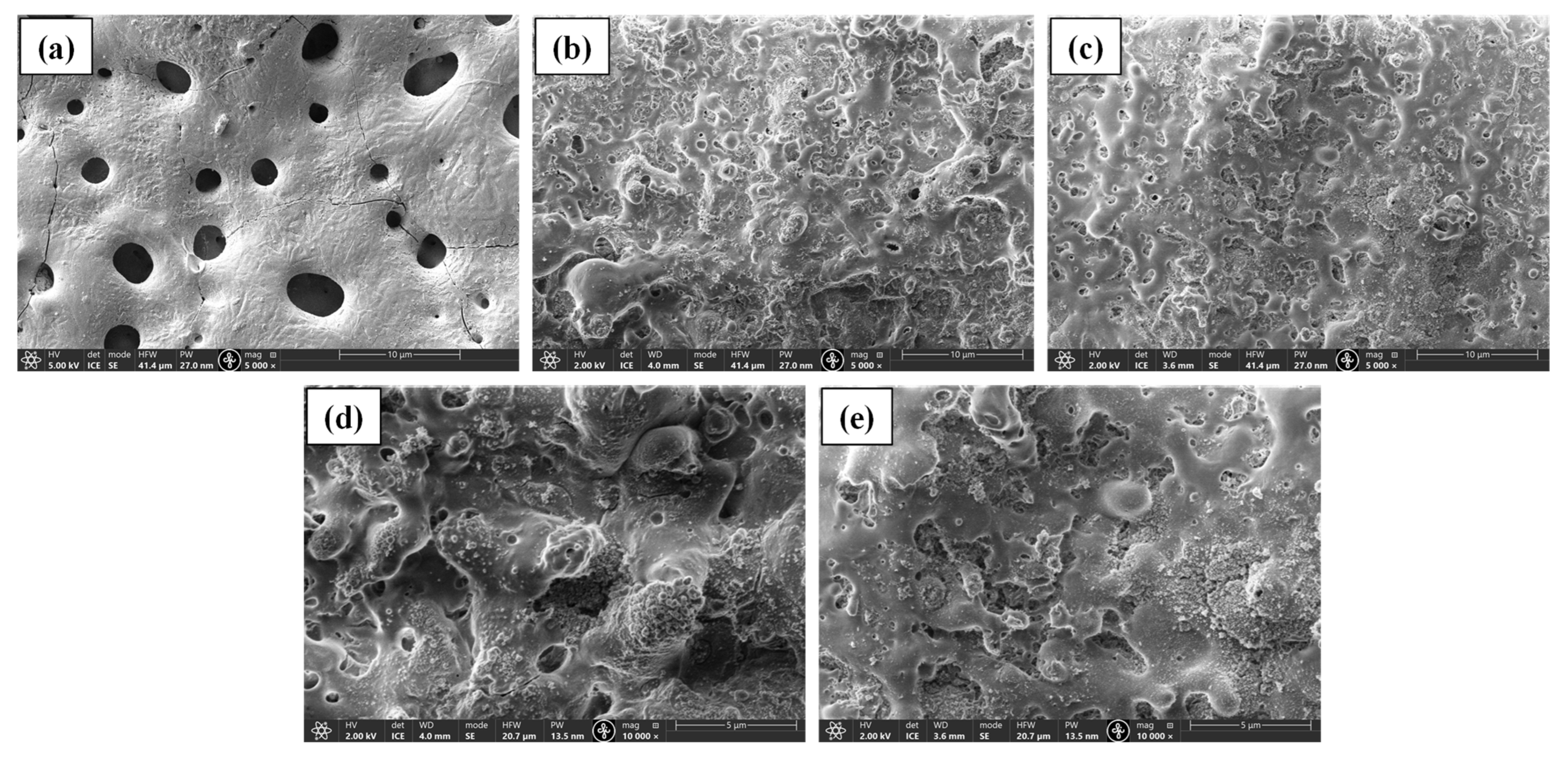
3.1. Coating Morphology
3.2. Coating Properties
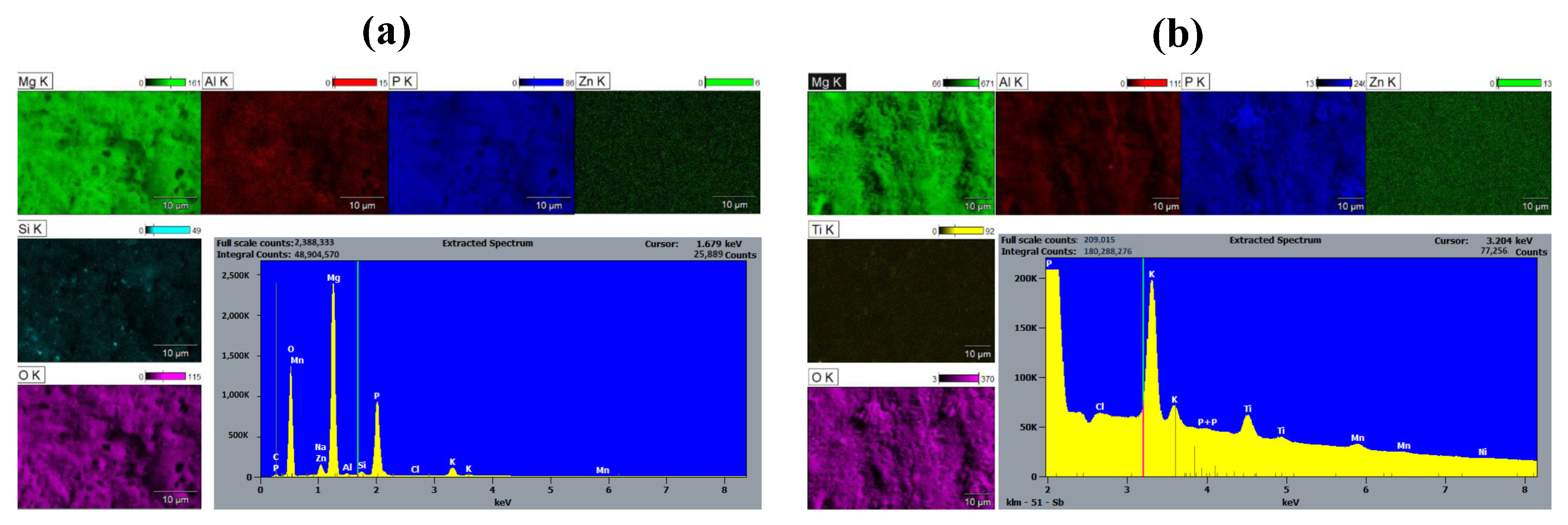
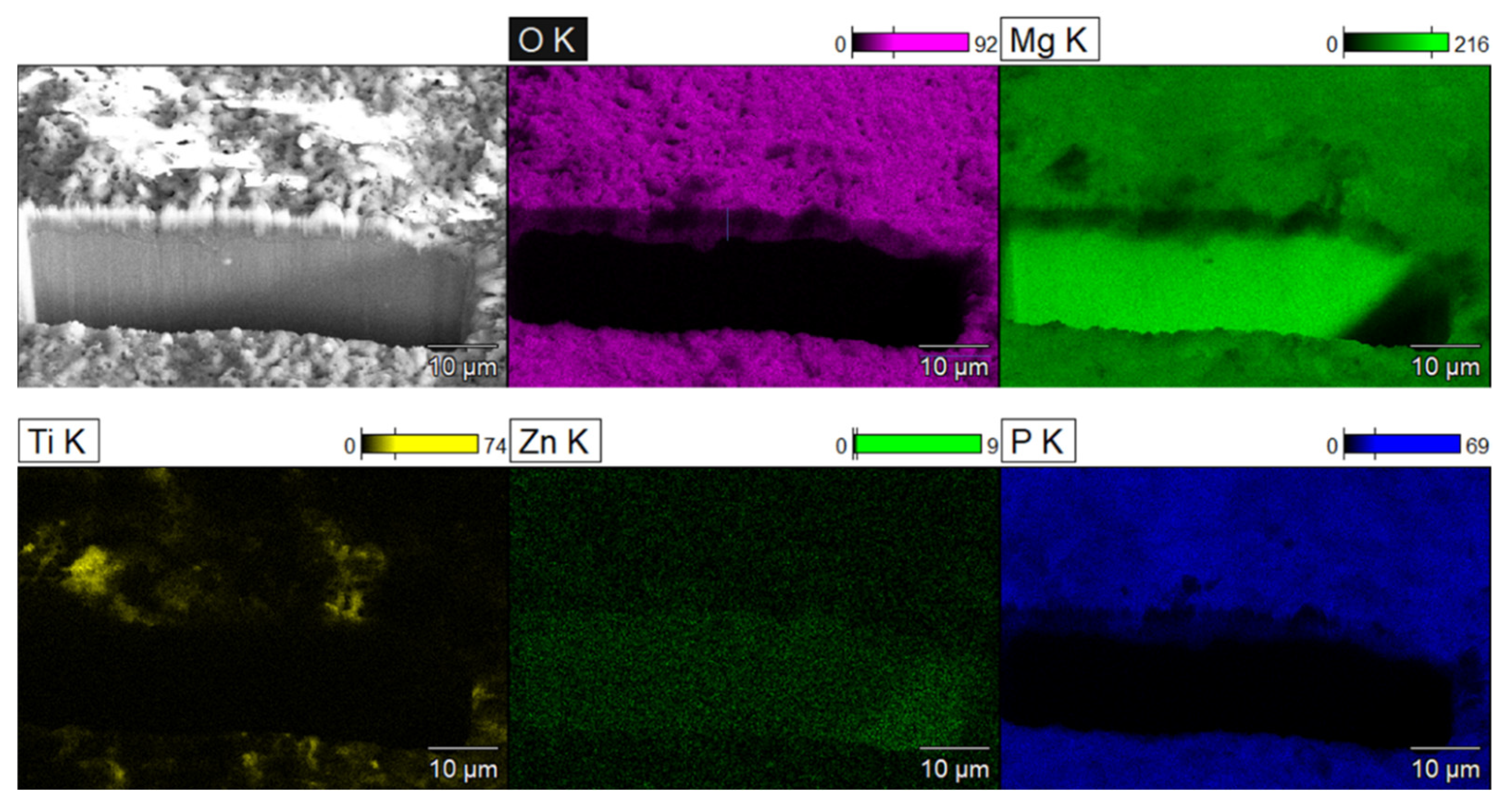
3.3. Energy Dispersive Spectroscopy
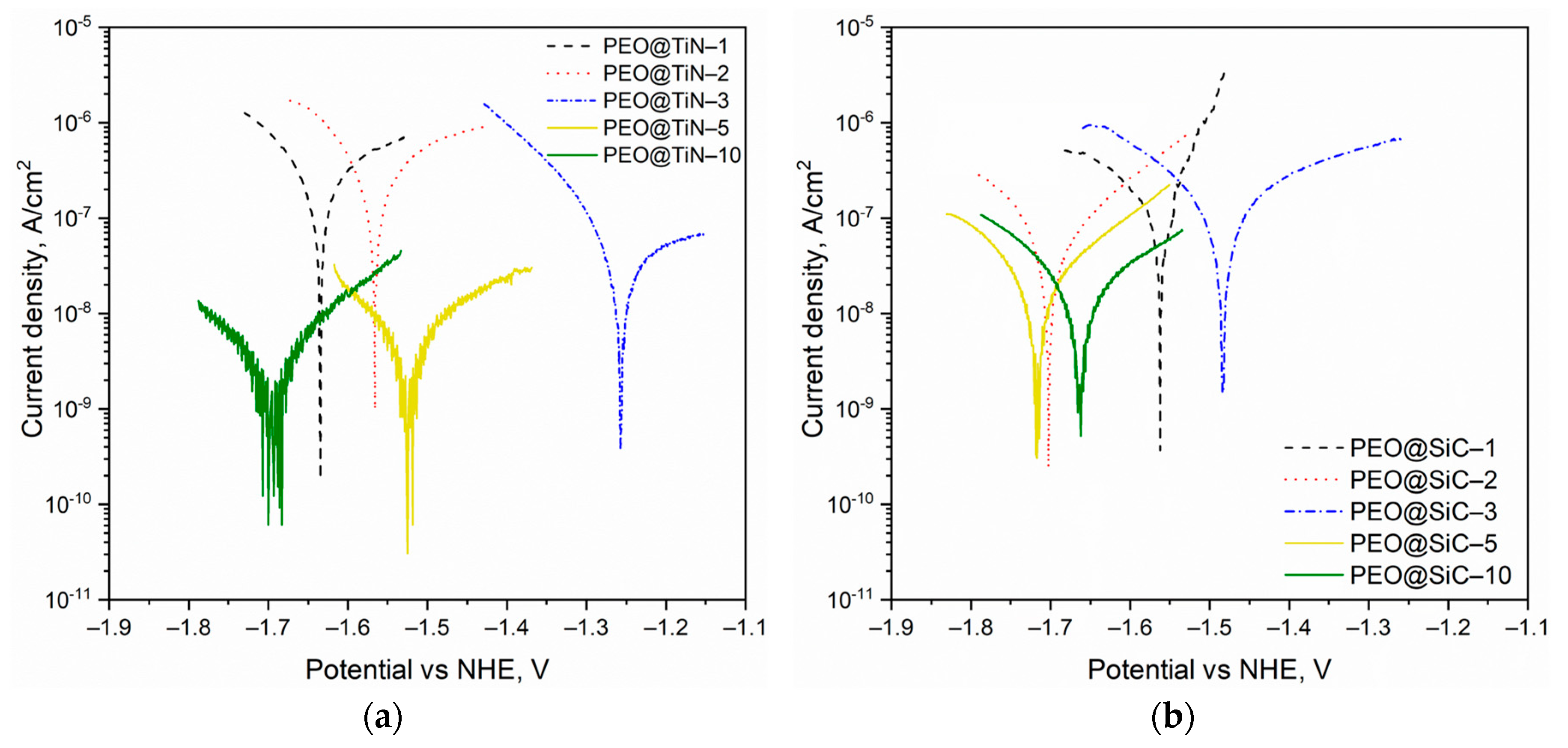
3.4. Electrochemical Corrosion Testing
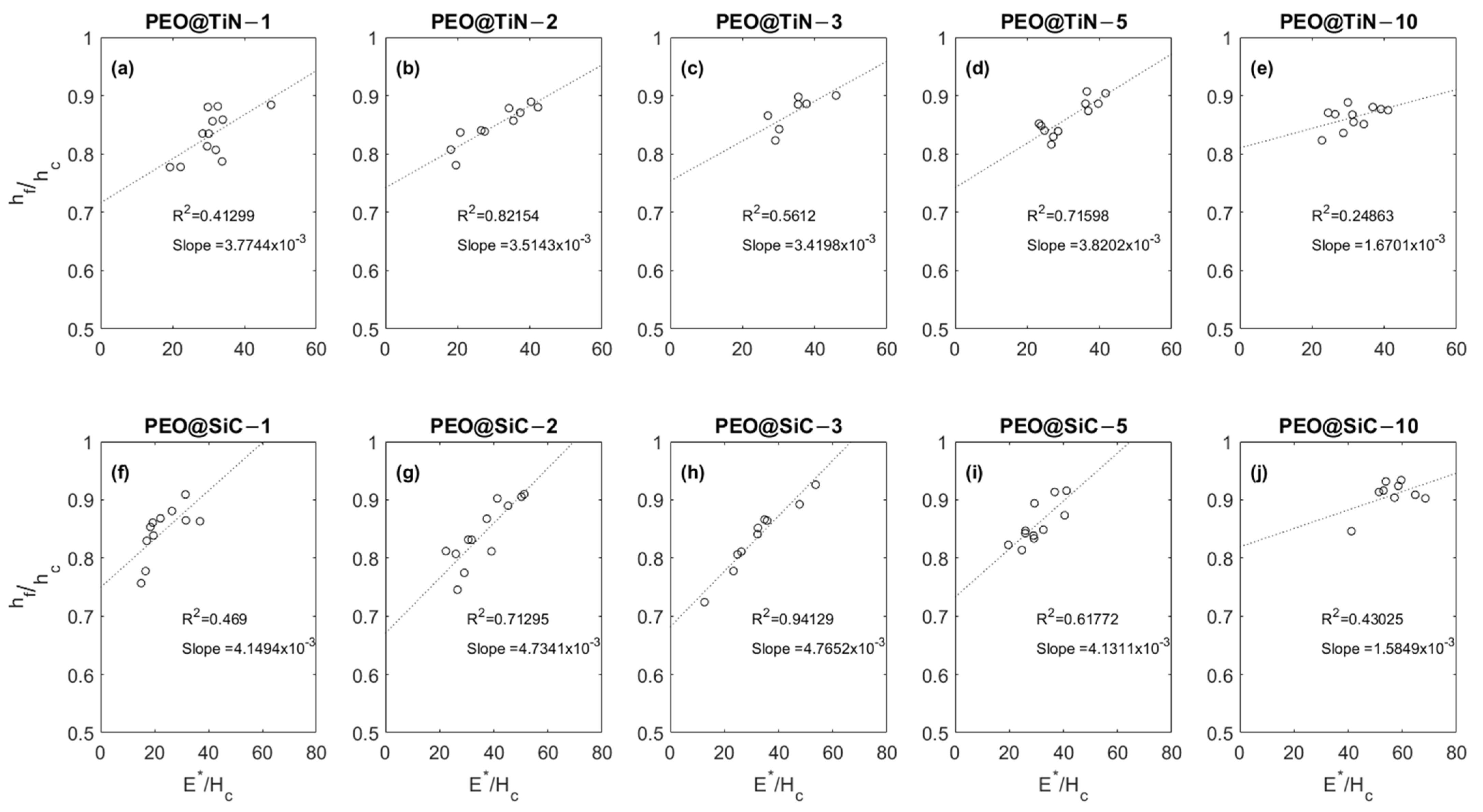
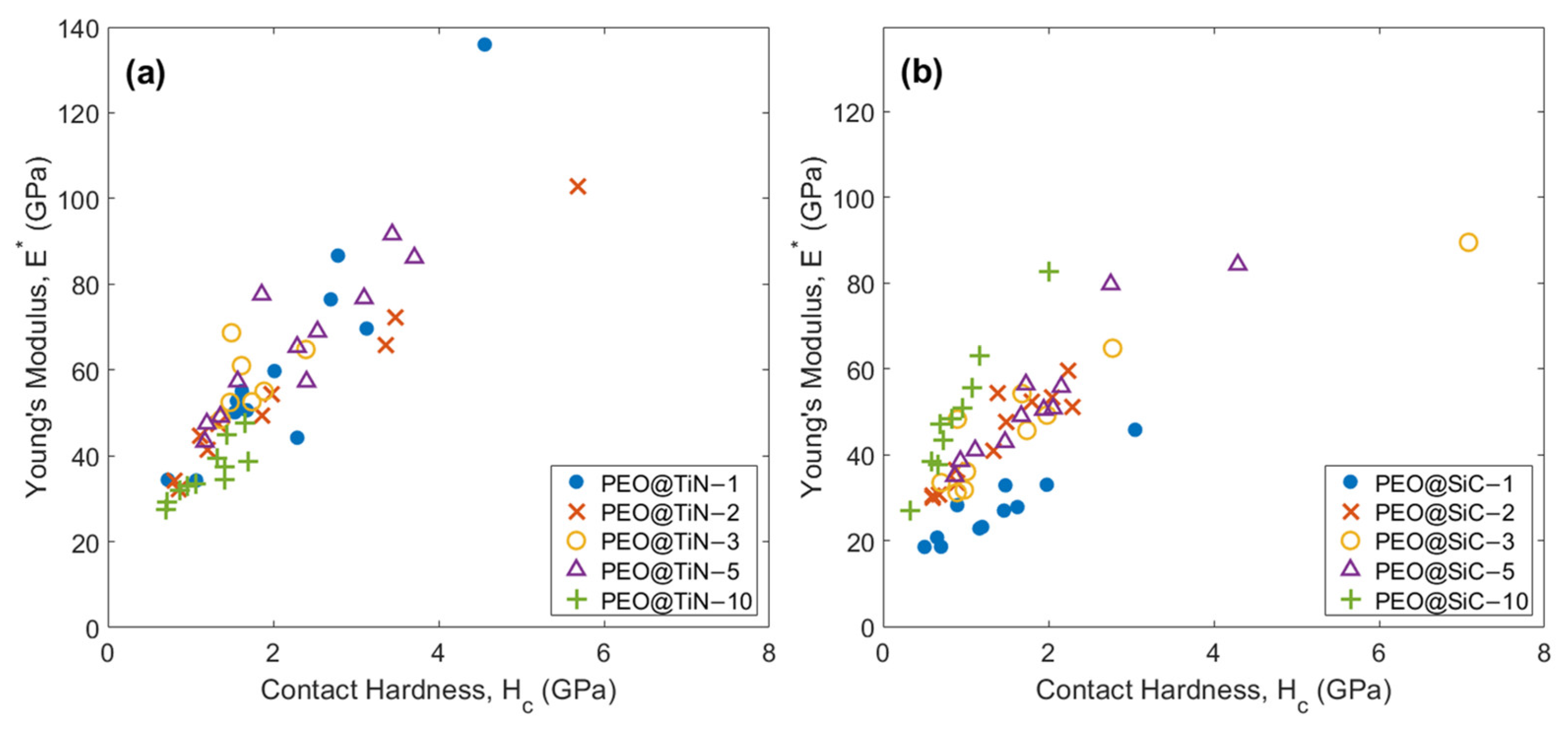
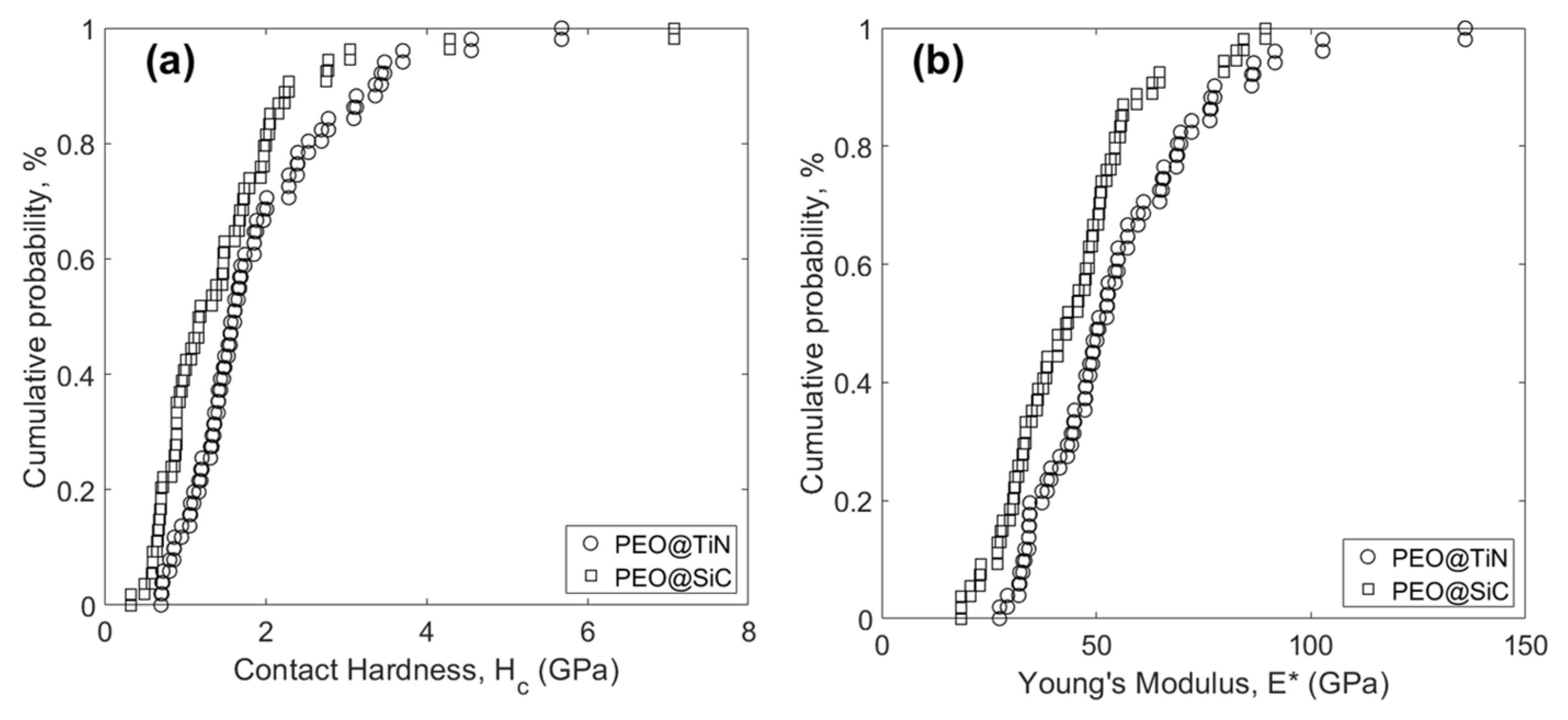
3.5. Nano-Mechanical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, J.; Chen, J.; Xiong, X.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys worldwide in 2021. J. Magnes. Alloys 2022, 10, 863–898. [Google Scholar] [CrossRef]
- Jayasathyakawin, S.; Ravichandran, M.; Baskar, N.; Anand Chairman, C.; Balasundaram, R. Mechanical properties and applications of Magnesium alloy—Review. Mater. Today Proc. 2020, 27, 909–913. [Google Scholar] [CrossRef]
- Ji, Q.; Wang, Y.; Wu, R.; Wei, Z.; Ma, X.; Zhang, J.; Hou, L.; Zhang, M. High specific strength Mg-Li-Zn-Er alloy processed by multi deformation processes. Mater. Charact. 2020, 160, 110135. [Google Scholar] [CrossRef]
- Tian, G.; Wang, J.; Wang, S.; Xue, C.; Yang, X.; Su, H. An ultra-light Mg-Li alloy with exceptional elastic modulus, high strength, and corrosion-resistance. Mater. Today Commun. 2023, 35, 105623. [Google Scholar] [CrossRef]
- Kiarasi, F.; Babaei, M.; Omidi Bidgoli, M.; Reza Kashyzadeh, K.; Asemi, K. Mechanical characterization and creep strengthening of AZ91 magnesium alloy by addition of yttrium oxide nanoparticles. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2022, 236, 1489–1500. [Google Scholar] [CrossRef]
- Tong, L.B.; Chu, J.H.; Zou, D.N.; Sun, Q.; Kamado, S.; Brokmeier, H.G.; Zheng, M.Y. Simultaneously Enhanced Mechanical Properties and Damping Capacities of ZK60 Mg Alloys Processed by Multi-Directional Forging. Acta Metall. Sin. (Engl. Lett.) 2021, 34, 265–277. [Google Scholar] [CrossRef]
- Wang, G.G.; Weiler, J.P. Recent developments in high-pressure die-cast magnesium alloys for automotive and future applications. J. Magnes. Alloys 2023, 11, 78–87. [Google Scholar] [CrossRef]
- Zahedi Asl, V.; Chini, S.F.; Zhao, J.; Palizdar, Y.; Shaker, M.; Sadeghi, A. Corrosion properties and surface free energy of the Zn Al LDH/rGO coating on MAO pretreated AZ31 magnesium alloy. Surf. Coat. Technol. 2021, 426, 127764. [Google Scholar] [CrossRef]
- Wu, T.; Blawert, C.; Lu, X.; Serdechnova, M.; Zheludkevich, M.L. Difference in formation of plasma electrolytic oxidation coatings on MgLi alloy in comparison with pure Mg. J. Magnes. Alloys 2021, 9, 1725–1740. [Google Scholar] [CrossRef]
- Kaseem, M.; Zehra, T.; Dikici, B.; Dafali, A.; Yang, H.W.; Ko, Y.G. Improving the electrochemical stability of AZ31 Mg alloy in a 3.5wt.% NaCl solution via the surface functionalization of plasma electrolytic oxidation coating. J. Magnes. Alloys 2022, 10, 1311–1325. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Dong, S.; Chen, X.-B.; Dong, J. Towards dense corrosion-resistant plasma electrolytic oxidation coating on Mg-Gd-Y-Zr alloy by using ultra-high frequency pulse current. Surf. Coat. Technol. 2022, 447, 128881. [Google Scholar] [CrossRef]
- Zehra, T.; Patil, S.A.; Shrestha, N.K.; Fattah-alhosseini, A.; Kaseem, M. Anionic assisted incorporation of WO3 nanoparticles for enhanced electrochemical properties of AZ31 Mg alloy coated via plasma electrolytic oxidation. J. Alloys Compd. 2022, 916, 165445. [Google Scholar] [CrossRef]
- Ali, W.; Echeverry-Rendón, M.; Dominguez, G.; van Gaalen, K.; Kopp, A.; González, C.; Llorca, J. Bioabsorbable WE43 Mg alloy wires modified by continuous plasma electrolytic oxidation for implant applications. Part II: Degradation and biological performance. Biomater. Adv. 2023, 147, 213325. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, L.; Yao, W.; Chen, Y.; Chen, Y.; Yuan, Y.; Wang, J.; Atrens, A.; Pan, F. Effect of electrolyte systems on plasma electrolytic oxidation coatings characteristics on LPSO Mg-Gd-Y-Zn alloy. Surf. Coat. Technol. 2023, 454, 129192. [Google Scholar] [CrossRef]
- Zafari, A.; Ghasemi, H.M.; Mahmudi, R. An investigation on the tribological behavior of AZ91 and AZ91+3wt% RE magnesium alloys at elevated temperatures. Mater. Des. (1980–2015) 2014, 54, 544–552. [Google Scholar] [CrossRef]
- Yu, L.; Cao, J.; Cheng, Y. An improvement of the wear and corrosion resistances of AZ31 magnesium alloy by plasma electrolytic oxidation in a silicate–hexametaphosphate electrolyte with the suspension of SiC nanoparticles. Surf. Coat. Technol. 2015, 276, 266–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Miao, C.; Tu, X.; Yu, J.; Li, J. Effect of Tungsten Carbide Particles on the Characteristics of PEO Coatings Formed on AZ31B Magnesium Alloy in Alkaline Electrolyte. Int. J. Electrochem. Sci. 2018, 13, 7923–7929. [Google Scholar] [CrossRef]
- NasiriVatan, H.; Ebrahimi-Kahrizsangi, R.; Asgarani, M.K. Tribological performance of PEO-WC nanocomposite coating on Mg Alloys deposited by Plasma Electrolytic Oxidation. Tribol. Int. 2016, 98, 253–260. [Google Scholar] [CrossRef]
- Tang, M.; Feng, Z.; Wu, X.; Wang, W.; Li, G.; Yan, Z.; Zhang, R. Microarc oxidation coatings containing TiC and NbC on magnesium alloy. Surf. Eng. 2019, 36, 1171–1179. [Google Scholar] [CrossRef]
- Wang, S.-y.; Si, N.-c.; Xia, Y.-p.; Liu, L. Influence of nano-SiC on microstructure and property of MAO coating formed on AZ91D magnesium alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 1926–1934. [Google Scholar] [CrossRef]
- Wu, M.; Jiang, F. Effect of SiC addition in electrolyte on the microstructure and tribological properties of micro-arc oxidation coatings on Al-Mg-Sc alloy. Surf. Topogr. Metrol. Prop. 2021, 9, 035043. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, H. Effects of Current Frequency on the Microstructure and Wear Resistance of Ceramic Coatings Embedded with SiC Nano-particles Produced by Micro-arc Oxidation on AZ91D Magnesium Alloy. J. Mater. Sci. Technol. 2010, 26, 865–871. [Google Scholar] [CrossRef]
- Golhin, A.P.; Kamrani, S.; Fleck, C.; Ghasemi, A. Corrosion protection of Mg-SiC nanocomposite through plasma electrolytic oxidation coating process. Mater. Corros. 2022, 73, 1813–1825. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Huang, Y.; Ovri, H.; Zheludkevich, M.L.; Kainer, K.U. Plasma electrolytic oxidation coatings on Mg alloy with addition of SiO2 particles. Electrochim. Acta 2016, 187, 20–33. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Mohedano, M.; Scharnagl, N.; Zheludkevich, M.L.; Kainer, K.U. Influence of electrical parameters on particle uptake during plasma electrolytic oxidation processing of AM50 Mg alloy. Surf. Coat. Technol. 2016, 289, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Mohedano, M.; Blawert, C.; Zheludkevich, M.L. Silicate-based Plasma Electrolytic Oxidation (PEO) coatings with incorporated CeO2 particles on AM50 magnesium alloy. Mater. Des. 2015, 86, 735–744. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, D.; Yu, J.; Di, S. Effects of Al2O3 Nano-additive on Performance of Micro-arc Oxidation Coatings Formed on AZ91D Mg Alloy. J. Mater. Sci. Technol. 2014, 30, 984–990. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.; Zhang, Y.; Du, C. Influence of graphene oxide additive on the tribological and electrochemical corrosion properties of a PEO coating prepared on AZ31 magnesium alloy. Tribol. Int. 2020, 146, 106135. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Imshinetskiy, I.M.; Puz’, A.V. Plasma electrolytic oxidation of the magnesium alloy MA8 in electrolytes containing TiN nanoparticles. J. Mater. Sci. Technol. 2017, 33, 461–468. [Google Scholar] [CrossRef]
- Mashtalyar, D.V.; Sinebryukhov, S.L.; Imshinetskiy, I.M.; Gnedenkov, A.S.; Nadaraia, K.V.; Ustinov, A.Y.; Gnedenkov, S.V. Hard wearproof PEO-coatings formed on Mg alloy using TiN nanoparticles. Appl. Surf. Sci. 2020, 503, 144062. [Google Scholar] [CrossRef]
- Doerner, M.F.; Nix, W.D. A method for interpreting the data from depth-sensing indentation instruments. J. Mater. Res. 1986, 1, 601–609. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Field, J.S.; Swain, M.V. A simple predictive model for spherical indentation. J. Mater. Res. 1993, 8, 297–306. [Google Scholar] [CrossRef]
- Gong, J.; Peng, Z.; Miao, H. Analysis of the nanoindentation load–displacement curves measured on high-purity fine-grained alumina. J. Eur. Ceram. Soc. 2005, 25, 649–654. [Google Scholar] [CrossRef]
- Drunka, R.; Iesalniece, P.; Savkovs, K.; Krumina, A. Plasma electrolytic oxidation of AZ31 Mg alloy in bipolar pulse mode and influence of corrosion to surface morphology of obtained coatings. Mater. Sci. (Medžiagotyra), 2022; Accepted. [Google Scholar]
- Drunka, R.; Iesalniece, P.; Steins, I.; Grase, L.; Eiduks, T.V.; Savkovs, K.; Blumbergs, I. Complex coating system for improving corrosion resistance of AZ31 magnesium alloy. J. Phys. Conf. Ser. 2023, 2423, 012020. [Google Scholar] [CrossRef]
- Lee, K.M.; Shin, K.R.; Namgung, S.; Yoo, B.; Shin, D.H. Electrochemical response of ZrO2-incorporated oxide layer on AZ91 Mg alloy processed by plasma electrolytic oxidation. Surf. Coat. Technol. 2011, 205, 3779–3784. [Google Scholar] [CrossRef]
- Molaei, M.; Babaei, K.; Fattah-alhosseini, A. Improving the wear resistance of plasma electrolytic oxidation (PEO) coatings applied on Mg and its alloys under the addition of nano- and micro-sized additives into the electrolytes: A review. J. Magnes. Alloys 2021, 9, 1164–1186. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Refractory Carbides & Nitrides: Properties, Characteristics, Processing and Applications; William Andrew: Norwich, NY, USA, 1996. [Google Scholar]
- Moriyama, M.; Kawazoe, T.; Tanaka, M.; Murakami, M. Correlation between microstructure and barrier properties of TiN thin films used Cu interconnects. Thin Solid Films 2002, 416, 136–144. [Google Scholar] [CrossRef]
- Daviau, K.; Lee, K.K.M. Decomposition of silicon carbide at high pressures and temperatures. Phys. Rev. B 2017, 96, 174102. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Lee, B.U.; Yoon, S.I.; Lee, E.S.; Yoo, B.; Shin, D.H. Evaluation of plasma temperature during plasma oxidation processing of AZ91 Mg alloy through analysis of the melting behavior of incorporated particles. Electrochim. Acta 2012, 67, 6–11. [Google Scholar] [CrossRef]
- Polunin, A.V.; Cheretaeva, A.O.; Borgardt, E.D.; Rastegaev, I.A.; Krishtal, M.M.; Katsman, A.V.; Yasnikov, I.S. Improvement of oxide layers formed by plasma electrolytic oxidation on cast Al Si alloy by incorporating TiC nanoparticles. Surf. Coat. Technol. 2021, 423, 127603. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Zheludkevich, M.L.; Kainer, K.U. Insights into plasma electrolytic oxidation treatment with particle addition. Corros. Sci. 2015, 101, 201–207. [Google Scholar] [CrossRef]
- O'Hara, M.; Troughton, S.C.; Francis, R.; Clyne, T.W. The incorporation of particles suspended in the electrolyte into plasma electrolytic oxidation coatings on Ti and Al substrates. Surf. Coat. Technol. 2020, 385, 125354. [Google Scholar] [CrossRef]
- Nikkam, N.; Saleemi, M.; Haghighi, E.B.; Ghanbarpour, M.; Khodabandeh, R.; Muhammed, M.; Palm, B.; Toprak, M.S. Fabrication, Characterization and Thermophysical Property Evaluation of SiC Nanofluids for Heat Transfer Applications. Nano-Micro Lett. 2014, 6, 178–189. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, L.; Jiang, D.; Lin, Q.; Iwasa, M. Dispersion of TiN in aqueous media. J. Colloid Interface Sci. 2005, 286, 209–215. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Li, Z.; Cheng, C.-M. Scaling relationships for indentation measurements. Philos. Mag. A 2002, 82, 1821–1829. [Google Scholar] [CrossRef]
- Lawn, B.R.; Howes, V.R. Elastic recovery at hardness indentations. J. Mater. Sci. 1981, 16, 2745–2752. [Google Scholar] [CrossRef]
- Chen, C.T.; Song, Y.C.; Yu, G.P.; Huang, J.H. Microstructure and hardness of hollow cathode discharge ion-plated titanium nitride film. J. Mater. Eng. Perform. 1998, 7, 324–328. [Google Scholar] [CrossRef]

| Mg | Al | Zn | Mn | Cu | Si | Fe |
|---|---|---|---|---|---|---|
| Bal. | 3.00 | 1.02 | 0.31 | 0.04 | 0.02 | 0.01 |
| Sample | Ecorr (V) | icorr (nA/cm2) | Corrosion Rate (mm/yr) |
|---|---|---|---|
| PEO@TiN-10 | −1.70 | 5.4 | 6.3 × 10−5 |
| PEO@TiN-5 | −1.52 | 8.6 | 1.0 × 10−4 |
| PEO@TiN-3 | −1.26 | 67.4 | 7.8 × 10−4 |
| PEO@TiN-2 | −1.57 | 276.8 | 3.2 × 10−3 |
| PEO@TiN-1 | −1.63 | 680.0 | 7.9 × 10−3 |
| PEO@SiC-10 | −1.66 | 15.3 | 1.8 × 10−4 |
| PEO@SiC-5 | −1.56 | 18.6 | 2.2 × 10−4 |
| PEO@SiC-3 | −1.72 | 53.1 | 6.2 × 10−4 |
| PEO@SiC-2 | −1.48 | 88.6 | 1.0 × 10−3 |
| PEO@SiC-1 | −1.70 | 215.4 | 2.5 × 10−3 |
| Coating | Contact Hardness, Hc (GPa) | Young’s Modulus, E* (GPa) | ||
|---|---|---|---|---|
| Mean, | Standard Deviation, | Mean, μ | Standard Deviation, σ | |
| PEO@TiN | 1.90 | 1.02 | 55.2 | 20.9 |
| PEO@SiC | 1.49 | 1.08 | 44.1 | 16.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.K.; Drunka, R.; Smits, K.; Vanags, M.; Iesalnieks, M.; Joksa, A.A.; Blumbergs, I.; Steins, I. Nanomechanical and Electrochemical Corrosion Testing of Nanocomposite Coating Obtained on AZ31 via Plasma Electrolytic Oxidation Containing TiN and SiC Nanoparticles. Crystals 2023, 13, 508. https://doi.org/10.3390/cryst13030508
Singh AK, Drunka R, Smits K, Vanags M, Iesalnieks M, Joksa AA, Blumbergs I, Steins I. Nanomechanical and Electrochemical Corrosion Testing of Nanocomposite Coating Obtained on AZ31 via Plasma Electrolytic Oxidation Containing TiN and SiC Nanoparticles. Crystals. 2023; 13(3):508. https://doi.org/10.3390/cryst13030508
Chicago/Turabian StyleSingh, Ashish Kumar, Reinis Drunka, Krisjanis Smits, Martins Vanags, Mairis Iesalnieks, Aiga Anna Joksa, Ilmars Blumbergs, and Ints Steins. 2023. "Nanomechanical and Electrochemical Corrosion Testing of Nanocomposite Coating Obtained on AZ31 via Plasma Electrolytic Oxidation Containing TiN and SiC Nanoparticles" Crystals 13, no. 3: 508. https://doi.org/10.3390/cryst13030508
APA StyleSingh, A. K., Drunka, R., Smits, K., Vanags, M., Iesalnieks, M., Joksa, A. A., Blumbergs, I., & Steins, I. (2023). Nanomechanical and Electrochemical Corrosion Testing of Nanocomposite Coating Obtained on AZ31 via Plasma Electrolytic Oxidation Containing TiN and SiC Nanoparticles. Crystals, 13(3), 508. https://doi.org/10.3390/cryst13030508








