Plasma Electrolytic Oxidation Treatment of AZ31 Magnesium Alloy for Biomedical Applications: The Influence of Applied Current on Corrosion Resistance and Surface Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Substrate
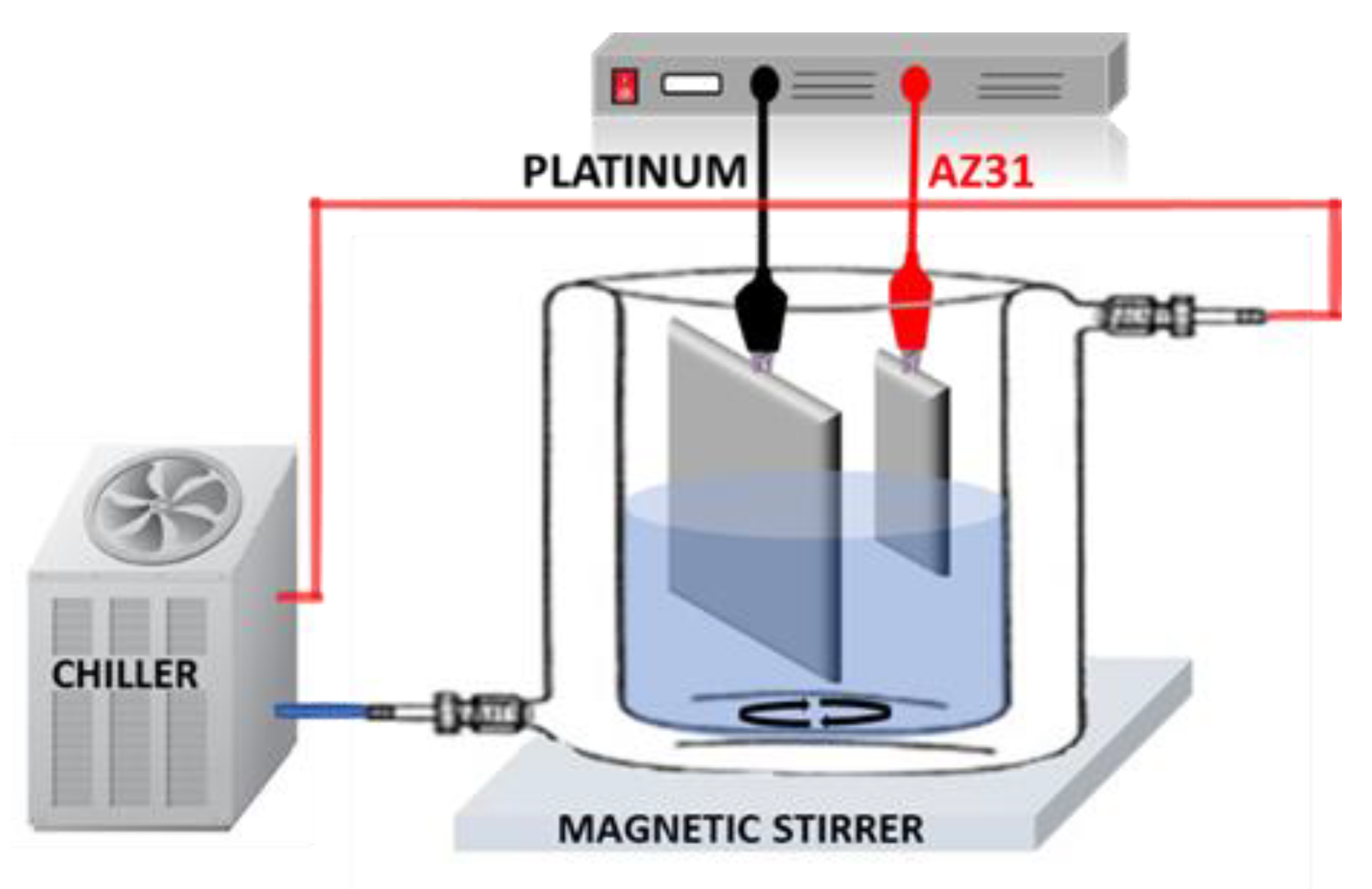
2.2. Plasma Electrolytic Oxidation
2.3. Characterization Techniques
3. Results and Discussion
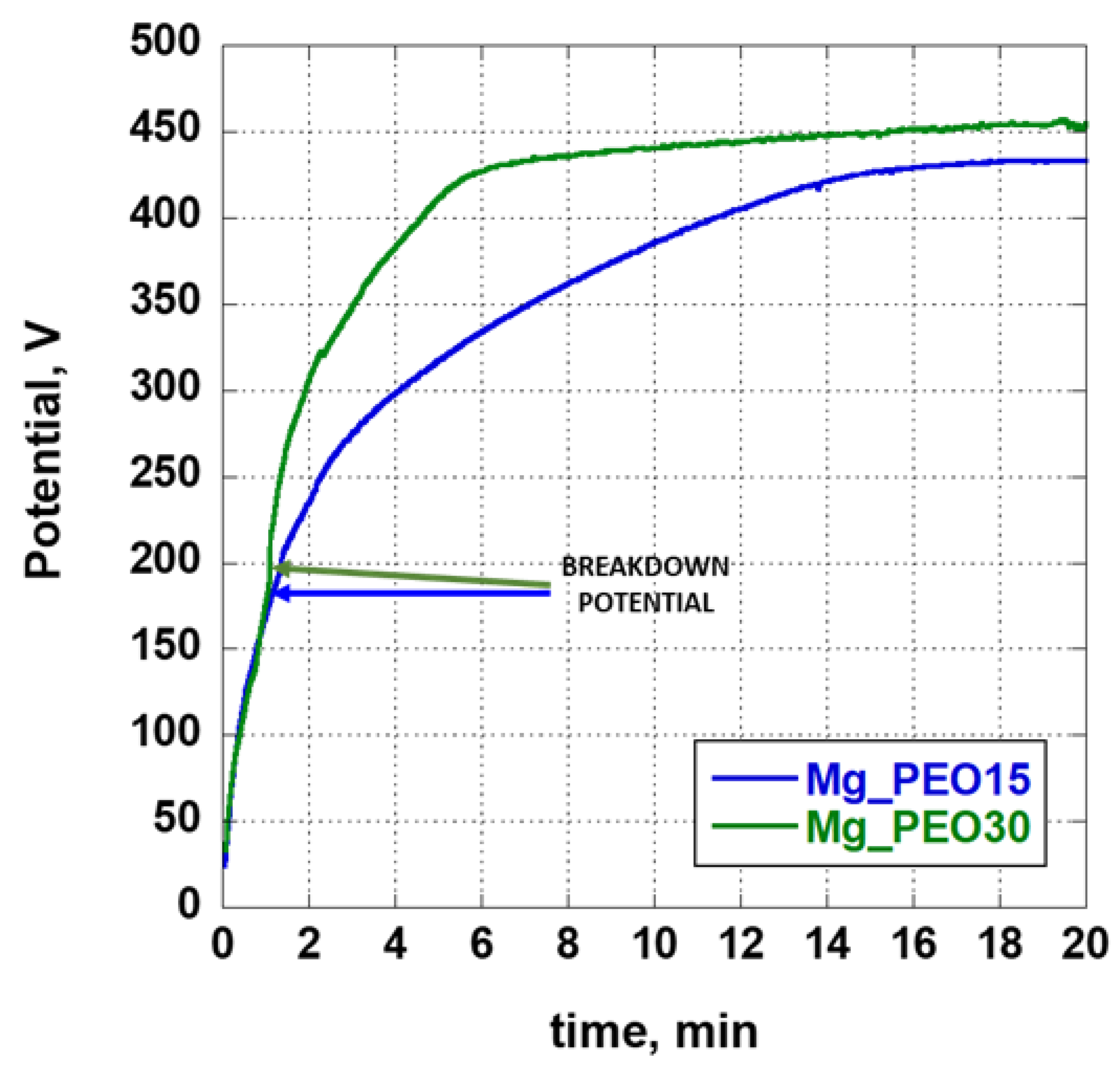
3.1. Potential-Time Curves
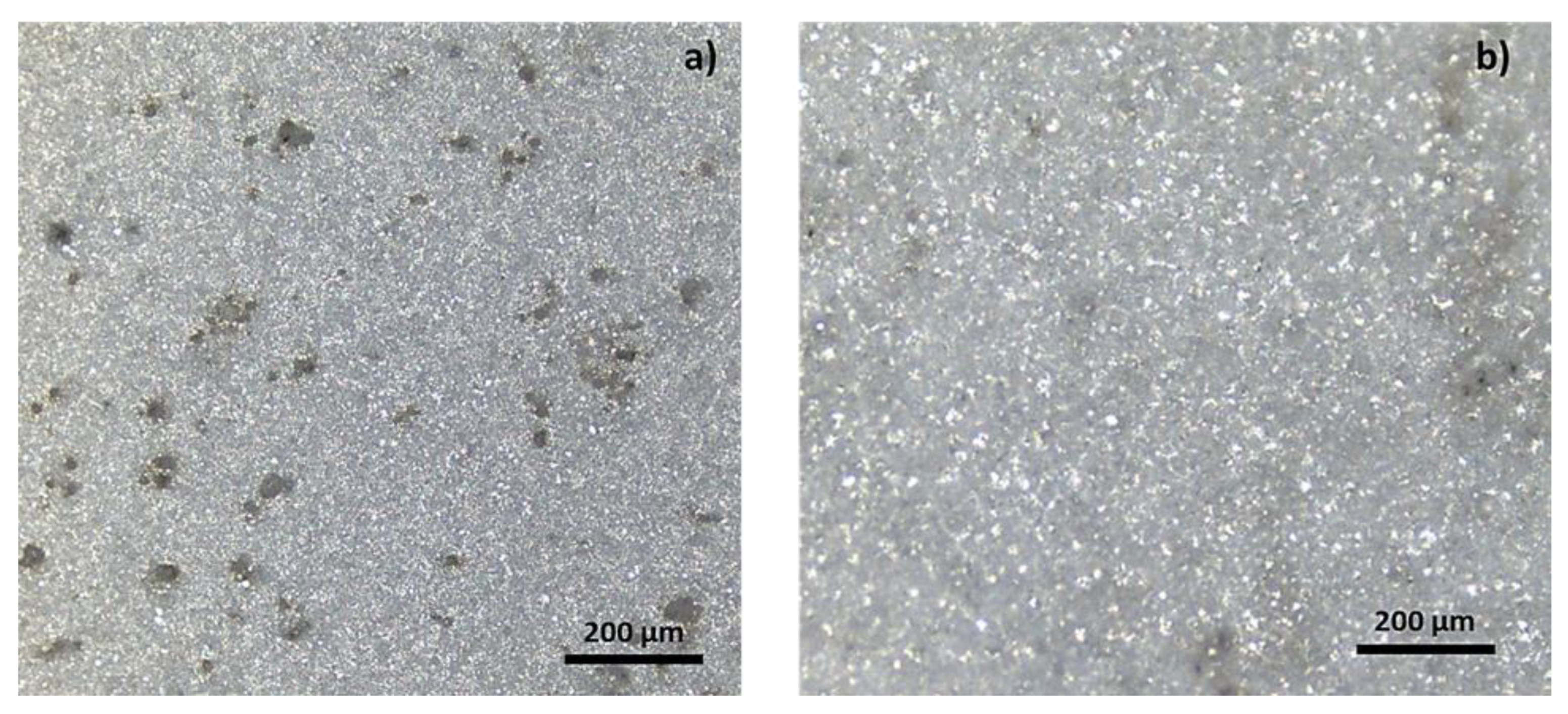
3.2. Morphological Analysis
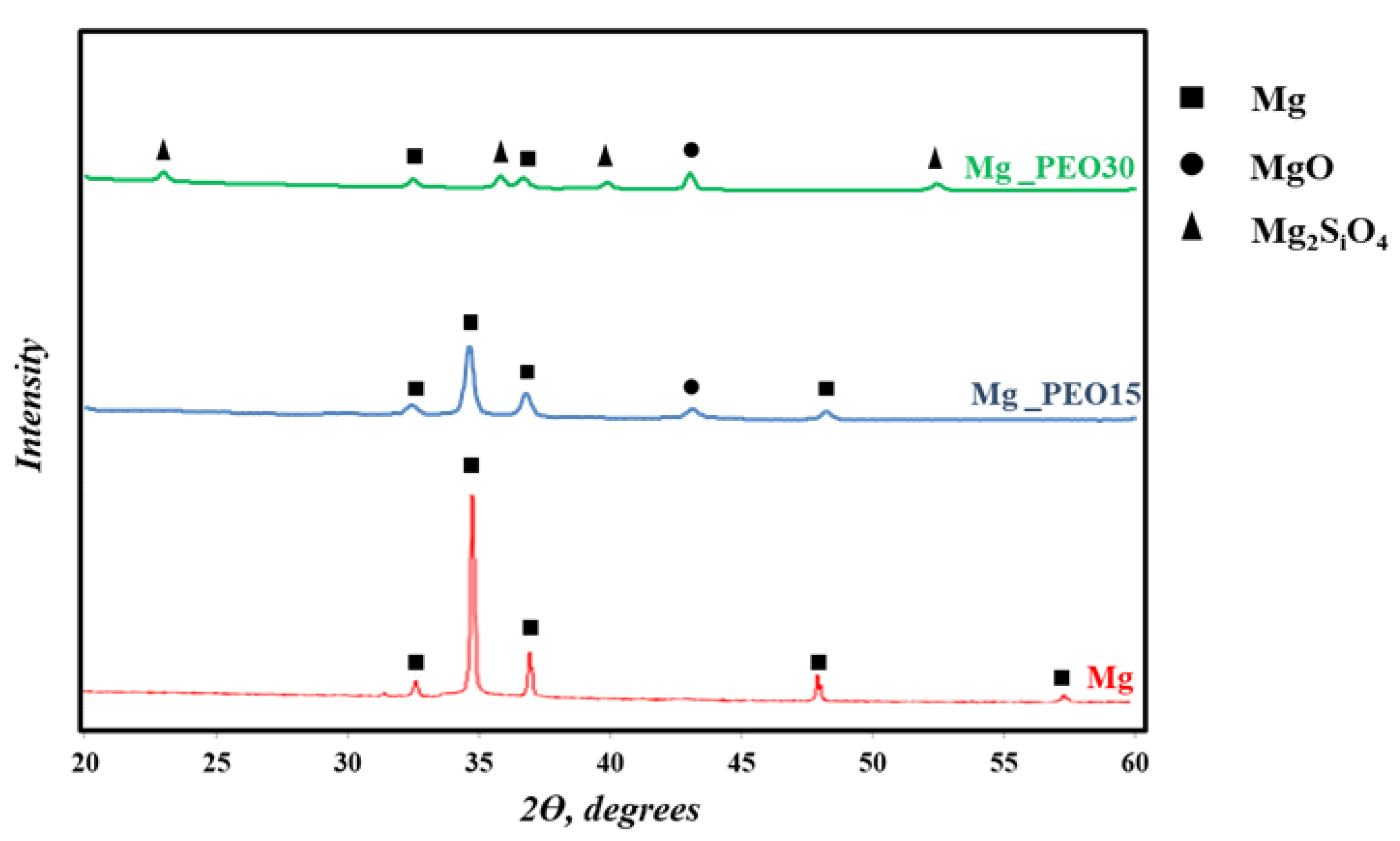
3.3. XRD Analysis
3.4. Electrochemical Analysis
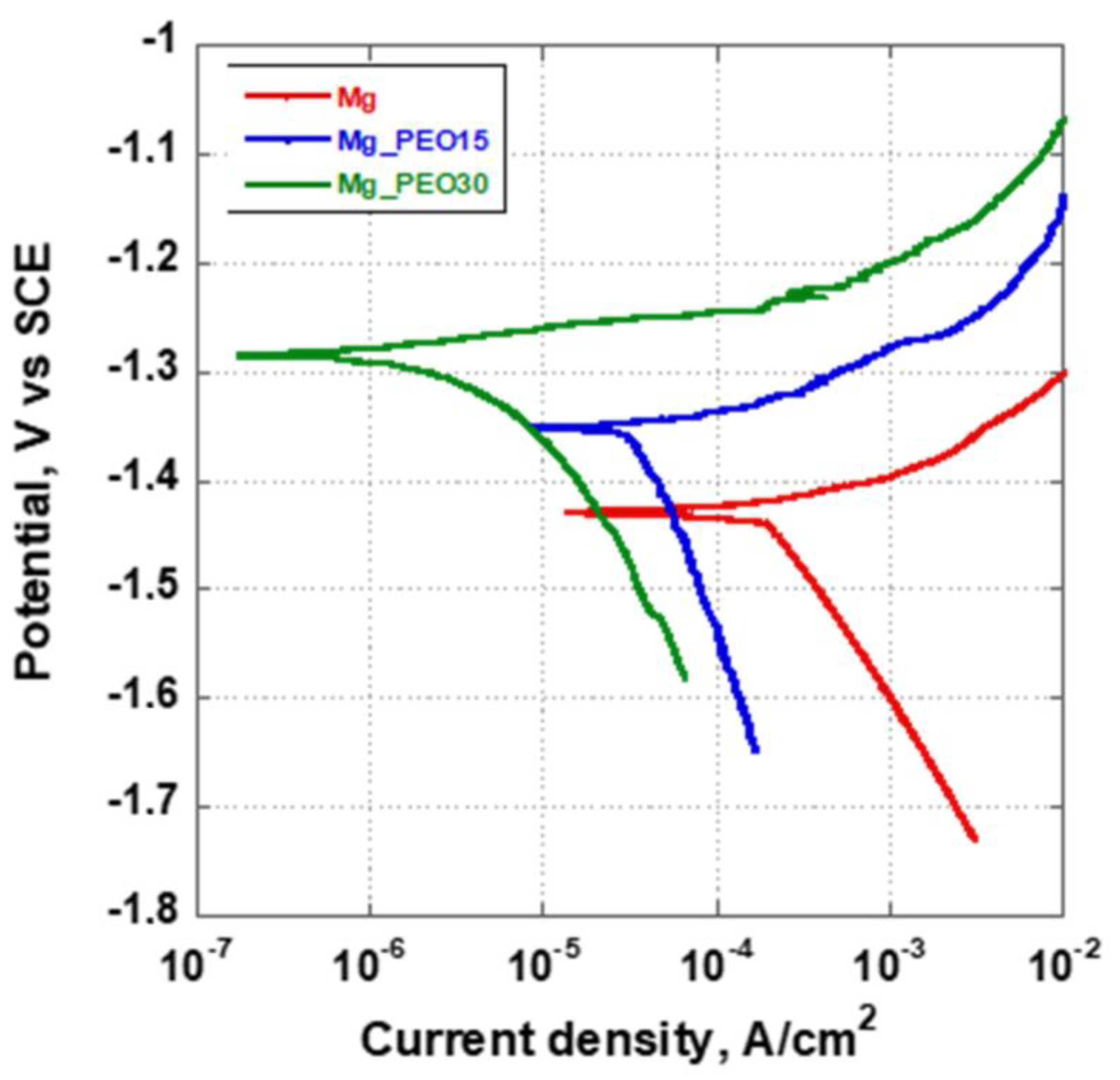
3.4.1. Potentiodynamic Polarization
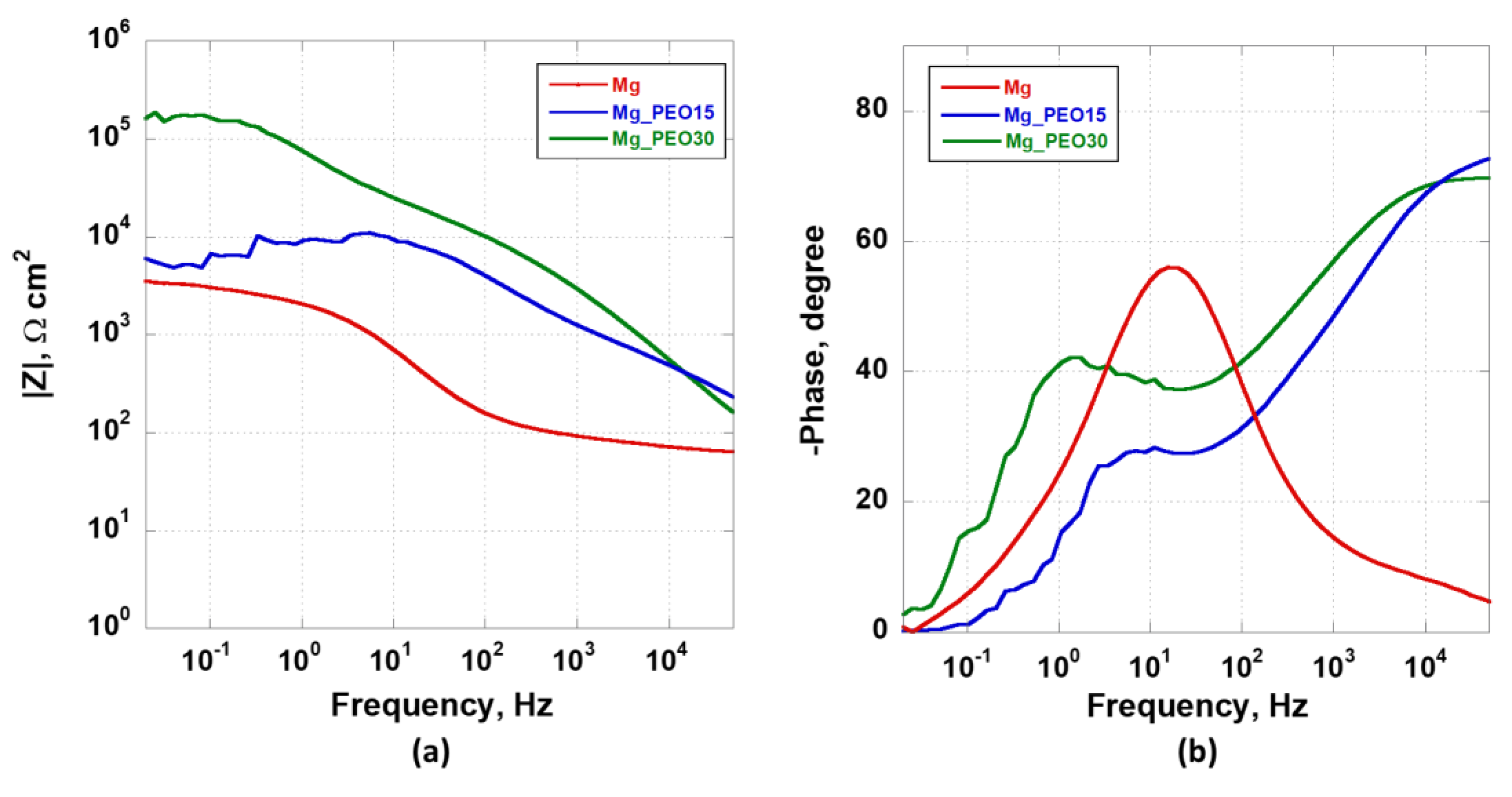
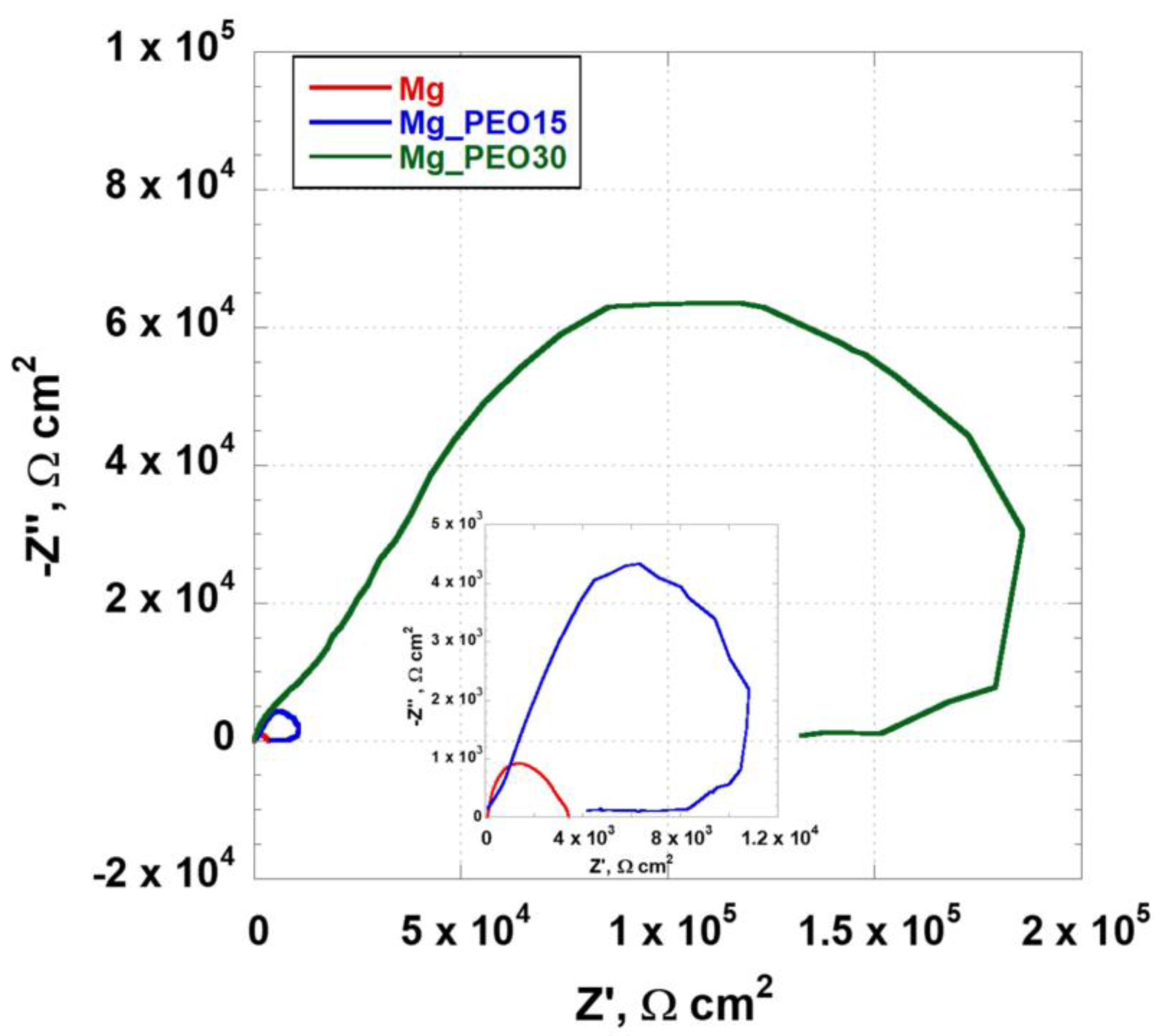
3.4.2. Electrochemical Impedance Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent Advances on the Development of Magnesium Alloys for Biodegradable Implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, H.; Hermawan, H. Biodegradable Metals: State of the Art. Biodegrad. Met. Concept Appl. 2012, 13–22. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Liu, J.; Wang, L.; Tang, Y.; Wang, K. A Review on Magnesium Alloys for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 106–127. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, V.V.; Ramasamy, P.; Kovukkal, M.D.; Myilsamy, G. Research and Development in Magnesium Alloys for Industrial and Biomedical Applications: A Review. Met. Mater. Int. 2019, 26, 409–430. [Google Scholar] [CrossRef]
- Carangelo, A.; Acquesta, A.; Monetta, T. In-Vitro Corrosion of AZ31 Magnesium Alloys by Using a Polydopamine Coating. Bioact. Mater. 2019, 4, 71–78. [Google Scholar] [CrossRef]
- Zhongling, W.; Peng, T.; Xuanyong, L.; Bangxin, Z. In Vitro Degradation, Hemolysis, and Cytocompatibility of PEO/PLLA Composite Coating on Biodegradable AZ31 Alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 342–354. [Google Scholar]
- Shi, P.; Niu, B.; Shanshan, E.; Chen, Y.; Li, Q. Preparation and Characterization of PLA Coating and PLA/MAO Composite Coatings on AZ31 Magnesium Alloy for Improvement of Corrosion Resistance. Surf. Coat. Technol. 2015, 262, 26–32. [Google Scholar] [CrossRef]
- Arrabal, R.; Mota, J.; Criado, A.; Pardo, A.; Mohedano, M.; Matykina, E. Assessment of Duplex Coating Combining Plasma Electrolytic Oxidation and Polymer Layer on AZ31 Magnesium Alloy. Surf. Coat. Technol. 2012, 206, 4692–4703. [Google Scholar] [CrossRef]
- Hou, F.; Gorthy, R.; Mardon, I.; Tang, D.; Goode, C. Low Voltage Environmentally Friendly Plasma Electrolytic Oxidation Process for Titanium Alloys. Sci. Rep. 2022, 12, 6037. [Google Scholar] [CrossRef]
- Jiao, Y.; Zou, G.-Y.; Mu, H.-X.; Ni, X.-H.; Yi, L.; Zhao, Q.-M. Cytocompatibility of Porous P-Containing Coating Prepared by Plasma Electrolytic Oxidation of Mg Alloy. Mater. Res. Express 2020, 7, 086404. [Google Scholar] [CrossRef]
- Toulabifard, A.; Rahmati, M.; Raeissi, K.; Hakimizad, A.; Santamaria, M. The Effect of Electrolytic Solution Composition on the Structure, Corrosion, and Wear Resistance of PEO Coatings on AZ31 Magnesium Alloy. Coatings 2020, 10, 937. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Golabadi, M.; Rouhaghdam, A.S. Effect of Lanthanum Nitrate on the Microstructure and Electrochemical Behavior of PEO Coatings on AZ31 Mg Alloy. J. Alloy Compd. 2017, 719, 242–255. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, R.; Liu, X.; Wang, D.; Zhang, H.; Shen, X.; Shen, D.; Li, D. Influence of Different Electrolyte Additives and Structural Characteristics of Plasma Electrolytic Oxidation Coatings on AZ31 Magnesium Alloy. Coatings 2020, 10, 817. [Google Scholar] [CrossRef]
- Zhuang, J.J.; Song, R.G.; Xiang, N.; Xiong, Y.; Hu, Q. Effect of Current Density on Microstructure and Properties of PEO Ceramic Coatings on Magnesium Alloy. Surf. Eng. 2017, 33, 744–752. [Google Scholar] [CrossRef]
- Lee, K.M.; Einkhah, F.; Sani, M.A.F.; Ko, Y.G.; Shin, D.H. Effects of Current Density on Microstructure and Corrosion Property of Coating on AZ31 Mg Alloy Processed via Plasma Electrolytic Oxidation. In Magnesium Technology 2014; Alderman, M., Manuel, M.V., Hort, N., Neelameggham, N.R., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 345–349. ISBN 978-3-319-48231-6. [Google Scholar]
- Kajánek, D.; Hadzima, B.; Brezina, M.; Jackova, M. Effect of Applied Current Density of Plasma Electrolytic Oxidation Process on Corrosion Resistance of AZ31 Magnesium Alloy. Commun.-Sci. Lett. Univ. Zilina 2019, 21, 32–36. [Google Scholar] [CrossRef]
- Bala Srinivasan, P.; Liang, J.; Blawert, C.; Störmer, M.; Dietzel, W. Effect of Current Density on the Microstructure and Corrosion Behaviour of Plasma Electrolytic Oxidation Treated AM50 Magnesium Alloy. Appl. Surf. Sci. 2009, 255, 4212–4218. [Google Scholar] [CrossRef] [Green Version]
- Monetta, T.; Acquesta, A.; Carangelo, A.; Donato, N.; Bellucci, F. Durability of AZ31 Magnesium Biodegradable Alloys Polydopamine Aided: Part 1. J. Magnes. Alloy. 2017, 5, 412–422. [Google Scholar] [CrossRef]
- Geels, K.; Fowler, D.B.; Kopp, W.-U.; Rückert, M. Metallographic and Materialographic Specimen Preparation, Light Microscopy, Image Analysis, and Hardness Testing; ASTM International: West Conshohocken, PA, USA, 2007; Volume 46. [Google Scholar]
- Liang, J.; Srinivasan, P.B.; Blawert, C.; Störmer, M.; Dietzel, W. Electrochemical Corrosion Behaviour of Plasma Electrolytic Oxidation Coatings on AM50 Magnesium Alloy Formed in Silicate and Phosphate Based Electrolytes. Electrochim. Acta 2009, 54, 3842–3850. [Google Scholar] [CrossRef]
- Monetta, T.; Parnian, P.; Acquesta, A. Recent Advances in the Control of the Degradation Rate of PEO Treated Magnesium and Its Alloys for Biomedical Applications. Metals 2020, 10, 907. [Google Scholar] [CrossRef]
- Hussein, R.O.; Zhang, P.; Nie, X.; Xia, Y.; Northwood, D.O. The Effect of Current Mode and Discharge Type on the Corrosion Resistance of Plasma Electrolytic Oxidation (PEO) Coated Magnesium Alloy AJ62. Surf. Coat. Technol. 2011, 206, 1990–1997. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wu, Y.; Chen, D.; Wang, R.; Li, D.; Guo, C.; Jiang, G.; Shen, D.; Yu, S.; Nash, P. Micro-Structures and Growth Mechanisms of Plasma Electrolytic Oxidation Coatings on Aluminium at Different Current Densities. Surf. Coat. Technol. 2017, 321, 236–246. [Google Scholar] [CrossRef]
- King, A.D.; Birbilis, N.; Scully, J.R. Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Fukuda, H.; Matsumoto, Y. Effects of Na2SiO3 on Anodization of Mg-Al-Zn Alloy in 3 M KOH Solution. Corros. Sci. 2004, 46, 2135–2142. [Google Scholar] [CrossRef]
- Walter, G. The Application of Impedance Spectroscopy to Study the Uptake of Sodium Chloride Solution in Painted Metals. Corros. Sci. 1991, 32, 1041–1058. [Google Scholar] [CrossRef]
- Schmitt, J.; Maheshwari, A.; Heck, M.; Lux, S.; Vetter, M. Impedance Change and Capacity Fade of Lithium Nickel Manganese Cobalt Oxide-Based Batteries during Calendar Aging. J. Power Sources 2017, 353, 183–194. [Google Scholar] [CrossRef]
- Amirudin, A.; Thieny, D. Application of Electrochemical Impedance Spectroscopy to Study the Degradation of Polymer-Coated Metals. Prog. Org. Coat. 1995, 26, 1–28. [Google Scholar] [CrossRef]
- Monetta, T.; Acquesta, A.; Carangelo, A.; Bellucci, F. TiO2 Nanotubes on Ti Dental Implant. Part 2: EIS Characterization in Hank’s Solution. Metals 2017, 7, 220. [Google Scholar] [CrossRef] [Green Version]
- Mansfeld, F.; Kendig, M.W.; Tsai, S. Evaluation of Corrosion Behavior of Coated Metals with AC Impedance Measurements. Corrosion 1982, 38, 478–485. [Google Scholar] [CrossRef]
- Walter, G.W. A Review of Impedance Plot Methods Used for Corrosion Performance Analysis of Painted Metals. Corros. Sci. 1986, 26, 681–703. [Google Scholar] [CrossRef]
- Sreekanth, D.; Rameshbabu, N.; Venkateswarlu, K. Effect of Various Additives on Morphology and Corrosion Behavior of Ceramic Coatings Developed on AZ31 Magnesium Alloy by Plasma Electrolytic Oxidation. Ceram. Int. 2012, 38, 4607–4615. [Google Scholar] [CrossRef]




| Element | Al | Zn | Mn | Si | Cu | Fe | Ni | Others | Mg |
|---|---|---|---|---|---|---|---|---|---|
| 2.5–3.5 | 0.7–1.3 | 0.2–1 | 0.05 | 0.01 | >0.05 | >0.05 | 0.4 | Balance |
| Acronym | Description of the Sample |
|---|---|
| Mg | Bare magnesium alloy sheet |
| Mg_PEO15 | Bare magnesium alloy treated by PEO applying a current density of 15 mA/cm2 |
| Mg_PEO30 | Bare magnesium alloy treated by PEO applying a current density of 30 mA/cm2 |
| Parameter | Mg | Mg_PEO15 | Mg_PEO30 |
|---|---|---|---|
| Ecorr (V vs. SCE) | −1.43 ± 0.03 | −1.35 ± 0.02 | −1.28 ± 0.04 |
| icorr (A/cm2) | 2 × 10−4 ± 0.6 × 10−4 | 4 × 10−5 ± 0. 5 × 10−5 | 5 × 10−6 ± 0. 3 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acquesta, A.; Russo, P.; Monetta, T. Plasma Electrolytic Oxidation Treatment of AZ31 Magnesium Alloy for Biomedical Applications: The Influence of Applied Current on Corrosion Resistance and Surface Characteristics. Crystals 2023, 13, 510. https://doi.org/10.3390/cryst13030510
Acquesta A, Russo P, Monetta T. Plasma Electrolytic Oxidation Treatment of AZ31 Magnesium Alloy for Biomedical Applications: The Influence of Applied Current on Corrosion Resistance and Surface Characteristics. Crystals. 2023; 13(3):510. https://doi.org/10.3390/cryst13030510
Chicago/Turabian StyleAcquesta, Annalisa, Pietro Russo, and Tullio Monetta. 2023. "Plasma Electrolytic Oxidation Treatment of AZ31 Magnesium Alloy for Biomedical Applications: The Influence of Applied Current on Corrosion Resistance and Surface Characteristics" Crystals 13, no. 3: 510. https://doi.org/10.3390/cryst13030510





