The Influence of Different Types of SiO2 Precursors and Ag Addition on the Structure of Selected Titania-Silica Gels
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. FTIR Spectroscopy Studies
3.2. X-ray Diffraction Studies
3.3. SEM Studies
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.E.L.; Zhang, R.; Liu, Z. The effect of SiO2 on TiO2-SiO2 composite film for self-cleaning application. Surf. Interfaces 2019, 16, 194–198. [Google Scholar] [CrossRef]
- Vishwas, M.; Narasimha Rao, K.; Arjuna Gowda, K.V.; Chakradhar, R.P.S. Optical, electrical and dielectric properties of TiO2–SiO2 films prepared by a cost effective sol–gel process. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 83, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, A.; Rokita, M. The structural studies of Ag containing TiO2-SiO2 gels and thin films deposited on steel. J. Mol. Str. 2016, 1114, 171–180. [Google Scholar] [CrossRef]
- Houmard, M.; Riassetto, D.; Roussel, F.; Bourgeois, A.; Berthomé, G.; Joud, J.C.; Langlet, M. Morphology and natural wettability properties of sol-gel derived TiO2-SiO2 composite thin films. Appl. Surf. Sci. 2007, 254, 1405–1414. [Google Scholar] [CrossRef]
- Eldural, B.; Bolukbasi, U.; Karakas, G. Photocatalytic antibacterial activity of TiO2–SiO2 thin films. J. Photochem. Photobiol. A Chem. 2014, 283, 29–37. [Google Scholar] [CrossRef]
- Khosravi, H.S.; Veerapandiyan, V.K.; Vallant, R.; Reichmann, K. Effect of processing conditions on the structural properties and corrosion behavior of TiO2–SiO2 multilayer coatings derived via the sol-gel method. Ceram. Intern. 2020, 46, 17741–17751. [Google Scholar] [CrossRef]
- Krishna, V.; Padmapreetha, R.; Chandrasekhar, S.B.; Murugan, K.; Johnson, R. Oxidation resistant TiO2–SiO2 coatings on mild steel by sol–gel. Surf. Coat. Technol. 2019, 378, 125031. [Google Scholar] [CrossRef]
- Sun, B.; Sun, S.Q.; Li, T.; Zhang, W.Q. Preparation and antibacterial activities of Ag-doped SiO2–TiO2 composite films by liquid phase deposition (LPD) method. J. Mater. Sci. 2007, 42, 10085–10089. [Google Scholar] [CrossRef]
- Kawashita, M.; Tsuneyama, S.; Miyaji, F.; Kokubo, T.; Kozuka, H.; Yamamoto, K. Antibacterial silver-containing silica glass prepared by sol–gel method. Biomaterials 2000, 213, 93–398. [Google Scholar] [CrossRef]
- Schierholz, J.M.; Lucas, L.J.; Rump, A.; Pulverer, G. Efficacy of silver-coated medical devices. J. Hosp. Infect. 1998, 40, 257–262. [Google Scholar] [CrossRef]
- Abd Aziz, R.; Sopyan, I. Synthesis of TiO2-SiO2 powder and thin film photocatalysts by sol-gel method. Indian J. Chem. 2009, 48A, 951–957. [Google Scholar]
- Zhai, J.; Zhang, L.; Yao, X. Efects of composition and temperature on gel-formed TiO2/SiO2 films. J. Non-Cryst. Solids 1999, 260, 160–163. [Google Scholar] [CrossRef]
- Cheng, X.M.; NIE, B.M.; Kumar, S. Preparation and bioactivity of SiO2 functional films on titanium by PACVD. Trans. Nonferrous Met. Soc. China 2008, 18, 627–630. [Google Scholar] [CrossRef]
- Fatimah, I.; Prakoso, N.I.; Sahroni, I.; Musawwa, M.M.; Sim, Y.L.; Kooli, F.; Muraza, O. Physicochemical characteristics and photocatalytic performance of TiO2/SiO2 catalyst synthesized using biogenic silica from bamboo leaves. Heliyon 2019, 5, e02766. [Google Scholar] [CrossRef] [PubMed]
- Bulla, D.A.P.; Morimoto, N.I. Deposition of thick TEOS PECVD silicon oxide layers for integrated optical waveguide applications. Thin Solid Film. 1998, 334, 60–64. [Google Scholar] [CrossRef]
- Choi, D.G.; Yang, S.M. Effect of two-step sol-gel reaction on the mesoporous silica structure. J. Colloid. Interface Sci. 2003, 26, 127–132. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Luo, Z.; Zhang, Q.; Wang, P.; Liang, X.; Li, C.; Chen, J. Effects of dimethyldiethoxysilane addition on the sol–gel process of tetraethylorthosilicate. J. Non-Cryst. Solids 2007, 353, 321–326. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Sun, Y. Sol-gel of methyl modified optical silica coatings and gels from DDS and TEOS. J. Sol-Gel Sci. Technol. 2005, 33, 19–24. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Yang, H.; Luo, Z.; Lv, W.; Li, C.; Qing, S. Spherical particles from tetraorthosilicate (TEOS) sol–gel process with dimethyldiethoxysilane (DDS) and diphenyldiethoxysilane (DPDS) addition. J. Non-Cryst. Solids 2008, 354, 5047–5052. [Google Scholar] [CrossRef]
- Peter, A.; Mihaly-Cozmuta, L.; Mihaly-Cozmuta, A.; Nicula, C.; Cadar, C.; Jastrzębska, A.; Kurtycz, P.; Olszyna, A.; Vulpoi, A.; Danciu, V.; et al. Silver functionalized titania-silica xerogels: Preparation, morphostructural and photocatalytic properties, kinetic modeling. J. Alloys Compd. 2015, 648, 890–902. [Google Scholar] [CrossRef]
- Massa, M.A.; Covarrubias, C.; Bittner, M.; Fuentevilla, I.A.; Capetillo, P.; Von Marttens, A.; Carvajal, J.C. Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Mater. Sci. Eng. 2014, C45, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Handke, M.; Mozgawa, W.; Nocuń, M. Specific features of the IR spectra of silicate glasses. J. Mol. Str. 1994, 325, 129–136. [Google Scholar] [CrossRef]
- Adamczyk, A. The influence of ZrO2 precursor type on the structure of ZrO2-TiO2-SiO2 gels and selected thin films. J. Mol. Str. 2018, 1171, 706–771. [Google Scholar] [CrossRef]
- Langauer-Lewowicka, H.; Pawlas, K. Nanocząstki, nanotechnologia—Potencjalne zagrożenia środowiskowe i zawodowe. Med. Sr.-Environ. Med. 2014, 17, 7–14. [Google Scholar]


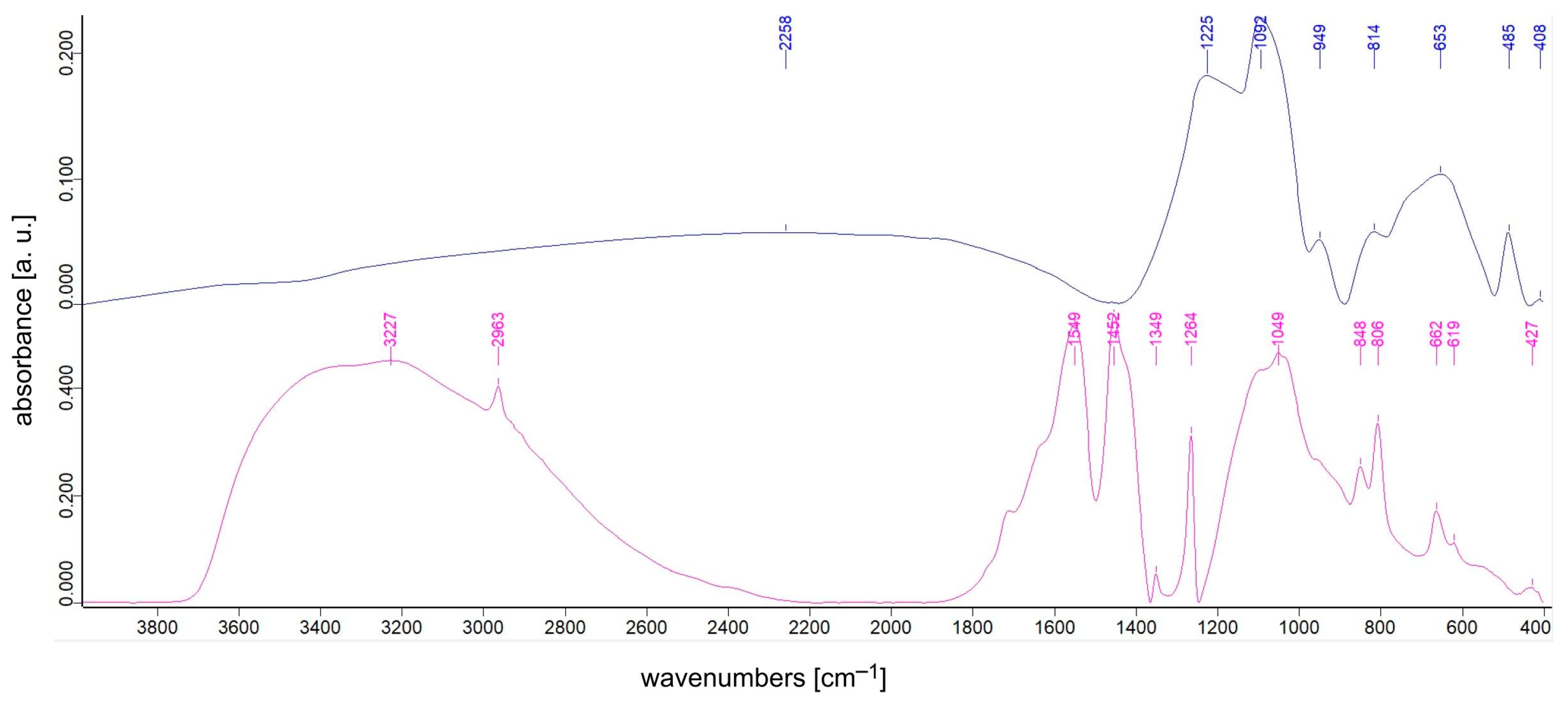
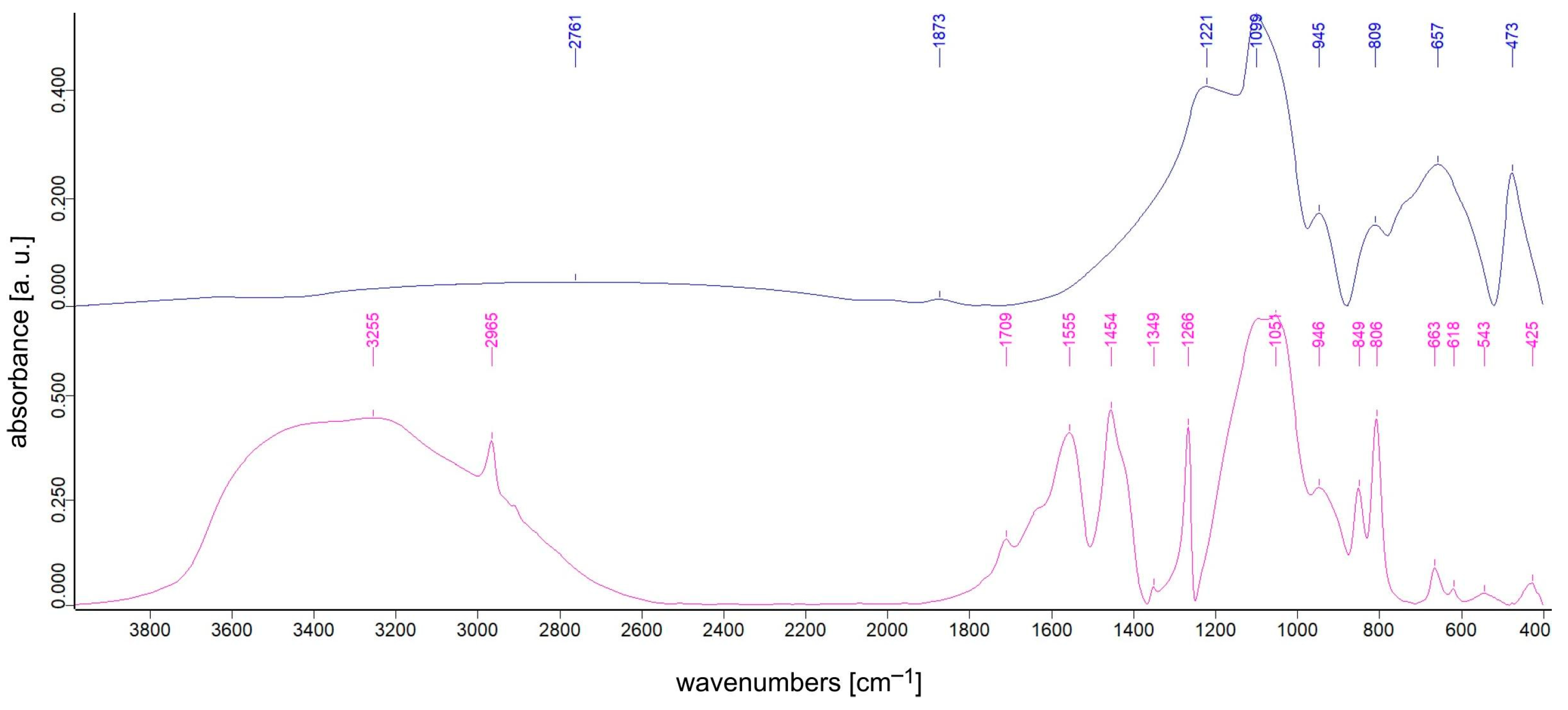










| Sample | TiO2 Content [% mol] | SiO2 Content [% mol] | TEOS:DDS Ratio [mol] | Ag Addition Ag:Si Ratio [mol] |
|---|---|---|---|---|
| TiO2:SiO2 = 1:2 | 33.3 | 66.7 | - | - |
| TiO2:SiO2 = 1:3 | 25 | 75 | - | - |
| TiO2:SiO2 = 1:2 | 33.3 | 66.7 | 1:1 | - |
| TiO2:SiO2 = 1:3 | 25 | 75 | 1:1 | - |
| TiO2:SiO2 = 1:3 + Ag | 25 | 75 | - | 1:24 |
| TiO2:SiO2 = 1:3 + Ag | 25 | 75 | 1:1 | 1:24 |
| Sample | Peak Position 2 θ [°] | βobs (FWHM) [°] | βstand [°] | Dhkl [nm] |
|---|---|---|---|---|
| TiO2:SiO2 = 1:3 + Ag synthesized with TEOS | 38.169 | 0.740 | 0.057 | 12.3 |
| 44.358 | 0.925 | 0.061 | 9.9 | |
| 64.489 | 0.855 | 0.078 | 12.1 | |
| TiO2:SiO2 = 1:3 + Ag synthesized with TEOS:DDS = 1 | 38.076 | 0.725 | 0.057 | 12.6 |
| 44.215 | 0.844 | 0.061 | 11.0 | |
| 64.395 | 0.803 | 0.078 | 12.9 |
| Sample | Peak Position 2 θ [°] | βobs (FWHM) [°] | βstand [°] | Dhkl [nm] |
|---|---|---|---|---|
| TiO2:SiO2 = 1:3 synthesized with TEOS | 25.401 | 0.801 | 0.054 | 10.9 |
| TiO2:SiO2 = 1:3 + Ag synthesized with TEOS | 25.425 | 0.525 | 0.054 | 17.3 |
| TiO2:SiO2 = 1:3 synthesized with TEOS:DDS = 1 | 25.383 | 0.378 | 0.054 | 25.1 |
| TiO2:SiO2 = 1:3 + Ag synthesized with TEOS:DDS = 1 | 25.350 | 0.456 | 0.054 | 20.3 |
| Sample | Peak Position 2 θ [°] | βobs (FWHM) [°] | βstand [°] | Dhkl [nm] |
|---|---|---|---|---|
| TiO2:SiO2 = 1:3 synthesized with TEOS | - | - | - | - |
| TiO2:SiO2 = 1:3 + Ag synthesized with TEOS | 27.554 | 0.293 | 0.058 | 34.4 |
| TiO2:SiO2 = 1:3 synthesized with TEOS:DDS = 1 | 27.504 | 0.276 | 0.058 | 37.0 |
| TiO2:SiO2 = 1:3 + Ag synthesized with TEOS:DDS = 1 | 27.463 | 0.339 | 0.066 | 28.8 |
| Sample | Peak Position 2 θ [°] | βobs (FWHM) [°] | βstand [°] | Dhkl [nm] |
|---|---|---|---|---|
| TiO2:SiO2 = 1:3 synthesized with TEOS | 22.059 | 0.328 | 0.054 | 29.5 |
| TiO2:SiO2 = 1:3 + Ag synthesized with TEOS | 21.877 | 0.655 | 0.054 | 13.5 |
| TiO2:SiO2 = 1:3 synthesized with TEOS:DDS = 1 | - | - | - | - |
| TiO2:SiO2 = 1:3 + Ag synthesized with TEOS:DDS = 1 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, A. The Influence of Different Types of SiO2 Precursors and Ag Addition on the Structure of Selected Titania-Silica Gels. Crystals 2023, 13, 811. https://doi.org/10.3390/cryst13050811
Adamczyk A. The Influence of Different Types of SiO2 Precursors and Ag Addition on the Structure of Selected Titania-Silica Gels. Crystals. 2023; 13(5):811. https://doi.org/10.3390/cryst13050811
Chicago/Turabian StyleAdamczyk, Anna. 2023. "The Influence of Different Types of SiO2 Precursors and Ag Addition on the Structure of Selected Titania-Silica Gels" Crystals 13, no. 5: 811. https://doi.org/10.3390/cryst13050811
APA StyleAdamczyk, A. (2023). The Influence of Different Types of SiO2 Precursors and Ag Addition on the Structure of Selected Titania-Silica Gels. Crystals, 13(5), 811. https://doi.org/10.3390/cryst13050811







