Impact of Polyamide Surface Preparation on the Formation of Mixed CdS-CdTe Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
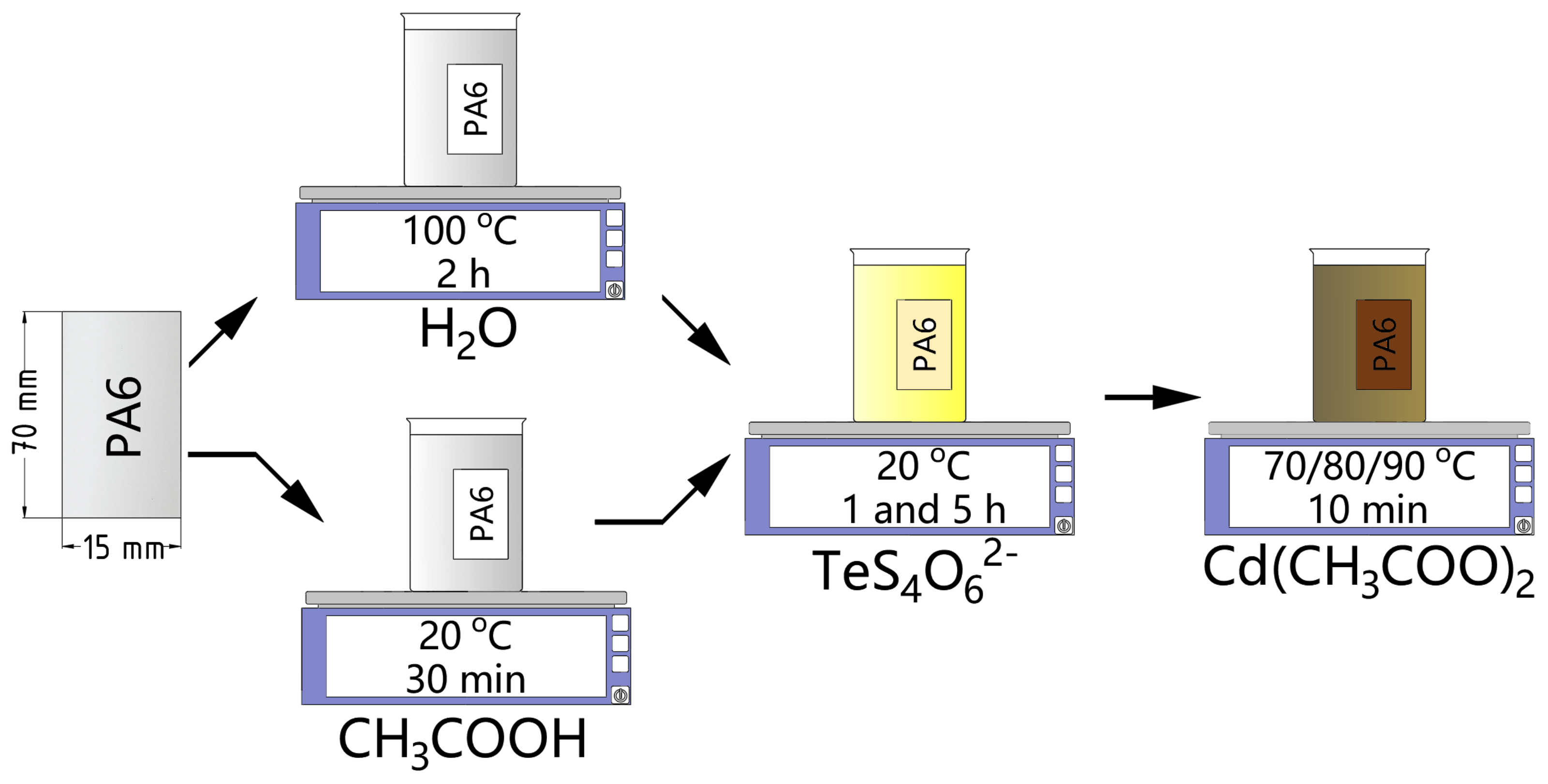
2.2. Synthesis of Cadmium Sulfide—Cadmium Telluride Layers
2.3. SEM/EDX Characterization
2.4. XRD Characterization
2.5. Elemental Composition
2.6. UV-Vis Spectroscopy
3. Results and Discussion
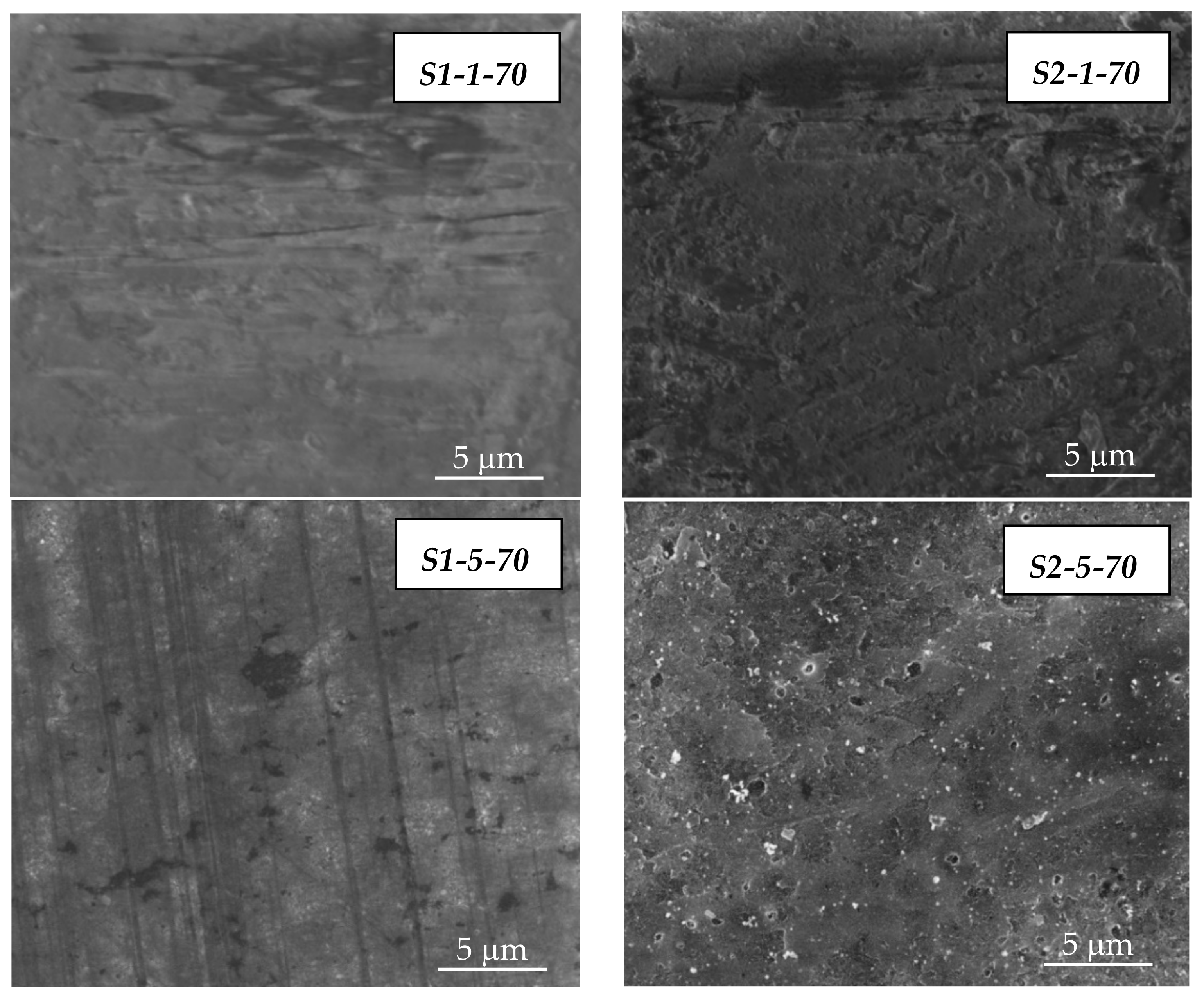
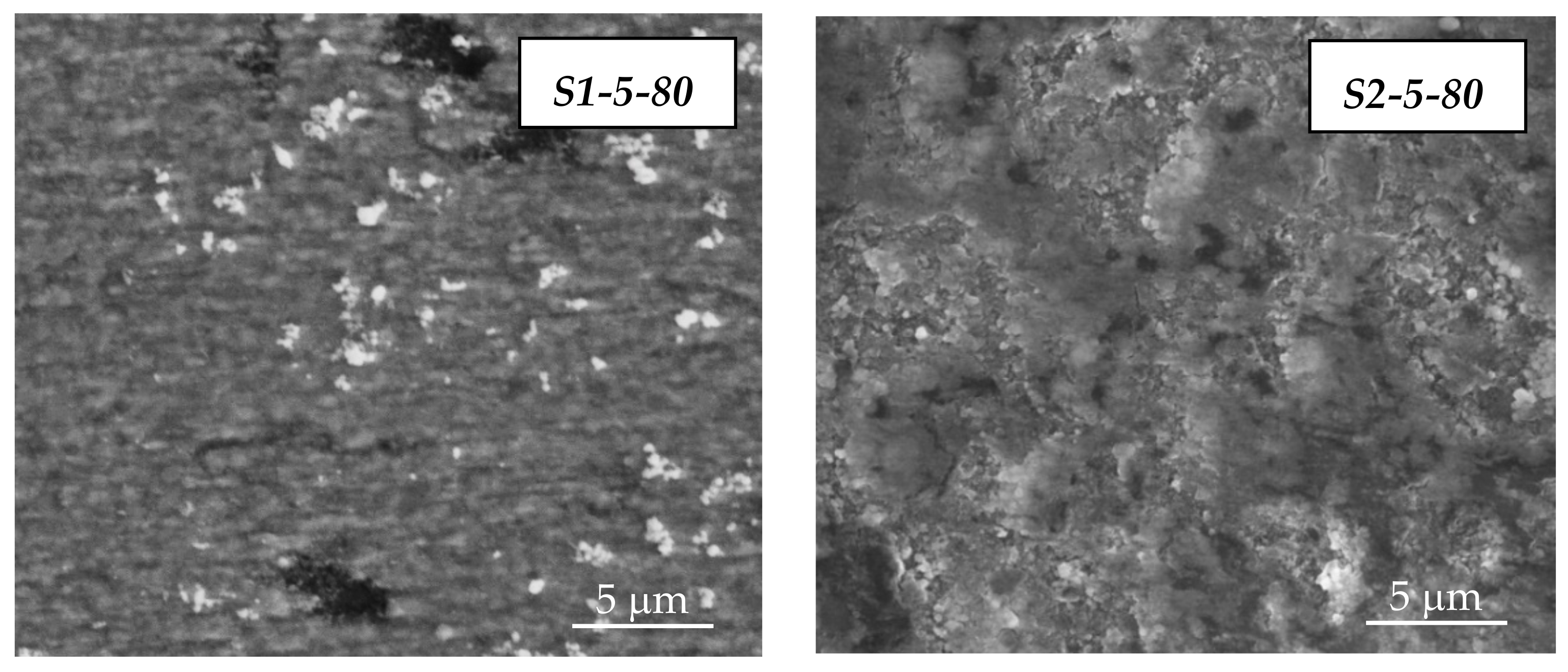
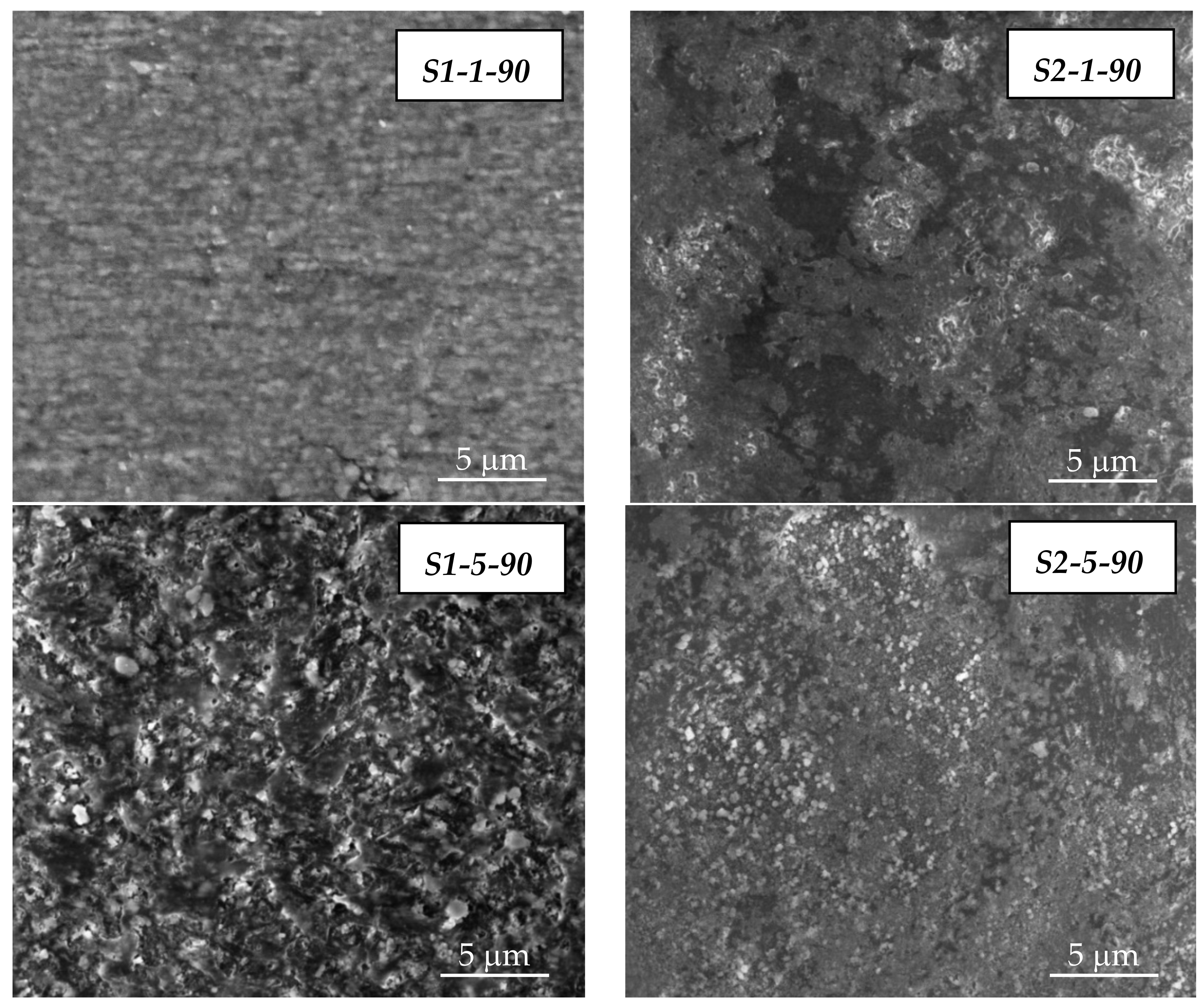
3.1. SEM/EDX Analysis
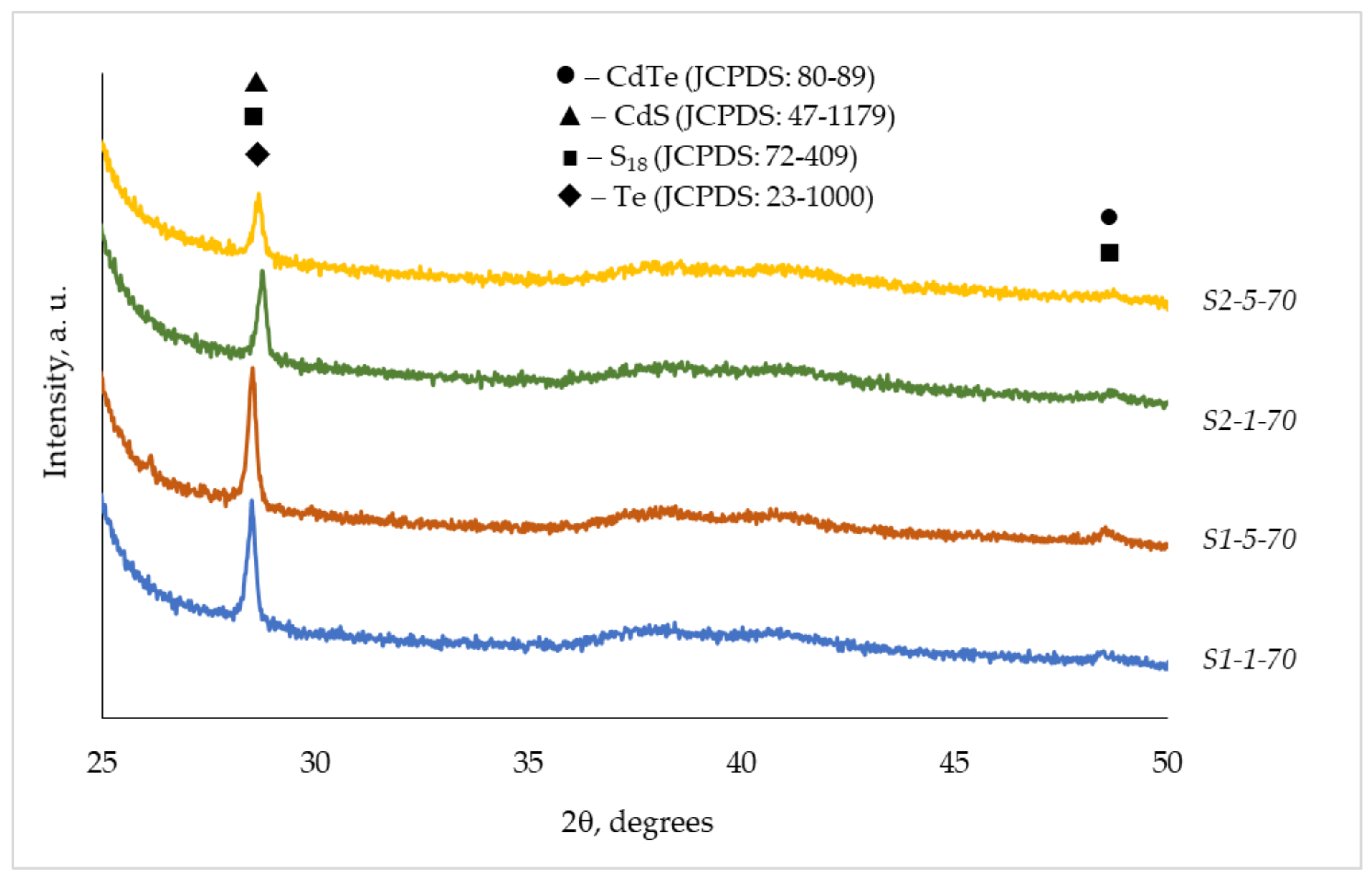
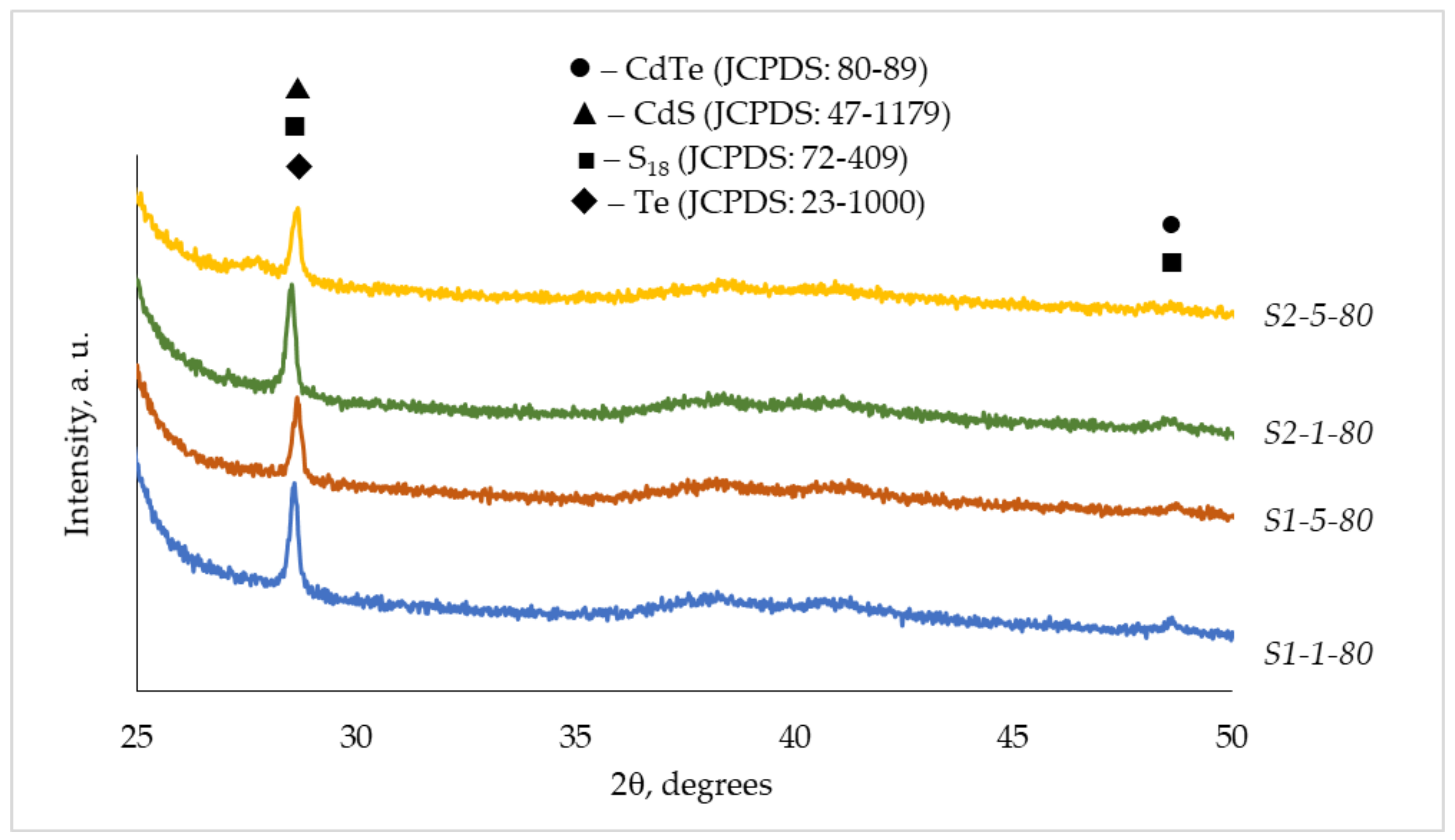
3.2. XRD Analysis
3.3. AAS Analysis
3.4. UV-Vis Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Liu, S.; Mu, Y.; Liu, L.; Runa, R.A.; Su, P.; Yang, J.; Zhu, G.; Fu, W.; Yang, H. Synthesis of Uniform Cadmium Sulphide Thin Film by the Homogeneous Precipitation Method on Cadmium Telluride Nanorods and Its Application in Three-Dimensional Heterojunction Flexible Solar Cells. J. Colloid Interface Sci. 2017, 505, 59–66. [Google Scholar] [CrossRef]
- Dang, H.; Singh, V.P.; Guduru, S.; Hastings, J.T. Embedded Nanowire Window Layers for Enhanced Quantum Efficiency in Window-Absorber Type Solar Cells like CdS/CdTe. Sol. Energy Mater. Sol. Cells 2016, 144, 641–651. [Google Scholar] [CrossRef]
- Stechmann, G.; Zaefferer, S.; Konijnenberg, P.; Raabe, D.; Gretener, C.; Kranz, L.; Perrenoud, J.; Buecheler, S.; Tiwari, A.N. 3-Dimensional Microstructural Characterization of CdTe Absorber Layers from CdTe/CdS Thin Film Solar Cells. Sol. Energy Mater. Sol. Cells 2016, 151, 66–80. [Google Scholar] [CrossRef]
- Ojo, A.A.; Dharmadasa, I.M. 15.3% Efficient Graded Bandgap Solar Cells Fabricated Using Electroplated CdS and CdTe Thin Films. Sol. Energy 2016, 136, 10–14. [Google Scholar] [CrossRef]
- Suchikova, Y.; Kovachov, S.; Bohdanov, I.; Popova, E.; Moskina, A.; Popov, A. Characterization of CdxTeyOz/CdS/ZnO Heterostructures Synthesized by the SILAR Method. Coatings 2023, 13, 639. [Google Scholar] [CrossRef]
- Jain, S.K.; Sharma, G.; Vyas, S. Influence of Hole Interface Layer on the Performance of Cadmium Telluride-Based Thin Film Solar Cell. Mater. Today Proc. 2023, 74, 231–233. [Google Scholar] [CrossRef]
- Himanshu; Patel, S.L.; Thakur, A.; Kannan, M.D.; Dhaka, M.S. Analysis of Different Annealing Conditions on Physical Properties of Bi Doped CdTe Thin Films for Potential Absorber Layer in Solar Cells. Sol. Energy 2020, 199, 772–781. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F. Photoelectric Properties of Highly Conductive Samarium-Doped Cadmium Telluride Thin Films for Photovoltaic Applications. Sol. Energy 2021, 213, 163–171. [Google Scholar] [CrossRef]
- Bosio, A.; Rosa, G.; Romeo, N. Past, Present and Future of the Thin Film CdTe/CdS Solar Cells. Sol. Energy 2018, 175, 31–43. [Google Scholar] [CrossRef]
- Danielson, A.; Reich, C.; Drayton, J.; Bothwell, A.; Shimpi, T.; Sites, J.; Sampath, W. A Comprehensive Material Study of CdSeTe Films Deposited with Differing Selenium Compositions. Thin Solid Films 2023, 768, 139684. [Google Scholar] [CrossRef]
- Alam, A.; Kumar, S.; Singh, D.K. Cadmium Sulphide Thin Films Deposition and Characterization for Device Applications. Mater. Today Proc. 2022, 62, 6102–6106. [Google Scholar] [CrossRef]
- Gangawane, S.A.; Malekar, V.P.; Fulari, V.J. Effect of Electron Irradiation on the Crystallite Size, Grain Size and Band Gap Energy of Electrodeposited Cadmium Sulfide Thin Films. Mater. Today Proc. 2021, 47, 5722–5725. [Google Scholar] [CrossRef]
- Raj, R.; Kumari, N.; Monalisa; Rai, B.C.; Karimi, N.A.; Singh, R.K.; Kr, N. Physical Properties of Quantum Dot Cadmium Sulphide Nanomaterials for Its Applications, Prepared by Low Cost Chemical Method. Mater. Today Proc. 2022, 66, 1750–1755. [Google Scholar] [CrossRef]
- Meysing, D.M.; Reese, M.O.; Warren, C.W.; Abbas, A.; Burst, J.M.; Mahabaduge, H.P.; Metzger, W.K.; Walls, J.M.; Lonergan, M.C.; Barnes, T.M.; et al. Evolution of Oxygenated Cadmium Sulfide (CdS:O) during High-Temperature CdTe Solar Cell Fabrication. Sol. Energy Mater. Sol. Cells 2016, 157, 276–285. [Google Scholar] [CrossRef]
- Sinha, T.; Lilhare, D.; Khare, A. A Review on the Improvement in Performance of CdTe/CdS Thin-Film Solar Cells through Optimization of Structural Parameters. J. Mater. Sci. 2019, 54, 12189–12205. [Google Scholar] [CrossRef]
- Hasaneen, M.F.; Taya, Y.A.; Ali, H.M.; Ahmed, M.R. Optical and Structure Properties of CdTe/CdS Films under Influence of Both CdCl2 Heat Treatment and (O2 + Ar) Atmosphere. Appl. Phys. A Mater. Sci. Process. 2020, 126, 496. [Google Scholar] [CrossRef]
- Himanshu; Patel, S.L.; Agrawal, D.; Chander, S.; Thakur, A.; Dhaka, M.S. Towards Cost Effective Absorber Layer to Solar Cells: Optimization of Physical Properties to Cu Doped Thin CdTe Films. Mater. Lett. 2019, 254, 141–144. [Google Scholar] [CrossRef]
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar Cell Efficiency Tables (Version 57). Prog. Photovolt. Res. Appl. 2021, 29, 3–15. [Google Scholar] [CrossRef]
- Sarkar, K.; Jahan, S.; Dutta, B.; Chatterjee, S.; Gain, S.; Ghosh, S. Effects of Very Thin CdS Window Layer on CdTe Solar Cell. J. Mech. Contin. Math. Sci. 2019, 14, 14–29. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Jiang, W.; Xu, W.; Deng, P.; Deng, J.; Yang, B. Application of Multi-Stage Vacuum Distillation for Secondary Resource Recovery: Potential Recovery Method of Cadmium Telluride Photovoltaic Waste. J. Mater. Res. Technol. 2020, 9, 6977–6986. [Google Scholar] [CrossRef]
- Nykyruy, L.I.; Yavorskyi, R.S.; Zapukhlyak, Z.R.; Wisz, G.; Potera, P. Evaluation of CdS/CdTe Thin Film Solar Cells: SCAPS Thickness Simulation and Analysis of Optical Properties. Opt. Mater. 2019, 92, 319–329. [Google Scholar] [CrossRef]
- Balakhayeva, R.; Akilbekov, A.; Baimukhanov, Z.; Usseinov, A.; Giniyatova, S.; Zdorovets, M.; Vlasukova, L.; Popov, A.I.; Dauletbekova, A. CdTe Nanocrystal Synthesis in SiO2/Si Ion-Track Template: The Study of Electronic and Structural Properties. Phys. Status Solidi 2021, 218, 2000231. [Google Scholar] [CrossRef]
- Yavorskyi, R.S.; Zapukhlyak, Z.R.; Yavorskyi, Y.S.; Nykyruy, L.I. Vapor Phase Condensation for Photovoltaic CdTe Films. Phys. Chem. Solid State 2017, 18, 410–416. [Google Scholar] [CrossRef]
- Han, J.; Jian, Y.; He, Y.; Liu, Y.; Xiong, X.; Cha, L.; Krishnakumar, V.; Schimper, H.J. Nanostructures of CdS Thin Films Prepared by Various Technologies for Thin Film Solar Cells. Mater. Lett. 2016, 177, 5–8. [Google Scholar] [CrossRef]
- Mccandless, B.E.; Moulton, L.V.; Birkmire, R.W. Recrystallization and Sulfur Diffusion in CdCl 2-Treated CdTe/CdS Thin Films. Prog. Photovolt. Res. Appl. 1997, 5, 249–260. [Google Scholar] [CrossRef]
- Compaan, A.D.; Gupta, A.; Lee, S.; Wang, S.; Drayton, J. High Efficiency, Magnetron Sputtered CdS/CdTe Solar Cells. Sol. Energy 2004, 77, 815–822. [Google Scholar] [CrossRef]
- Yimamu, A.U.; Afrassa, M.A.; Dejene, B.F.; Echendu, O.K.; Terblans, J.J.; Swart, H.C.; Motloung, S.J. Electrodeposition of CdTe Thin FIlms Using an Acetate Precursor for Solar Energy Application: The Effect of Deposition Voltage. Mater. Today Commun. 2023, 35, 105673. [Google Scholar] [CrossRef]
- Huang, L.; Wei, Z.L.; Zhang, F.M.; Wu, X.S. Electronic and Optical Properties of CdS Films Deposited by Evaporation. J. Alloys Compd. 2015, 648, 591–594. [Google Scholar] [CrossRef]
- Feldmeier, E.M.; Fuchs, A.; Schaffner, J.; Schimper, H.J.; Klein, A.; Jaegermann, W. Comparison between the Structural, Morphological and Optical Properties of CdS Layers Prepared by Close Space Sublimation and RF Magnetron Sputtering for CdTe Solar Cells. Thin Solid Films 2011, 519, 7596–7599. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, L.; Zhang, Y.; Liu, S.; Liu, G. Fabrication of CdS/CdSe Bilayer Thin Films by Chemical Bath Deposition and Electrodeposition, and Their Photoelectrochemical Properties. Appl. Surf. Sci. 2014, 319, 278–284. [Google Scholar] [CrossRef]
- Han, J.F.; Liao, C.; Cha, L.M.; Jiang, T.; Xie, H.M.; Zhao, K.; Besland, M.P. TEM and XPS Studies on CdS/CIGS Interfaces. J. Phys. Chem. Solids 2014, 75, 1279–1283. [Google Scholar] [CrossRef]
- Kariper, A.; Güneri, E.; Göde, F.; Gümüş, C.; Özpozan, T. The Structural, Electrical and Optical Properties of CdS Thin Films as a Function of PH. Mater. Chem. Phys. 2011, 129, 183–188. [Google Scholar] [CrossRef]
- Mukherjee, A.; Satpati, B.; Bhattacharyya, S.R.; Ghosh, R.; Mitra, P. Synthesis of Nanocrystalline CdS Thin Film by SILAR and Their Characterization. Phys. E Low Dimens. Syst. Nanostruct. 2015, 65, 51–55. [Google Scholar] [CrossRef]
- Enríquez, J.P.; Mathew, X. Influence of the Thickness on Structural, Optical and Electrical Properties of Chemical Bath Deposited CdS Thin Films. Sol. Energy Mater. Sol. Cells 2003, 76, 313–322. [Google Scholar] [CrossRef]
- Schaffner, J.; Motzko, M.; Tueschen, A.; Swirschuk, A.; Schimper, H.J.; Klein, A.; Modes, T.; Zywitzki, O.; Jaegermann, W. 12% Efficient CdTe/CdS Thin Film Solar Cells Deposited by Low-Temperature Close Space Sublimation. J. Appl. Phys. 2011, 110, 064508. [Google Scholar] [CrossRef]
- Olav, F. Salts of Monotelluropentathionic Acid. Acta Chem. Scand. 1949, 3, 708–716. [Google Scholar]
- Zhu, Y.; Li, Z.; Chen, M.; Cooper, H.M.; Lu, G.Q.M.; Xu, Z.P. One-Pot Preparation of Highly Fluorescent Cadmium Telluride/Cadmium Sulfide Quantum Dots under Neutral-PH Condition for Biological Applications. J. Colloid Interface Sci. 2013, 390, 3–10. [Google Scholar] [CrossRef]




| Sample No | Elements | ||
|---|---|---|---|
| Cd at. % | S at. % | Te at. % | |
| S1-1-70 | 52.9 ± 0.1 | 36.4 ± 0.0 | 10.7 ± 0.1 |
| S1-5-70 | 42.1 ± 0.3 | 26.2 ± 0.1 | 31.7 ± 0.3 |
| S1-1-80 | 50.1 ± 0.2 | 21.9 ± 0.1 | 28.0 ± 0.2 |
| S1-5-80 | 38.2 ± 0.6 | 17.2 ± 0.1 | 44.6 ± 0.9 |
| S1-1-90 | 28.0 ± 0.5 | 17.4 ± 0.1 | 54.6 ± 1.2 |
| S1-5-90 | 31.7 ± 0.6 | 16.0 ± 0.1 | 52.3 ± 1.1 |
| S2-1-70 | 25.8 ± 0.1 | 74.1 ± 0.0 | 0.1 ± 0.0 |
| S2-5-70 | 34.2 ± 0.5 | 16.8 ± 0.1 | 49.0 ± 1.0 |
| S2-1-80 | 21.5 ± 0.1 | 77.2 ± 0.1 | 1.3 ± 0.0 |
| S2-5-80 | 35.9 ± 0.6 | 17.4 ± 0.6 | 46.7 ± 1.0 |
| S2-1-90 | 19.6 ± 0.1 | 80.1 ± 0.1 | 0.3 ± 0.0 |
| S2-5-90 | 18.4 ± 0.2 | 29.4 ± 0.1 | 52.2 ± 0.5 |
| Observed Data | CdTe (●) | CdS (▲) | S18 (■) | Te (♦) | ||||
|---|---|---|---|---|---|---|---|---|
| JCPDS: 80-89 | JCPDS: 47-1179 | JCPDS: 72-409 | JCPDS: 23-1000 | |||||
| d (Å) | d (Å) | hkl | d (Å) | hkl | d (Å) | hkl | d (Å) | hkl |
| 3.1209 | 3.1260 | 2 1 4 4 1 2 | 3.1251 | 1 2 2 | 3.1100 | 0 1 2 | ||
| 1.8711 | 1.8702 | 2 0 0 | 1.8698 | 0 1 4 | ||||
| Sample No | Average Crystallite Size, nm | Sample No | Average Crystallite Size, nm |
|---|---|---|---|
| S1-1-70 | 37.8 ± 1.8 | S2-1-70 | 38.2 ± 1.9 |
| S1-5-70 | 36.5 ± 0.7 | S2-5-70 | 39.7 ± 3.5 |
| S1-1-80 | 37.9 ± 1.7 | S2-1-80 | 36.4 ± 0.9 |
| S1-5-80 | 40.1 ± 2.3 | S2-5-80 | 37.6 ± 2.6 |
| S1-1-90 | 39.0 ± 2.9 | S2-1-90 | 42.0 ± 3.1 |
| S1-5-90 | 40.1 ± 3.4 | S2-5-90 | 37.5 ± 2.8 |
| Sample No | Elements | Sample No | Elements | ||||
|---|---|---|---|---|---|---|---|
| Cd, μg/g | S, μg/g | Te, μg/g | Cd, μg/g | S, μg/g | Te, μg/g | ||
| S1-1-70 | 2.65 | 41.54 | 5.20 | S2-1-70 | 5.78 | 44.92 | 8.70 |
| S1-5-70 | 5.94 | 45.54 | 18.41 | S2-5-70 | 11.87 | 69.99 | 11.37 |
| S1-1-80 | 8.03 | 42.41 | 9.34 | S2-1-80 | 7.81 | 43.73 | 10.54 |
| S1-5-80 | 11.50 | 53.85 | 24.37 | S2-5-80 | 31.28 | 107.24 | 77.26 |
| S1-1-90 | 5.09 | 38.56 | 6.51 | S2-1-90 | 10.99 | 59.53 | 23.57 |
| S1-5-90 | 8.12 | 51.38 | 19.52 | S2-5-90 | 26.93 | 105.09 | 62.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liudziute, M.; Zalenkiene, S.; Ivanauskas, R.; Ancutiene, I. Impact of Polyamide Surface Preparation on the Formation of Mixed CdS-CdTe Layers. Crystals 2023, 13, 730. https://doi.org/10.3390/cryst13050730
Liudziute M, Zalenkiene S, Ivanauskas R, Ancutiene I. Impact of Polyamide Surface Preparation on the Formation of Mixed CdS-CdTe Layers. Crystals. 2023; 13(5):730. https://doi.org/10.3390/cryst13050730
Chicago/Turabian StyleLiudziute, Migle, Skirma Zalenkiene, Remigijus Ivanauskas, and Ingrida Ancutiene. 2023. "Impact of Polyamide Surface Preparation on the Formation of Mixed CdS-CdTe Layers" Crystals 13, no. 5: 730. https://doi.org/10.3390/cryst13050730
APA StyleLiudziute, M., Zalenkiene, S., Ivanauskas, R., & Ancutiene, I. (2023). Impact of Polyamide Surface Preparation on the Formation of Mixed CdS-CdTe Layers. Crystals, 13(5), 730. https://doi.org/10.3390/cryst13050730







