Phase Transition and Energy Storage Density in Lead-Free Ferroelectric Ba1−xSrxTiO3 (x = 0.1, 0.3, and 0.7) Capacitors
Abstract
:1. Introduction
2. Materials and Methods
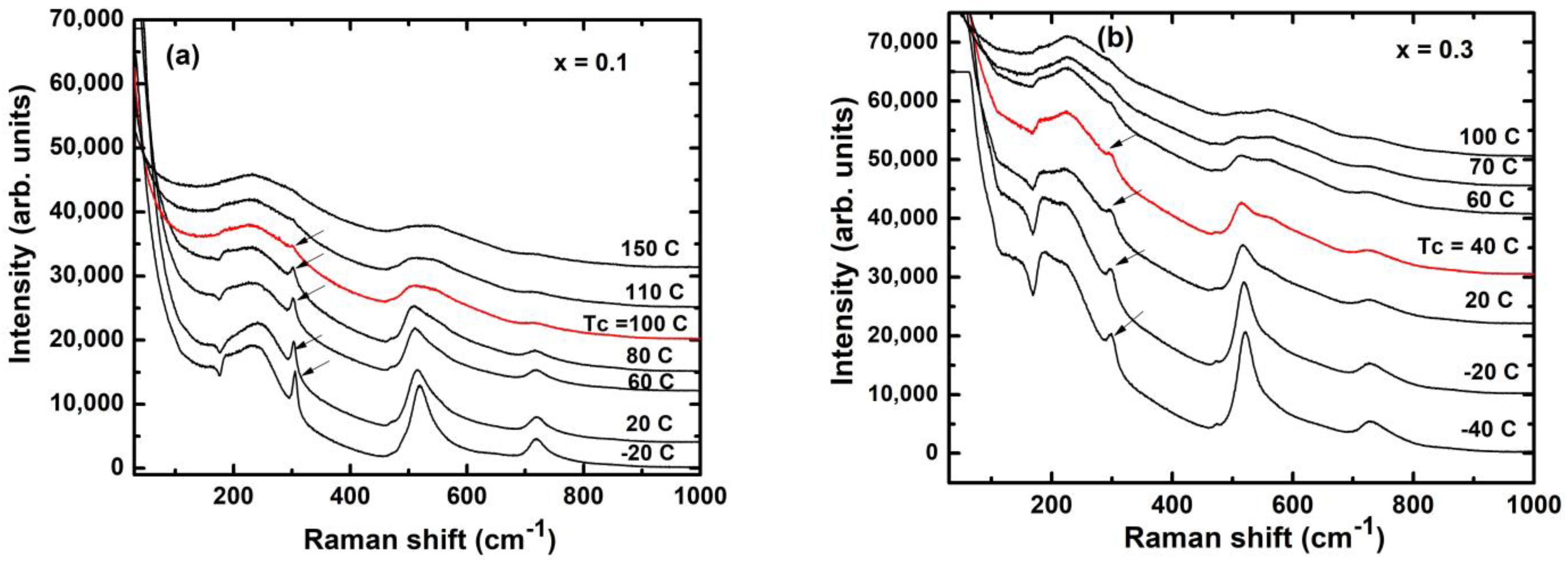
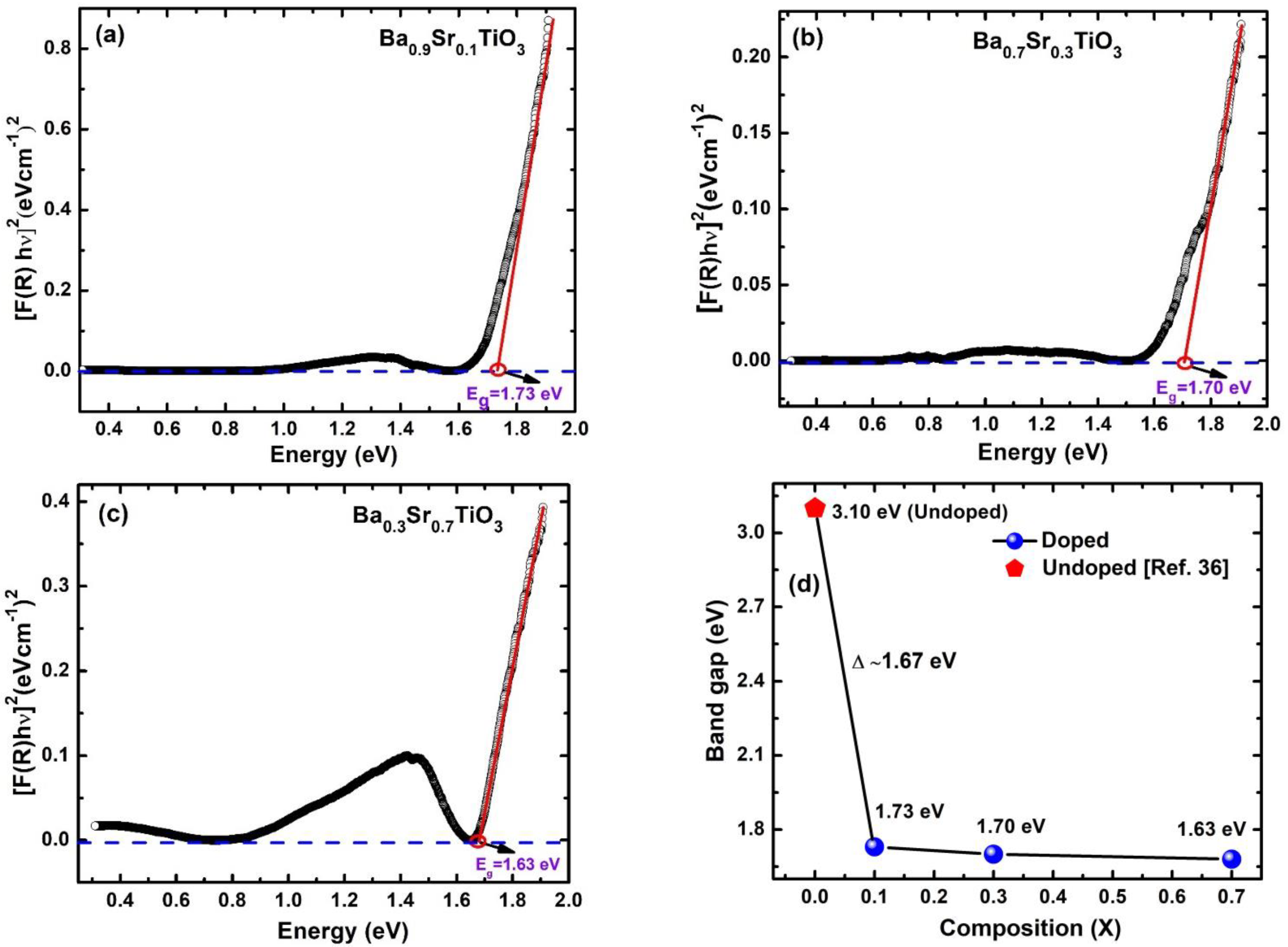
3. Results and Discussion
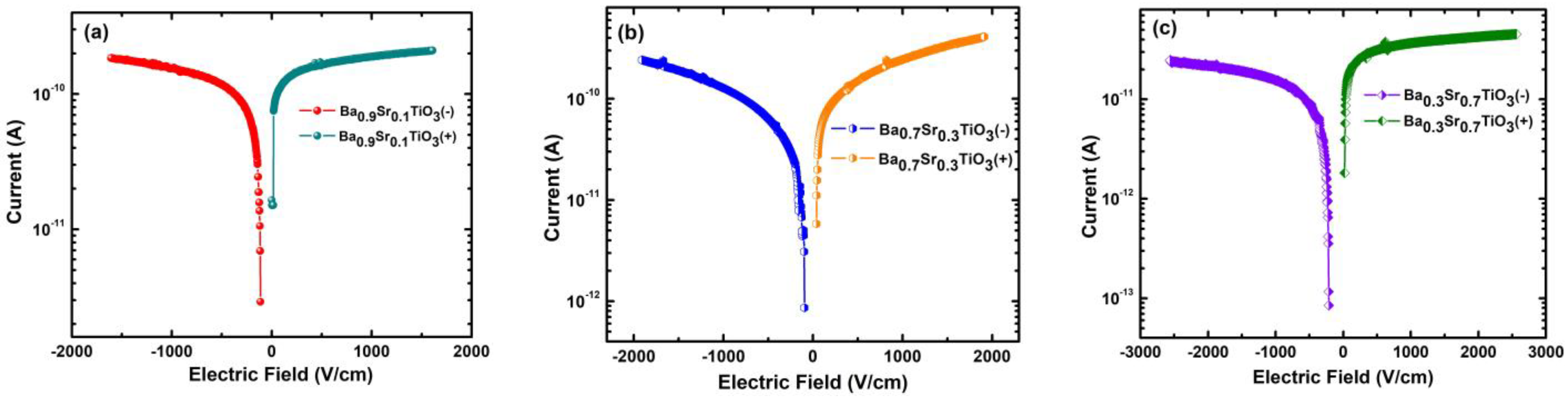
3.1. Current–Voltage (I–V) Behaviors
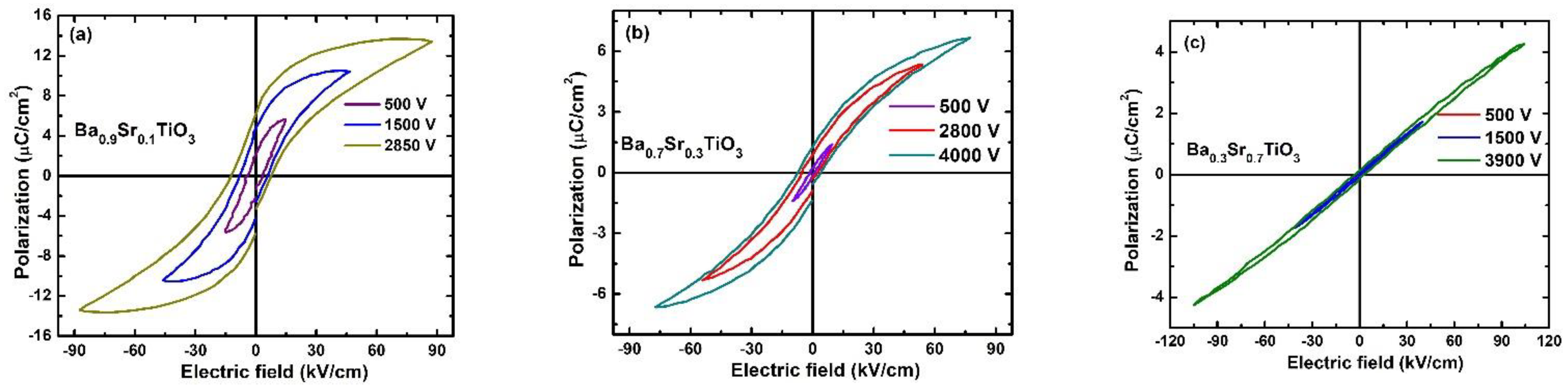
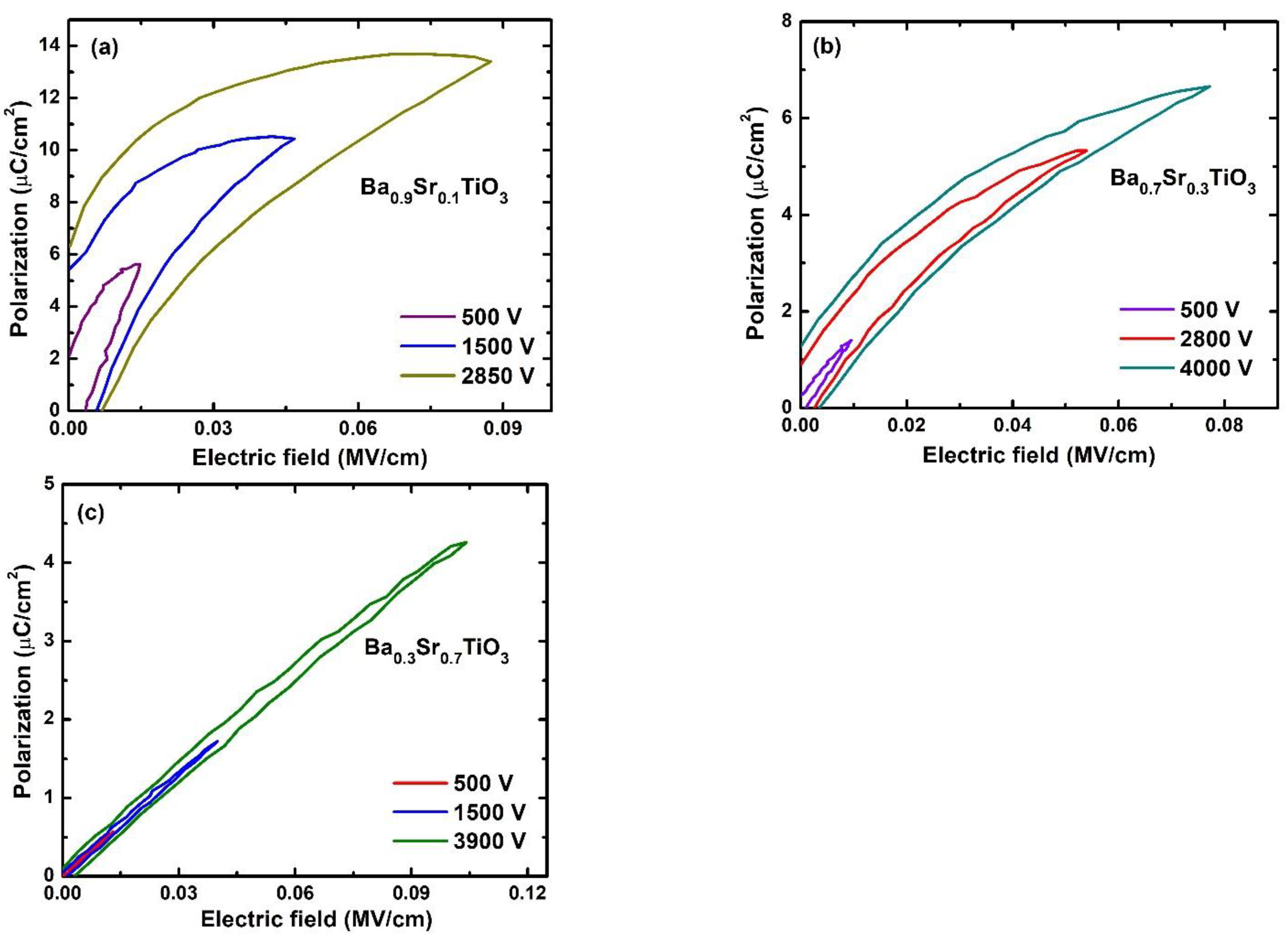
3.2. Ferroelectricity, and Energy Storage Density Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chu, B.; Zhou, X.; Ren, K.; Neese, B.; Lin, M.; Wang, Q.; Bauer, F.; Zhang, Q.M. A Dielectric Polymer with Hight Electric Energy Density and Fast Discharge Speed. Science 2006, 313, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liang, R.; Zhou, Z.; Dong, X. Novel BaTiO3-based lead-free ceramic capacitors featuring high energy storage density, high power density, and excellent stability. J. Mater. Chem. C 2018, 6, 8528–8537. [Google Scholar] [CrossRef]
- Yao, Z.; Song, Z.; Hao, H.; Yu, Z.; Cao, M.; Zhang, S.; Lanagan, M.T.; Liu, H. Homogeneous/Inhomogeneous-Structured Dielectrics and their Energy-Storage Performances. Adv. Mater. 2017, 1601727, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hao, X. A review on the dielectric materials for high energy-storage application. J. Adv. Dielectr. 2013, 3, 1330001. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Z.; Chen, Y.; Huang, W.; Dong, X. A novel lead-free and high-performance barium strontium titanate-based thin film capacitor with ultrahigh energy storage density and giant power density. J. Mater. Chem. C 2020, 50, 50. [Google Scholar] [CrossRef]
- Han, F.; Meng, G.; Zhou, F.; Song, L.; Li, X.; Hu, X.; Zhu, X.; Wu, B.; Wei, B. Dielectric capacitors with three-dimensional nanoscale interdigital electrodes for energy storage. Sci. Adv. 2015, 1, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Liang, R.; Zhou, Z.; Dong, X. Designing lead-free bismuth ferrite-based ceramics learning from relaxor ferroelectric behavior for simultaneous high energy density and efficiency under low electric field. J. Mater. Chem. C 2018, 6, 10211–10217. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, Y.; Liu, N.; Peng, P.; Zhou, M.; Yan, S.; Cao, F.; Dong, X.; Wang, G. Enhanced energy storage properties in sodium bismuth titanate-based ceramics for dielectric capacitor applications. J. Mater. Chem. C 2019, 7, 6222–6230. [Google Scholar] [CrossRef]
- Wei, J.; Yang, T.; Wang, H. Excellent Energy Storage and Charge-discharge Performances in PbHfO3 Antiferroelectric Ceramics. J. Eur. Ceram. Soc. 2019, 39, 624–630. [Google Scholar] [CrossRef]
- Shao, T.; Du, H.; Ma, H.; Qu, S.; Wang, J.; Wang, J.; Wei, X.; Xu, Z. Potassium-Sodium Niobate Based Lead-free Ceramic: Novel Electrical Ennergy Storage Materials. J. Mater. Chem. A 2017, 5, 554–563. [Google Scholar] [CrossRef]
- Yang, H.; Yan, F.; Lin, Y.; Wang, T.; Wang, F.; Yilin, W.; Guo, L.; Tai, W.; Wei, H. Lead-free BaTiO3-Bi0.5Na0.5TiO3-Na0.73Bi0.09NbO3 relaxor ferroelectric ceramics for high energy storage. J. Eur. Ceram. Soc. 2017, 37, 3303–3311. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Xu, Z.; Zhang, S. Multilayer Lead-Free Ceramic Capacitors with Ultrahigh Energy Density and Efficiency. Adv. Mater. 2018, 1802155, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Hu, Z.; Koritala, R.E.; Lee, T.H.; Dorris, S.E.; Balachandran, U. PLZT film capacitors for power electronics and energy storage applications. J. Mater. Sci.: Mater. Electron. 2015, 15, 3025. [Google Scholar] [CrossRef]
- Ozaki, T.; Kitagawa, S.; Nishihara, S.; Hosokoshi, Y.; Suzuki, M.; Noguchi, Y.; Miyayama, M.; Mori, S. Ferroelectric Properties and Nano-Scaled Domain Structures in (1−x)BiFeO. Ferroelectrics 2009, 385, 155–161. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, X. Dielectric properties and energy-storage performances of (1−x)(Na0.5Bi 0.5)TiO3-xSrTiO3 thick films prepared by screen printing technique. J. Alloys Compd. 2014, 586, 674–678. [Google Scholar] [CrossRef]
- Mishra, A.; Majumdar, B.; Ranjan, R. A Complex lead-free (Na, Bi, Ba)(Ti, Fe)O3 single phase perovskite ceramic with a high energy-density and high discharge-efficiency for solid state capacitor applications. J. Eur. Ceram. Soc. 2017, 37, 2379–2384. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.; Patel, S.; Vaish, R.; Bowen, C.R. Anti-Ferroelectric Ceramics for High Energy Density Capacitors. Materials 2015, 8, 8009–8031. [Google Scholar] [CrossRef] [Green Version]
- Dobal, P.S.; Dixit, A.; Katiyar, R.S.; Garcia, D.; Guo, R.; Bhalla, A.S. Micro-Raman study of Ba1−xSrxTiO3 ceramics. J. Raman Spectrosc. 2001, 32, 147–149. [Google Scholar] [CrossRef]
- Naik, R.; Nazarko, J.J. Temperature dependence of the Raman spectra of polycrystalline Ba1−xSixTiO3. Phys. Rev. B 2000, 61, 11367–11372. [Google Scholar] [CrossRef]
- Kamba, S.; Samoukhina, P.; Kadlec, F.; Pokorny, J.; Petzelt, J.; Reaney, I.M.; Wise, P.L. Composition dependence of the lattice vibrations in Srn+1TinO3n+1 Ruddlesden-Popper homologous series. J. Eur. Ceram. Soc. 2003, 23, 2639–2645. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Zeng, L.Z.; Wang, R.P.; Zhu, Y.; Liu, Y.L. Fundamental and second-order Raman spectra of BaTiO3. J. Raman Spectrosc. 1996, 27, 31–34. [Google Scholar] [CrossRef]
- Sood, A.K.; Chandrabhas, N.; Muthu DV, S.; Jayaraman, A. Phonon interference in BaTiO3: High-pressure Raman study. Phys. Rev. B 1995, 51, 8892–8896. [Google Scholar] [CrossRef] [PubMed]
- Sanjurjo, J.A.; Katiyar, R.S.; Porto, S.P.S. Temperature dependece of dipolar modes in ferroelectric BaTi03 by infrared studies. Phys. Rev. B 1980, 22, 2396–2403. [Google Scholar] [CrossRef] [Green Version]
- Maslova, O.A.; Shirokov, F.V.; Yuzyuk, Y.I.; Marssi, M.E.; Jain, M.; Ortega, N.; Katiyar, R.S. Raman spectroscopy study of lattice dynamics of macro-, micro-, and nanostructured barium titanates. Phys. Solid State 2014, 56, 310–316. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, C.J.; Zhou, X.H.; Tang, B.; Zhang, S.R. High-temperature stable dielectrics in Mn-modified (1−x)Bi0.5Na0.5TiO3-xCaTiO3 ceramics. J. Electroceram. 2010, 25, 212–217. [Google Scholar] [CrossRef]
- Mishra, K.K.; Satya, A.T.; Bharathi, A.; Sivasubramanian, V.; Murthy VR, K.; Arora, A.K. Vibrational, magnetic, and dielectric behavior of La-substituted BiFeO3-PbTiO3. J Appl. Phys. 2011, 110, 123529. [Google Scholar] [CrossRef]
- Berbecaru, C.; Alexandru, H.V.; Porosnicu, C.; Velea, A.; Ioachim, A.; Nedelcu, L.; Toacsan, M. Ceramic materials Ba(1−x)SrxTiO3 for electronics-Synthesis and characterization. Thin Solid Films 2008, 516, 8210–8214. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, H.I.; Lee, M.H.; Park, J.S.; Kim, D.J.; Do, D.; Kim, M.H.; Song, T.K.; Kim, W.J. Electrical properties and phase of BaTiO3-SrTiO3 solid solution. Ceram. Int. 2013, 39, S487–S490. [Google Scholar] [CrossRef]
- Wodecka-Dus, B.; Lisinska-Czekaj, A.; Orkisz, T.; Adamzcyk, M.; Osinska, K.; Kozielski, L.; Czekaj, D. The sol-gel synthesis of barium strontium titannate ceramics. Mater. Sci.-Poland 2007, 25, 791–799. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Puli, V.S.; Li, P.; Adireddy, S.; Chrisey, D.B. Crystal structure, dielectric, ferroelectric and energy storage properties of La-doped BaTiO3 semiconducting ceramics. J. Adv. Dielectr. 2015, 5, 1550027. [Google Scholar] [CrossRef]
- Mishra, K.K.; Sivasubramanian, V.; Sarguna, R.M.; Ravindran, T.R.; Arora, A.K. Raman scattering from La-substituted BiFeO3-PbTiO3. J. Solid State Chem. 2011, 184, 2381–2386. [Google Scholar] [CrossRef]
- Mishra, K.K.; Achary, S.N.; Chandra, S.; Ravindran, T.R.; Sinha, A.K.; Singh, M.N.; Tyagi, A.K. Structural and Thermal Properties of BaTe2O6: Combined Variable Temperature Synchrotron X-ray Diffraction, Raman Spectroscopy, and ab Initio Calculations. Inorg. Chem. 2016, 55, 8994–9005. [Google Scholar] [CrossRef] [PubMed]
- Landi, S., Jr.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misue of the Kubelka-function to obtain the band gap ennergy diffuse relectance measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Lopez, R.; Gomez, R. Band-gap energy estimations from diffuse reflectance measurements on sol-gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Boubaia, A.; Assali, A.; Berrah, S.; Bennacer, H.; Zerifi, I.; Boukortt, A. Band gap emission wavelenght tuning of Sr-doped Ba TiO3(BST) perovskites for high-efficiency visible-light emitters and solar cells. Mater. Sci. Semicon. Proc. 2021, 130, 105837. [Google Scholar] [CrossRef]
- Zheng, D.; Deng, H.; Pan, Y.; Guo, Y.; Zhao, F.; Yang, P.; Chu, J. Modified multiferroic properties in narrow bandgap (1−x)BaTiO3-xBaNb1/3Cr2/3O3−δ ceramics. Ceram. Int. 2020, 46, 26823–26828. [Google Scholar] [CrossRef]
- Putra, I.R.; Syafutra, H.; Alatas, H. Development of Ferroelectric Solar Cells of Barium Strontium Titanate (BaxSr1−xTiO3) for Subtituting Conventional Battery in LAPAN-IPB Satellite (LISAT). Procedia Environ. Sci. 2016, 33, 607–614. [Google Scholar]
- Puli, V.S.; Pradhan, D.K.; Adireddy, S.; Martínez, R.; Silwal, P.; Scott, J.F.; Ramana, C.V.; Chrisey, D.B.; Katiyar, R.S. Nanoscale polarisation switching and leakage currents in (Ba0.955Ca0.045)(Zr0.17Ti0.83)O3 epitaxial thin films. J. Phys. D Appl. Phys. 2015, 48, 355502. [Google Scholar] [CrossRef]
- Yun, S.; Wang, X.; Xu, D. Influence of processing parameters on the structure and properties of barium strontium titanate ceramics. Mater. Res. Bull. 2008, 43, 1989–1995. [Google Scholar] [CrossRef]
- Fu, C.; Yang, C.; Chen, H.; Wang, Y.; Hu, L. Microstructure and dielectric properties of BaxSr1−xTiO3 ceramics. Mater Sci Eng. B. 2005, 119, 185–188. [Google Scholar] [CrossRef]
- Sindhu, M.; Ahlawat, N.; Sanghi, S.; Kumari, R.; Agarwal, A. Crystal structure refinement and investigation of electrically heterogeneous microstructure of single phased Sr substituted BaTiO3 ceramics. J. Alloys Compd. 2013, 575, 109–114. [Google Scholar] [CrossRef]
- Liu, X.Q.; Chen, T.T.; Wu, Y.J.; Chen, X.M. Enhanced Electrocaloric Effects in Spark Plasma-Sintered Ba0.65 Sr0.35TiO3-Based Ceramics at Room Temperature. J. Am. Ceram. Soc. 2013, 96, 1021–1023. [Google Scholar] [CrossRef]
- Xu, X.; Hilmas, G.E. Effects of Ba6Ti17O40 on the Dielectric Properties of Nb-Doped BaTiO3 Ceramics. J. Am. Ceram. Soc. 2006, 89, 2496–2501. [Google Scholar] [CrossRef]
- Pontes, F.M.; Leite, E.R.; Longo, E.; Varela, J.A.; Araujo, E.B.; Eiras, J.A. Effects of the postannealing atmosphere on the dieelectric properties of (Ba,Sr)TiO3 capacitors: Evidence of an interfacial space charger layer. Appl. Phys. Lett. 2000, 76, 2433. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Zhang, S.; Liu, H.; Hao, H.; Cao, M.; Li, Q.; Wang, Q.; Yao, Z.; Wang, Z.; Lanagan, M.T. Improved Energy Storage Properties Accompanied by Enhanced Interface Polarizations in Annealed Microwave-Sintered BST. J. Am. Ceram. Soc. 2015, 98, 3212–3222. [Google Scholar] [CrossRef]
- Jin, Q.; Pu, Y.P.; Wang, C.; Gao, Z.Y.; Zheng, H.Y. Enhanced energy storage performance of Ba0.4Sr0.6TiO3 ceramic: Influence of sintering atmosphere. Ceram. Int. 2017, 10, 1016. [Google Scholar]
- Huang, Y.H.; Wu, Y.J.; Qiu, W.J.; Li, J.; Chen, X.M. Enhanced energy storage density of Ba0.4Sr0.6TiO3–MgO composite prepared by spark plasma sintering. J. Eur. Ceram. Soc. 2015, 35, 1469–1476. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Shen, B.; Zhai, J. Property optimization of BST-based composite glass ceramics for energy-storage applications. Ceram. Int. 2014, 40, 2261–2266. [Google Scholar] [CrossRef]
- Fletcher, N.H.; Hilton, A.D.; Ricketts, B.W. Optimization of energy storage density in ceramic capacitors. J. Phys. D: Appl. Phys. 1996, 29, 253–258. [Google Scholar] [CrossRef]
- Song, Z.; Liu, H.; Zhang, S.; Wang, Z.; Shi, Y.; Hao, H.; Cao, M.; Yao, Z.; Yu, Z. Effect of grain size on the energy storage properties of (Ba0.4Sr0.6)TiO3 paraelectric ceramics. J. Eur. Ceram. Soc. 2014, 34, 1209–1217. [Google Scholar] [CrossRef]
- Dai, Z.; Xie, J.; Liu, W.; Wang, X.; Zhang, L.; Zhou, Z.; Li, J.; Ren, X. Effective Strategy to Achieve Excellent Energy Storage Properties in Lead-Free BaTiO3-Based Bulk Ceramics. ACS Appl. Mater. Interfaces 2020, 12, 30289–33029. [Google Scholar] [CrossRef] [PubMed]





| x | c/a | Rp | Rwp | ||||
|---|---|---|---|---|---|---|---|
| 0.1 | 3.9871(1) | 4.0166(1) | 63.851(1) | 1.0073 | 2.57 | 3.99 | 1.55 |
| 0.3 | 3.9673(1) | 3.9822(1) | 62.677(1) | 1.0037 | 2.37 | 3.6 | 1.51 |
| 0.7 | 3.9357(1) | 60.963(2) | - | 1.9 | 2.80 | 1.44 |
| BSTx Sample | Pr (μC/cm2) | Ec (kV/cm) | Ferroelectricity |
|---|---|---|---|
| BST1 | 5.964 | 7.63 | ferroelectric |
| BST3 | 1.279 | 4.0 | ferroelectric |
| BST7 | 0.125 | 2.76 | relaxor-ferroelectric |
| Material Composition | Udis (J/cm3) | η (%) | Electric Field | Worked by |
|---|---|---|---|---|
| Ba0.4Sr0.6TiO3 | 1.15 | 82 | 180 kV/cm | Song et al. [46] |
| Ba0.4Sr0.6TiO3-MgO | 1.5 | - | 300 kV/cm | Huang et al. [48] |
| Ba0.4Sr0.6TiO3 | 0.33 | - | 514.2 kV/cm | Wang et al. [49] |
| Ba0.3Sr0.8TiO3 | 8 | - | 600 kV/cm | Fletcher et al. [50] |
| Ba0.4Sr0.6TiO3 | 1.28 | - | 243 kV/cm | Song et al. [51] |
| 0.9Ba0.65Sr0.35TiO3-0.1Bi(Mg2/3Nb1/3)O3 | 3.34 | 85.7 | 400 kV/cm | Dai et al. [52] |
| Ba0.9Sr0.1TiO3 | 0.11 | 20 | 86.79 kV/cm | Present work |
| Ba0.7Sr0.3TiO3 | 0.14 | 62 | 76.66 kV/cm | Present work |
| Ba0.3Sr0.7TiO3 | 0.20 | 88 | 104.65 kV/cm | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, I.; Mishra, K.K.; Katiyar, R.S. Phase Transition and Energy Storage Density in Lead-Free Ferroelectric Ba1−xSrxTiO3 (x = 0.1, 0.3, and 0.7) Capacitors. Crystals 2023, 13, 630. https://doi.org/10.3390/cryst13040630
Castillo I, Mishra KK, Katiyar RS. Phase Transition and Energy Storage Density in Lead-Free Ferroelectric Ba1−xSrxTiO3 (x = 0.1, 0.3, and 0.7) Capacitors. Crystals. 2023; 13(4):630. https://doi.org/10.3390/cryst13040630
Chicago/Turabian StyleCastillo, Ivan, Karuna Kara Mishra, and Ram S. Katiyar. 2023. "Phase Transition and Energy Storage Density in Lead-Free Ferroelectric Ba1−xSrxTiO3 (x = 0.1, 0.3, and 0.7) Capacitors" Crystals 13, no. 4: 630. https://doi.org/10.3390/cryst13040630






