Preparation of a Flexible Reduced Graphene Oxide-Si Composite Film and Its Application in High-Performance Lithium Ion Batteries
Abstract
:1. Introduction
2. Experimental
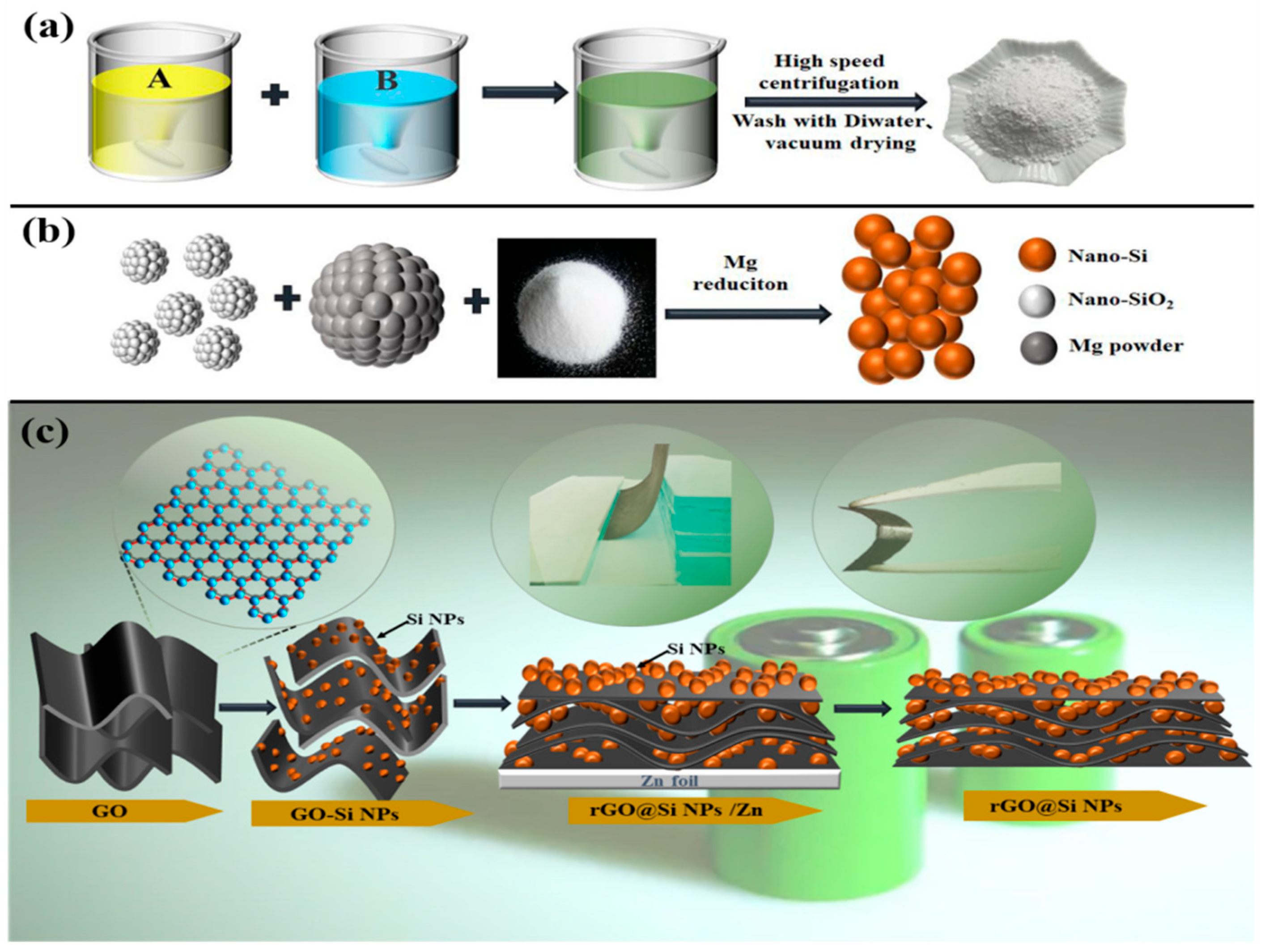
2.1. Preparation of Nano-SiO2
2.2. Preparation of Si NPs
2.3. Preparation of rGO@Si NPs Composite Films
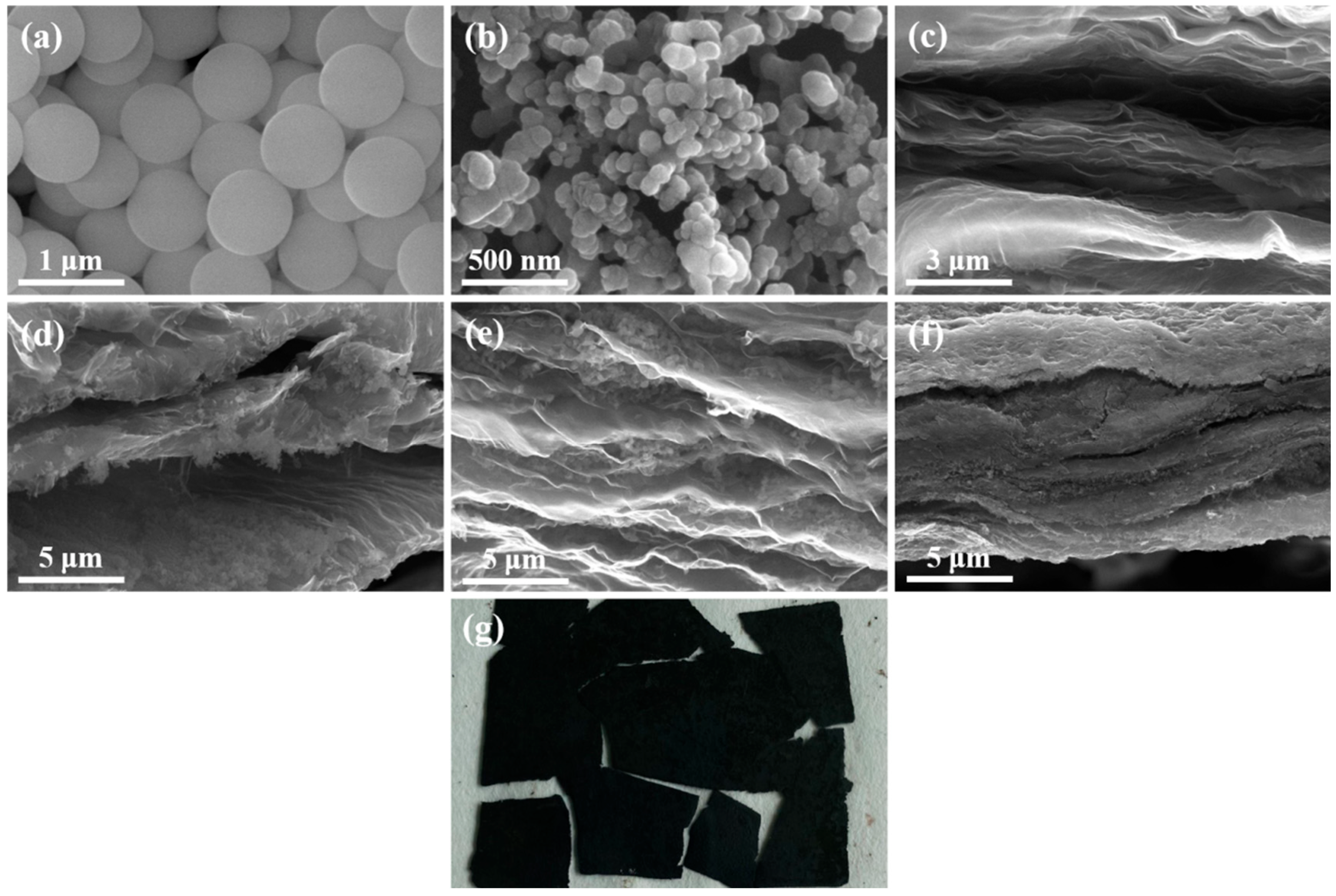
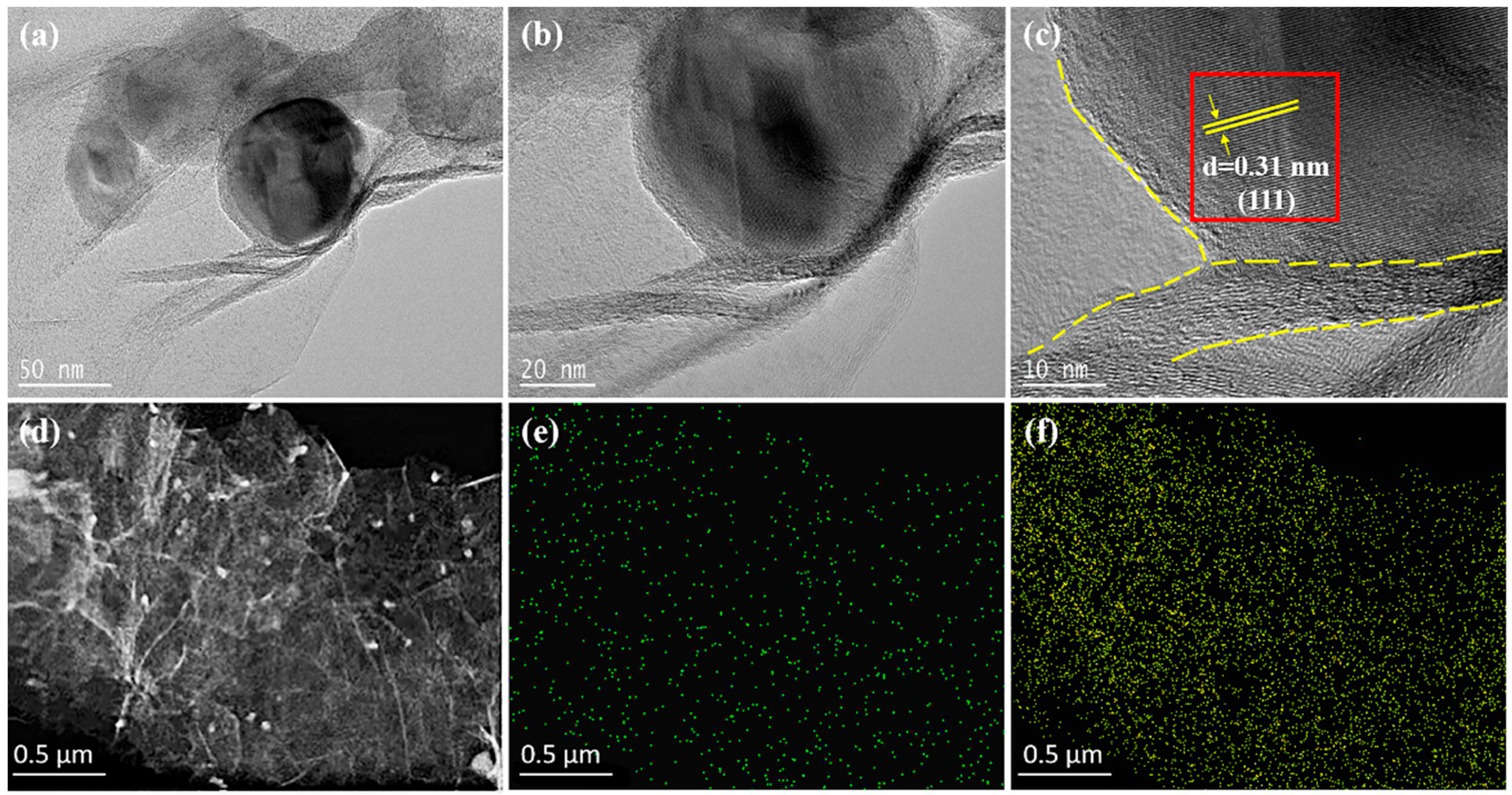
2.4. Materials Characterization
2.5. Electrochemical Measurements
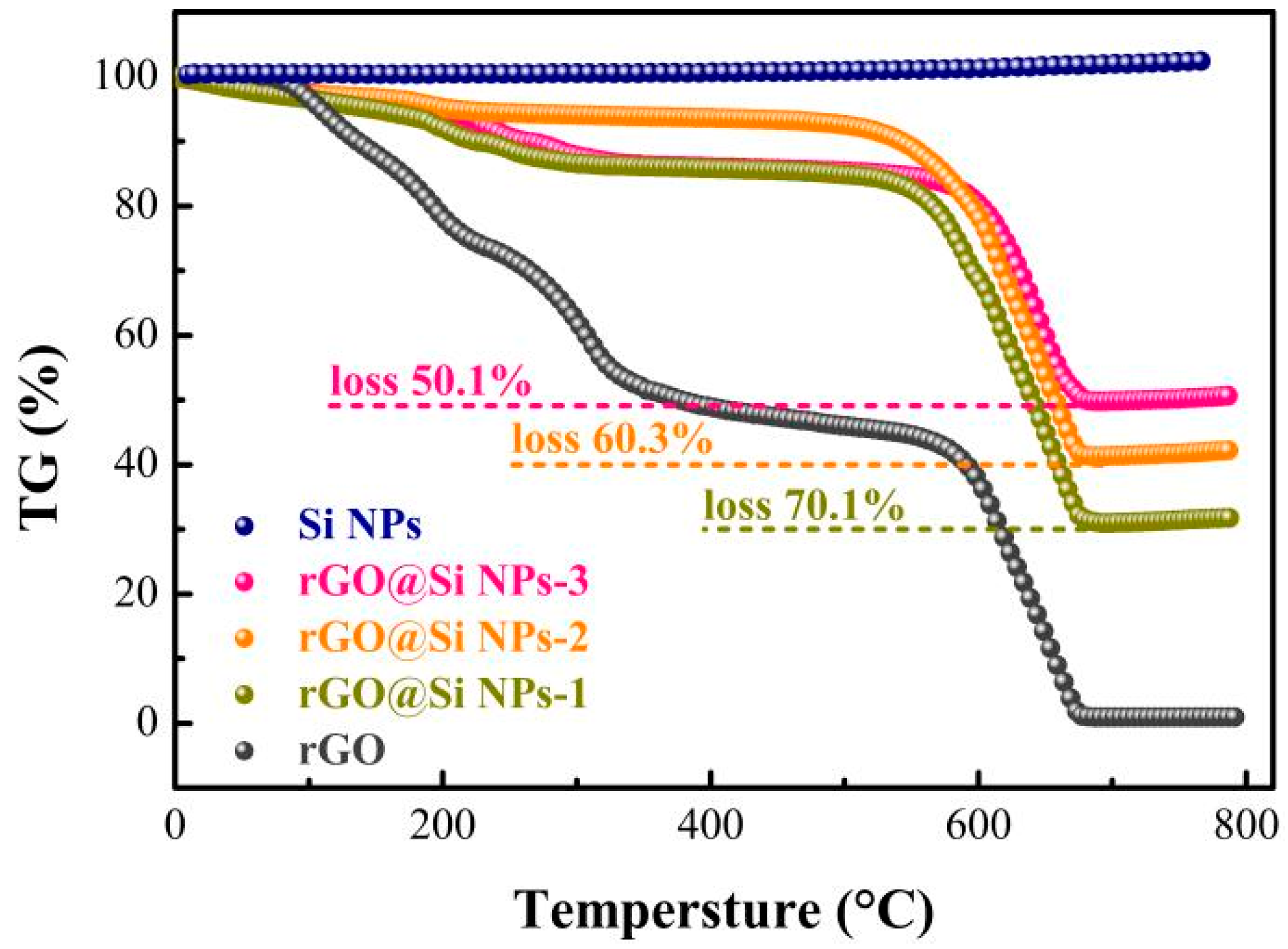
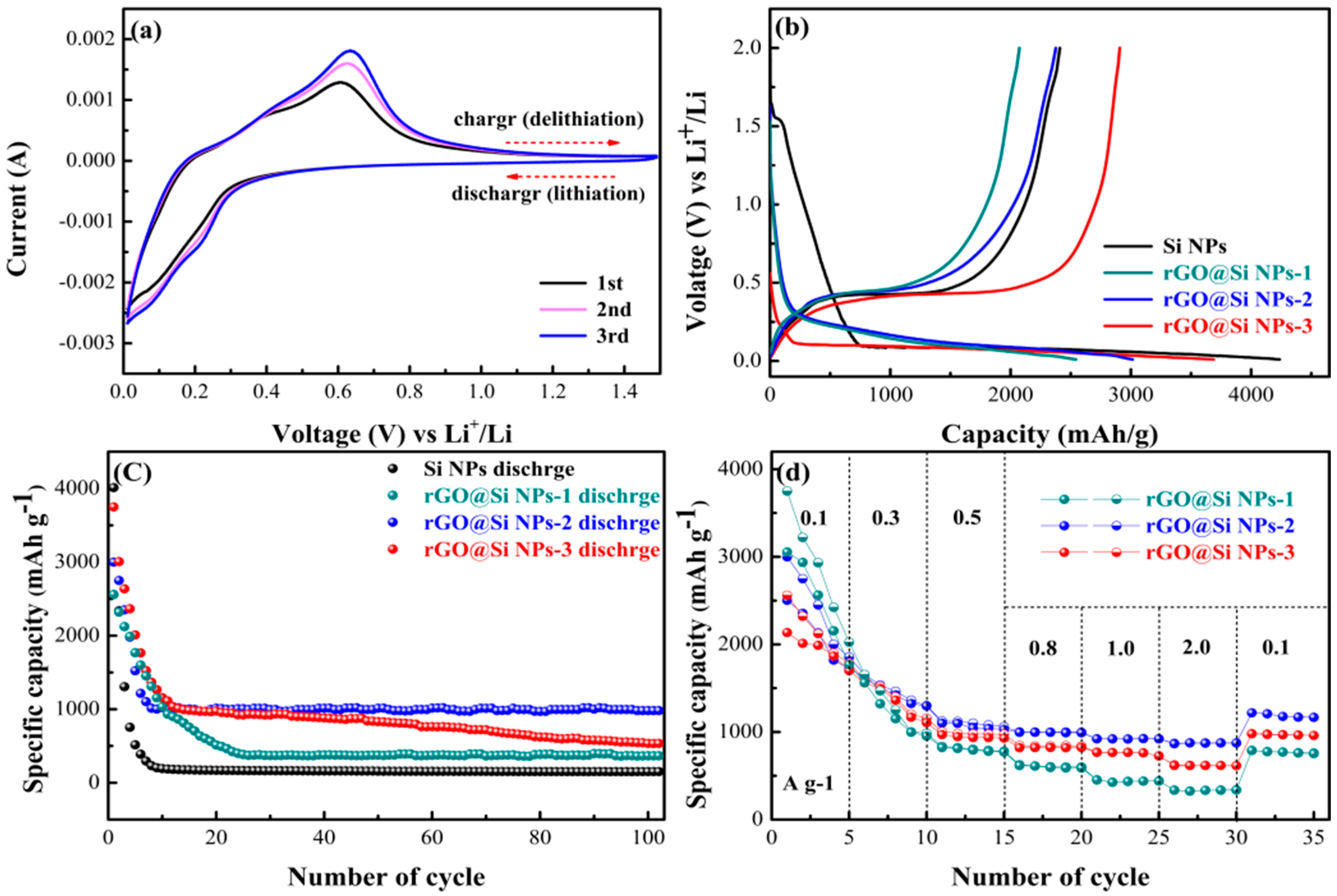
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, Y.; Li, Y.T.; Peng, L.L.; Byon, H.R.; Goodenough, J.B.; Yu, G.H. A chemistry and material perspective on lithium redox flow batteries towards high-density electrical energy storage. Chem. Soc. Rev. 2015, 44, 7968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.L.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Nitta, N.; Yushin, G. High-Capacity Anode Materials for Lithium-Ion Batteries: Choice of Elements and Structures for Active Particles. Part. Part. Syst. Charact. 2014, 31, 317–336. [Google Scholar] [CrossRef]
- Kang, B.; Ceder, G. Battery materials for ultrafast chargingand discharging. Nat. Lett. 2009, 458, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Saw, L.H.; Ye, Y.; Tay, A.A.O. Integration issues of lithium-ion battery into electric vehicles battery pack. J. Clean. Prod. 2016, 113, 1032–1045. [Google Scholar] [CrossRef]
- Liu, X.L.; Gao, Y.F.; Jin, R.H.; Luo, H.J.; Peng, P.; Liu, Y. Scalable synthesis of Si nanostructures by low-temperature magnesiothermic reduction of silica for application in lithium ion batteries. Nano Energy 2014, 4, 31–38. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Kasavajjula, U.; Wang, C.; Appleby, A.J. Nano-and bulk-silicon-based insertion anodes for lithium-ion secondary cells. J. Power Sources 2007, 163, 1003–1039. [Google Scholar] [CrossRef]
- Boukamp, B.A.; Lesh, G.C.; Huggins, R.A. All Solid Lithium Electrodes with Mixed Conductor Matrix. J. Eletrochem. Soc. 1981, 128, 725. [Google Scholar] [CrossRef]
- He, Y.; Yu, X.; Wang, Y.; Li, H.; Huang, X. Alumina-coated patterned amorphous silicon as the anode for a lithium-ion battery with high coulombic efficiency. Adv. Mater. 2011, 23, 4938–4941. [Google Scholar] [CrossRef] [PubMed]
- Aghajamali, M.; Xie, H.; Javadi, M.; Kalisvaart, W.P.; Buriak, J.M.; Veinot, J.G.C. Size and Surface Effects of Silicon Nanocrystals in Graphene Aerogel Composite Anodes for Lithium Ion Batteries. Chem. Mater. 2018, 30, 7782–7792. [Google Scholar] [CrossRef]
- Liu, L.; Lyu, J.; Li, T.; Zhao, T. Well-constructed silicon-based materials as high-performance lithium-ion battery anodes. Nanoscale 2016, 8, 701–722. [Google Scholar] [CrossRef] [PubMed]
- Szczech, J.R.; Jin, S. Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 2011, 4, 56. [Google Scholar] [CrossRef]
- Lee, S.W.; McDowell, M.T.; Choi, J.W.; Cui, Y. Anomalous Shape Changes of Silicon Nanopillars by Electrochemical Lithiation. Nano Lett. 2011, 11, 3034–3039. [Google Scholar] [CrossRef]
- Bordes, A.; Vito, E.D.; Haon, C.; Boulineau, A.; Montani, A.; Marcus, P. Multiscale Investigation of Silicon Anode Li Insertion Mechanisms by Time-of-Flight Secondary Ion Mass Spectrometer Imaging Performed on an In Situ Focused Ion Beam Cross Section. Chem. Mater. 2016, 28, 1566–1573. [Google Scholar] [CrossRef]
- Dong, H.; Wang, J.; Ding, H.; Zong, F.F.; Wang, P.; Song, R.; Zhang, N.S.; Cui, X.L.; Cui, X.C.; Li, S.Y. Exploring the practical applications of silicon anodes: A review of silicon-based composites for lithium-ion batteries. Ionics 2022, 28, 3057–3077. [Google Scholar] [CrossRef]
- Johnson, D.C.; Mosby, J.M.; Riha, S.C.; Prieto, A.L. Synthesis of copper silicide nanocrystallites embedded in silicon nanowires for enhanced transport properties. J. Mater. Chem. 2010, 20, 1993–1998. [Google Scholar] [CrossRef]
- Laïk, B.; Ung, D.; Caillard, A.; Cojocaru, C.S.; Pribat, D.; Pereira-Ramos, J.P. An electrochemical and structural investigation of silicon nanowires as negative electrode for Li-ion batteries. J. Solid State Electrochem. 2010, 14, 1835–1839. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, Y.; Wang, L.; Yang, Y. Silicon nanowires coated with copper layer as anode materials for lithium-ion batteries. J. Power Sources 2011, 196, 6657–6662. [Google Scholar] [CrossRef]
- Hu, L.; Wu, H.; Hong, S.S.; Cui, L.; McDonough, J.R.; Bohy, S.; Cui, Y. Si nanoparticle-decorated Si nanowire networks for Li-ion battery anodes. Chem. Commun. 2011, 47, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Park, M.H.; Wang, Y.; Lee, J.Y.; Cho, J. Nanoscale Si coating on the pore walls of SnO2 nanotube anode for Li rechargeable batteries. Chem. Commun. 2010, 46, 622–624. [Google Scholar] [CrossRef] [Green Version]
- Song, T.; Xia, J.; Lee, J.H.; Lee, D.H.; Kwon, M.S.; Choi, J.M.; Wu, J.; Doo, S.K.; Chang, H.; Park, W.I.; et al. Arrays of sealed silicon nanotubes as anodes for lithium ion batteries. Nano Lett. 2010, 10, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jin, Y.; Zhu, B.; Liu, Z.; Xu, D.; Guan, Y.; Sun, M.; Liu, F. Tuning density of Si nanoparticles on graphene sheets in graphene-Si aerogels for stable lithium ion batteries. J. Colloid Interface Sci. 2018, 532, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Liu, X.; Zhao, Q.; Huang, Y.; Huang, Y.; Hu, X. Constructing Three-Dimensional Honeycombed Graphene/Silicon Skeletons for High-Performance Li-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 31879–31886. [Google Scholar] [CrossRef]
- Fang, G.; Deng, X.; Zou, J.; Zeng, X. Amorphous/ordered dual carbon coated silicon nanoparticles as anode to enhance cycle performance in lithium ion batteries. Electrochim. Acta 2019, 295, 498–506. [Google Scholar] [CrossRef]
- Wang, W.; Gu, L.; Qian, H.; Zhao, M.; Ding, X.; Peng, X.; Sha, J.; Wang, Y. Carbon-coated silicon nanotube arrays on carbon cloth as a hybrid anode for lithium-ion batteries. J. Power Sources 2016, 307, 410–415. [Google Scholar] [CrossRef]
- Gong, X.H.; Zheng, Y.B.; Zheng, J.; Cao, S.P.; Wen, H.; Lin, B.P.; Sun, Y.M. Yolk-shell silicon/carbon composites prepared from aluminum-silicon alloy as anode materials for lithium-ion batteries. Ionics 2021, 27, 1939–1948. [Google Scholar] [CrossRef]
- Ji, L.W.; Zhang, X.W. Fabrication of porous carbon/Si composite nanofibers as high-capacity battery electrodes. Electrochem. Commun. 2009, 6, 1146–1149. [Google Scholar] [CrossRef]
- Gao, R.S.; Tang, J.; Zhang, K.; Ozawa, K.; Qin, L.C. A sandwich-like silicon–carbon composite prepared by surface-polymerization for rapid lithium-ion storage. Nano Energy 2020, 78, 105341. [Google Scholar] [CrossRef]
- Lu, Y.H.; Ye, Z.T.; Zhao, Y.T.; Li, Q.; He, M.Y.; Bai, C.C.; Wang, X.T.; Han, Y.L.; Wan, X.C.; Zhang, S.L.; et al. Graphene supported double-layer carbon encapsulated silicon for high-performance lithium-ion battery anode materials. Carbon 2023, 201, 962–971. [Google Scholar] [CrossRef]
- Lee, J.K.; Smith, K.B.; Hayner, C.M.; Kung, H.H. Silicon nanoparticles-graphene paper composites for Li ion battery anodes. Chem. Commun. 2010, 46, 2025–2027. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Kuniyil, M.; Shaik, M.R.; Adil, S.F.; Warthan, A.A.; Alkhahlan, H.A.; Tremel, W.; Tahir, M.N.; Siddiqui, M.R.H. Plant extract mediated eco-friendly synthesis of Pd@graphene nanocatalyst: An efficient and reusable catalyst for the Suzuki-Miyaura coupling. Catalysts 2017, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Al-Marri, A.H.; Mohri, N.; Adil, S.F.; Al-Warthan, A.; Siddiqui, M.R.H.; Alkhathlan, H.Z.; Berger, R.; Tremel, W.; Tahir, M.N. Pulicaria glutinosa plant extract: A green and eco-friendly reducing agent for the preparation of highly reduced graphene oxide. RSC Adv. 2014, 4, 24119. [Google Scholar] [CrossRef]
- Nzabahimana, J.; Chang, P.; Hu, X. Porous carbon-coated ball-milled silicon as high-performance anodes for lithium-ion batteries. J. Mater. Sci. 2018, 54, 4798–4810. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Z.; Cai, T.; Yang, Y.; Wu, H.; Cao, S.; Yang, Z.; Zhang, W. An integrated highly stable anode enabled by carbon nanotube-reinforced all-carbon binder for enhanced performance in lithium-ion battery. Carbon 2021, 182, 749–757. [Google Scholar] [CrossRef]
- Cao, J.; Chen, C.; Zhao, Q.; Zhang, N.; Lu, Q.; Wang, X.; Niu, Z.; Chen, J. A Flexible Nanostructured Paper of a Reduced Graphene Oxide-Sulfur Composite for High-Performance Lithium-Sulfur Batteries with Unconventional Configurations. Adv. Mater. 2016, 28, 9629–9636. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Qi, D.; Yin, S.; Bu, J.; Li, F.; Goh, C.F.; Zhang, S.; Chen, X. Ambient fabrication of large-area graphene films via a synchronous reduction and assembly strategy. Adv. Mater. 2013, 25, 2957–2962. [Google Scholar] [CrossRef] [PubMed]
- Maiti, U.N.; Lim, J.; Lee, K.E.; Lee, W.J.; Kim, S.O. Three-dimensional shape engineered, interfacial gelation of reduced graphene oxide for high rate, large capacity supercapacitors. Adv. Mater. 2014, 26, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, C.; Chen, K.; Lu, Q.; Wang, Q.; Zhou, P.; Liu, D.; Song, L.; Niu, Z.; Chen, J. High-strength graphene composite films by molecular level couplings for flexible supercapacitors with high volumetric capacitance. J. Mater. Chem. A 2017, 5, 15008–15016. [Google Scholar] [CrossRef]
- Li, Q.; Mu, J.; Zhou, J.; Zhao, Y.; Zhuo, S. Avoiding the use of corrosive activator to produce nitrogen-doped hierarchical porous carbon materials for high-performance supercapacitor electrode. J. Electroanal. Chem. 2019, 832, 284–292. [Google Scholar] [CrossRef]
- Chen, W.T.; Muruganantham, R.; Liu, W.R. Construction of 3D porous graphene aerogel wrapped silicon composite as anode materials for high-efficient lithium-ion storage. Surf. Coat. Technol. 2022, 434, 128147. [Google Scholar] [CrossRef]
- Madito, M.J.; Ismail, M.Y.A.; Hlatshwayo, T.T.; Mtshali, C.B. The nature of surface defects in Xe ion-implanted glassy carbon annealed at high temperatures: Raman spectroscopy analysis. Appl. Surf. Sci. 2020, 506, 145001. [Google Scholar] [CrossRef]
- Gao, M.; Tang, Z.; Wu, M.; Chen, J.; Xue, Y.; Guo, X.; Liu, Y.; Kong, Q.; Zhang, J. Self-supporting N, P doped Si/CNTs/CNFs composites with fiber network for high-performance lithium-ion batteries. J. Alloys Compd. 2021, 857, 157554. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Lin, Y.G.; Chiang, C.L.; Liu, W.R. Carbon-coated porous Si/C composite anode materials via two-step etching/coating processes for lithium-ion batteries. Ceram. Int. 2020, 46, 26598–26607. [Google Scholar] [CrossRef]
- He, W.; Luo, H.; Jing, P.; Wang, H.M.; Xu, C.; Wu, H.; Wang, Q.; Zhang, Y. Embedding silicon in biomass-derived porous carbon framework as high performance anode of lithium-ion batteries. J. Alloys Compd. 2022, 15, 165464. [Google Scholar] [CrossRef]
- Chen, S.; Ma, C.; Zhu, Y.; Cao, C. Interpenetrated tunnel routes in silicon/carbon hollow sphere anodes to boost their lithium storage. Mater. Chem. Front. 2020, 4, 2782–2790. [Google Scholar] [CrossRef]
- Xu, H.; Ding, M.; Li, D.; Liu, Y.; Jiang, Y.; Li, F.; Xue, B. Silicon nanoparticles coated with nanoporous carbon as a promising anode material for lithium ion batteries. New J. Chem. 2020, 44, 17323–17332. [Google Scholar] [CrossRef]
- Su, M.; Wang, Z.; Guo, H.; Li, X.; Huang, S.; Xiao, W.; Gan, L. Enhancement of the Cyclability of a Si/Graphite@Graphene composite as anode for Lithium-ion batteries. Electrochim. Acta 2014, 116, 230–236. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, H.; Yang, Y.; Wang, Z.; Li, X.; Zhou, R.; Peng, W. Facile synthesis of silicon/carbon nanospheres composite anode materials for lithium-ion batteries. Mater. Lett. 2016, 168, 138–142. [Google Scholar] [CrossRef]
- Ruffo, R.; Hong, S.S.; Chan, C.K.; Huggins, R.A.; Cui, Y. Impedance Analysis of Silicon Nanowire Lithium Ion Battery Anodes. J. Phys. Chem. C 2009, 113, 11390–11398. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zheng, G.; Liu, N.; Carney, T.J.; Yang, Y.; Cui, Y. Engineering empty space between Si nanoparticles for lithium-ion battery anodes. Nano Lett. 2012, 12, 904–909. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Ge, M.; Zhang, A.; Fang, X.; Liu, Y.; Rong, J.; Zhou, C. Silicon(lithiated)–sulfur full cells with porous silicon anode shielded by Nafion against polysulfides to achieve high capacity and energy density. Nano Energy 2016, 19, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Han, X.; Cheng, F.; Hu, Y.; Chen, C.; Chen, J. Phase and composition controllable synthesis of cobalt manganese spinel nanoparticles towards efficient oxygen electrocatalysis. Nat. Commun. 2015, 6, 7345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Li, W.; Wu, Z.; Li, H.; Zeng, P.; Chen, G.; Chang, B.; Zhang, X.; Wang, X. Si/C composite embedded nano-Si in 3D porous carbon matrix and enwound by conductive CNTs as anode of lithium-ion batteries. Sustain. Mater. Technol. 2022, 32, e00410. [Google Scholar] [CrossRef]
- Yan, M.Y.; Li, G.; Zhang, J.; Tian, Y.F.; Yin, Y.X.; Zhang, C.J.; Jiang, K.C.; Xu, Q.; Li, H.L.; Guo, Y.G. Enabling SiO(x)/C Anode with High Initial Coulombic Efficiency through a Chemical Pre-Lithiation Strategy for High-Energy-Density Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 27202–27209. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Chae, S.; Ma, J.; Ko, M.; Cho, J. Fast-charging high-energy lithium-ion batteries via implantation of amorphous silicon nanolayer in edge-plane activated graphite anodes. Nat. Commun. 2017, 8, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqsed, S.; Almegmadi, F.A.; Mustafa, J.; Husain, S. Effect of nano phase change materials on the cooling process of a triangular lithium battery pack. J. Energy Storage 2022, 51, 104326. [Google Scholar] [CrossRef]
- Xu, S.; Hou, X.; Wang, D.; Zuin, L.; Zhou, J.; Hou, Y.; Mann, M. Insights into the Effect of Heat Treatment and Carbon Coating on the Electrochemical Behaviors of SiO Anodes for Li−Ion Batteries. Adv. Energy Mater. 2022, 12, 2200127. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Chen, H.; Wang, Y.; Xia, L.; Tan, Q.; Li, H.; Zhong, Z.; Su, F.; Zhao, X.S. Growth of silicon/carbon microrods on graphite microspheres as improved anodes for lithium-ion batteries. J. Mater. Chem. A 2013, 1, 4483–4489. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.H.; Yang, Z.; Zhang, M.; Li, Z.L.; Zhao, H.L. Carbon-coated mesoporous silicon shell-encapsulated silicon nanograins for high performance lithium-ion batteries anode. Carbon 2022, 192, 277–284. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Du, N.; Zhu, S.J.; Chen, Y.F.; Lin, Y.F.; Wu, S.L.; Yang, E.E. Porous silicon in carbon cages as high-performance lithium-ion battery anode Materials. Electrochim. Acta 2017, 252, 438–445. [Google Scholar] [CrossRef]
- Almehmadi, F.A.; Alqaed, S.; Mustafa, J.; Jamil, B.; Sharifpur, M.; Cheraghian, G. Combining an active method and a passive method in cooling lithium-ion batteries and using the generated heat in heating a residential unit. J. Energy Storage 2022, 49, 104181. [Google Scholar] [CrossRef]
- Pei, Y.X.; Wang, Y.X.; Chang, A.Y.; Liao, Y.X.; Zhang, S.; Wen, X.F.; Wang, S.N. Nanofiber-in-microfiber carbon/silicon composite anode with high silicon content for lithium-ion batteries. Carbon 2023, 203, 436–444. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Z.; Zhao, X.; Wang, Q.; Bao, T.; Li, H.; Cao, Y.; Zhang, B.; Cao, J.; Si, W. Preparation of a Flexible Reduced Graphene Oxide-Si Composite Film and Its Application in High-Performance Lithium Ion Batteries. Crystals 2023, 13, 547. https://doi.org/10.3390/cryst13030547
Chu Z, Zhao X, Wang Q, Bao T, Li H, Cao Y, Zhang B, Cao J, Si W. Preparation of a Flexible Reduced Graphene Oxide-Si Composite Film and Its Application in High-Performance Lithium Ion Batteries. Crystals. 2023; 13(3):547. https://doi.org/10.3390/cryst13030547
Chicago/Turabian StyleChu, Zhaoyun, Xiangchuan Zhao, Qi Wang, Tianshuang Bao, Hongxiang Li, Yue Cao, Boming Zhang, Jun Cao, and Weimeng Si. 2023. "Preparation of a Flexible Reduced Graphene Oxide-Si Composite Film and Its Application in High-Performance Lithium Ion Batteries" Crystals 13, no. 3: 547. https://doi.org/10.3390/cryst13030547
APA StyleChu, Z., Zhao, X., Wang, Q., Bao, T., Li, H., Cao, Y., Zhang, B., Cao, J., & Si, W. (2023). Preparation of a Flexible Reduced Graphene Oxide-Si Composite Film and Its Application in High-Performance Lithium Ion Batteries. Crystals, 13(3), 547. https://doi.org/10.3390/cryst13030547






