Abstract
BaSixO1+2x (1.61 ≤ x ≤ 1.90) and LiF-doped BaSi1.63O4.26 ceramics were prepared by using a traditional solid-state method at the optimal sintering temperatures. The evolution of phase compositions of BaSixO1+2x (1.61 ≤ x ≤ 1.9) ceramics was revealed. The coexistence of Ba5Si8O21 and Ba3Si5O13 phases was obtained in BaSixO1+2x (1.61 ≤ x ≤ 1.67) ceramics. The BaSi2O5 phase appeared inBaSixO1+2x (1.68 ≤ x ≤ 1.90) ceramics. At 1.68 ≤ x ≤ 1.69, only BaSi2O5 and Ba3Si5O13 phases existed. With the further increase in x, the Ba5Si8O21 phase appeared, and BaSi2O5, Ba5Si8O21 and Ba3Si5O13 phases coexisted in BaSixO1+2x (1.70 ≤ x ≤ 1.90) ceramics. The phase compositions of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics were controlled by the ratio of Ba:Si. The BaSixO1+2x (x = 1.68) ceramics with 98.15 wt% Ba3Si5O13 and 1.85 wt% BaSi2O5 phases exhibited a negative τf value (−37.53 ppm/°C), and the good microwave dielectric properties of εr = 7.51, Q × f = 13,038 GHz and τf = +3.95 ppm/°C were obtained for BaSi1.63O4.26 ceramics with 70.05 wt% Ba5Si8O21 and 29.95 wt% Ba3Si5O13 phases. The addition of LiF sintering aids were able to reduce the sintering temperatures of BaSi1.63O4.26 ceramics to 800 °C. The phase composition of BaSi1.63O4.26 ceramics was affected by the sintering temperature, and the coexistence of Ba5Si8O21, Ba2Si3O8, BaSi2O5 and SiO2 phases was achieved in BaSi1.63O4.26-3 wt% LiF ceramics. The BaSi1.63O4.26-3 wt% LiF ceramics sintered at 800 °C exhibited dense microstructures and excellent microwave dielectric properties (εr = 7.10, Q × f = 12,463 GHz and τf = +5.75 ppm/°C), and no chemical reaction occurred between BaSi1.63O4.26-3 wt% LiF ceramics and the Ag electrodes, which indicates their potential for low-temperature co-fired ceramic (LTCC) applications.
1. Introduction
The rapid development of telecommunication has promoted the high demand for multi-layer devices and microwave dielectric ceramics [1,2,3,4,5]. Barium silicates with excellent luminescence and dielectric properties are widely used in optical glasses and microwave dielectric ceramics [6,7,8,9,10,11,12]. The low cost and free of variable valence elements of barium silicates indicated their great application potential, especially in communication applications [13,14]. The Si–O bond was also strong because it is approximately 55% covalent and 45% ionic [15,16,17]. Barium silicates with high contents of SiO4 tetrahedra and Si–O bonds demonstrated their low permittivity (εr < 15) and high-quality factor (Q × f), which attracted considerable attention regarding microwave dielectric materials [18,19,20,21,22]. However, 13 crystalline phases were known in the BaO–SiO2 system [23], which indicates that the complex phase compositions might exist in barium silicates. Ambiguous phase compositions might limit the application of barium silicates in microwave dielectric materials.
The microwave dielectric properties of barium silicates were first reported by Wen Lei et al. [9], and a BaSi2O5 ceramic with excellent microwave dielectric properties (εr = 6.7, Q × f = 59,500 GHz and τf = −28.0 ppm/°C) was synthesised through sintering at 1250 °C. Enzhu Li et al. pointed out that the synthesis temperature of a single-phase BaSi2O5 ceramic was more than 1100 °C, and the coexistence of BaSi2O5, Ba5Si8O21 (BaSi1.6O4.2) and SiO2 phases was identified in BaSi2O5 ceramics. The Ba5Si8O21 phases existed in BaSi2O5-2 wt% Li2O–B2O3–CaO–CuO glass ceramics when their sintering temperature was below 800 °C, which means that the Ba5Si8O21 phase was easier to synthesise than the BaSi2O5 phase [10]. A single-phase BaSi2O5 ceramic sintered at 1225 °C was prepared by Yun Zhang [11]. The Ba5Si8O21 ceramic with a stable phase composition and novel positive temperature coefficient of the resonance frequency (τf) was used as a τf regulator to control the negative τf values of many low-εr microwave dielectric ceramics [12]. The Ba5Si8O21 ceramics always exhibited the Ba5Si8O21 phase at a high sintering temperature (above 800 °C). However, the Ba3Si5O13 (BaSi1.667O4.334) ceramic, as an intermediate compound between Ba5Si8O21 (BaSi1.6O4.2) and BaSi2O5, exhibited the highest structural and topological complexity in barium silicates [8]. The Ba3Si5O13 (BaSi1.667O4.334) ceramics sintering at different temperatures exhibited the opposite τf values (+37.0 ppm/°C sintering at 1200 °C and −36.0 ppm/°C sintering at 1250 °C) and different phase compositions (Ba5Si8O21 and BaSi2O5 phases sintered at 1200 °C and Ba3Si5O13 phase sintered at 1250 °C) [9]. Toshihiro Moriga et al. pointed out that Ba3Si5O13 single-phase ceramics sintered at 1000 °C could be synthesised at stoichiometric ratios [7]. The small difference in the Ba:Si ratios of the Ba5Si8O21, Ba3Si5O13 and BaSi2O5 phases caused difficulty in synthesizing Ba3Si5O13 ceramics.
Considering the excellent application potential of barium silicate ceramics in LTCC technology, the variation in the phase compositions of BaSixO1+2x ceramics with stoichiometric ratios and sintering temperatures needs to be investigated. This study aimed to clarify the phase compositions of BaSixO1+2x (1.61 ≤ x ≤ 1.9) ceramics with different stoichiometric ratios and investigate the evolution of their phase compositions on microwave dielectric properties. Simultaneously, high-performance BaSixO1+2x-based LTCC materials with LiF doped were prepared.
2. Materials and Methods
BaSixO1+2x (1.61 ≤ x ≤ 1.9)- and LiF-doped BaSixO1+2x ceramics were prepared using the solid reaction method. BaCO3 (99.9%), SiO2 (99.9%) and LiF (99.9%) were weighed according to the stoichiometric formulation of BaSixO1+2x (1.61 ≤ x ≤ 1.9) and ground in deionised water for 5 h. After drying, BaSixO1+2x-prepared powders were calcined at 1050 °C for 5 h to obtain barium silicates. After being re-milled for 5 h and dryed, the as-prepared powders together with 8 wt% PVA were pressed into cylindrical samples. The BaSixO1+2x samples were then sintered at 1300–1325 °C for 5 h. The BaSixO1+2x calcined powders and LiF were weighed according to the mass percentage formulation of BaSixO1+2x-y wt% LiF (1 ≤ y ≤ 3), which were sintered at 800–1025 °C for 3 h.
The sintered samples of BaSixO1+2x and BaSixO1+2x-y wt% LiF (1 ≤ y ≤ 3) ceramics were broken up and ground into powders. The phase and crystal structure of powders were obtained using an X-ray diffractometer (X’Pert PRO). The Rietveld refinement of samples was conducted via Fullprof software [24]. The polished surfaces of samples were observed via scanning electron microscopy (SEM; Sirion 200) after thermal etching. The microwave dielectric properties of as-sintered BaSixO1+2x (1.61 ≤ x ≤ 1.9) and BaSixO1+2x-y wt% LiF (1 ≤ y ≤ 3) ceramics were measured in the range of 10–14 GHz by employing the Hakki–Coleman methods with a network analyser (Keysight E5063A) [25].
3. Results
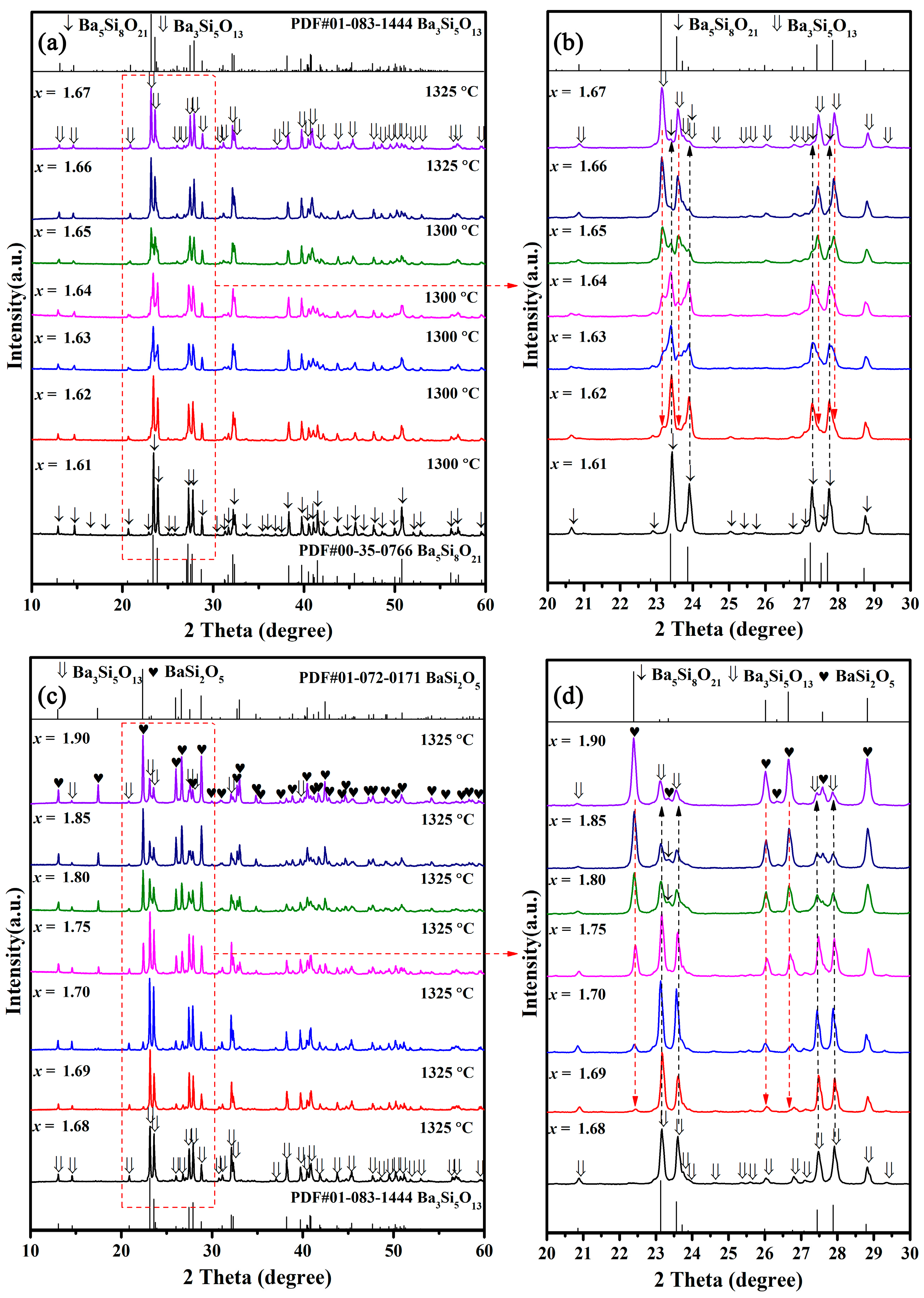
The BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics at the optimal sintering temperature (Tsint) were broken up and ground into powders for X-ray analysis. As shown in Figure 1a, the optimal sintering temperature of BaSixO1+2x (1.61 ≤ x ≤ 1.67) ceramics was between 1300 and 1325 °C. Only Ba5Si8O21 phase seemed to exist in BaSixO1+2x (1.61 ≤ x ≤ 1.62) ceramics, and the BaSixO1+2x (1.66 ≤ x ≤ 1.67) ceramics exhibited the Ba3Si5O13 single phase. The evident coexistence of Ba5Si8O21 and Ba3Si5O13 phases was observed in BaSixO1+2x (1.63 ≤ x ≤ 1.65) ceramics. As illustrated in Figure 1c, the Ba3Si5O13 single phase existed in the BaSixO1+2x (x = 1.68) ceramics, and the obvious coexistence of Ba3Si5O13 and BaSi2O5 phases was observed in the BaSixO1+2x (1.69 ≤ x ≤ 1.90) ceramics. However, the similar XRD patterns between Ba3Si5O13 and Ba5Si8O21 phases caused difficulty in distinguishing the phase compositions of BaSixO1+2x ceramics. The enlarged XRD patterns of BaSixO1+2x (1.61 ≤ x ≤ 1.9) ceramics at a 20°–30° scanning range can be seen in Figure 1b,d, along with the evolution of the main XRD peaks. The evident Ba3Si5O13 second phase existed in BaSixO1+2x (x = 1.62) ceramics, and the content of the Ba3Si5O13 phase in BaSixO1+2x (1.61 ≤ x ≤ 1.67) ceramics increased gradually with the rise in x. In the BaSixO1+2x (x = 1.67) ceramics, the Ba5Si8O21 second phase was present. The single-phase Ba3Si5O13 ceramics might be seen in the BaSixO1+2x (x = 1.68) ceramics (Figure 1d). With the further increase in x, the intensity of the XRD patterns of the BaSi2O5 and Ba3Si5O13 phases gradually rose and decreased, respectively. The evident Ba5Si8O21 phase also existed in BaSixO1+2x (1.80 ≤ x ≤ 1.85) ceramics, which indicates the complex evolution of the phase compositions of BaSixO1+2x (1.68 ≤ x ≤ 1.90) ceramics. Moreover, the Ba3Si5O13 ceramics were able to be synthesised via sintering at 1325 °C.
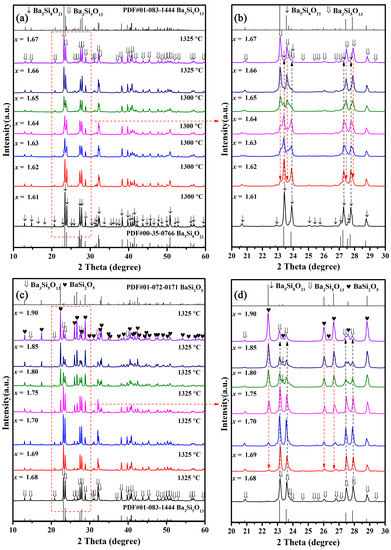
Figure 1.
XRD patterns of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics sintered at their densification temperature: (a) the XRD patterns of BaSixO1+2x (1.61 ≤ x ≤ 1.67) ceramics; (b) the enlarged XRD patterns of BaSixO1+2x (1.61 ≤ x ≤ 1.67) ceramics at 20°~30°; (c) the XRD patterns of BaSixO1+2x (1.68 ≤ x ≤ 1.90) ceramics; (d) the enlarged XRD patterns of BaSixO1+2x (1.68 ≤ x ≤ 1.90) ceramics at 20°~30°.
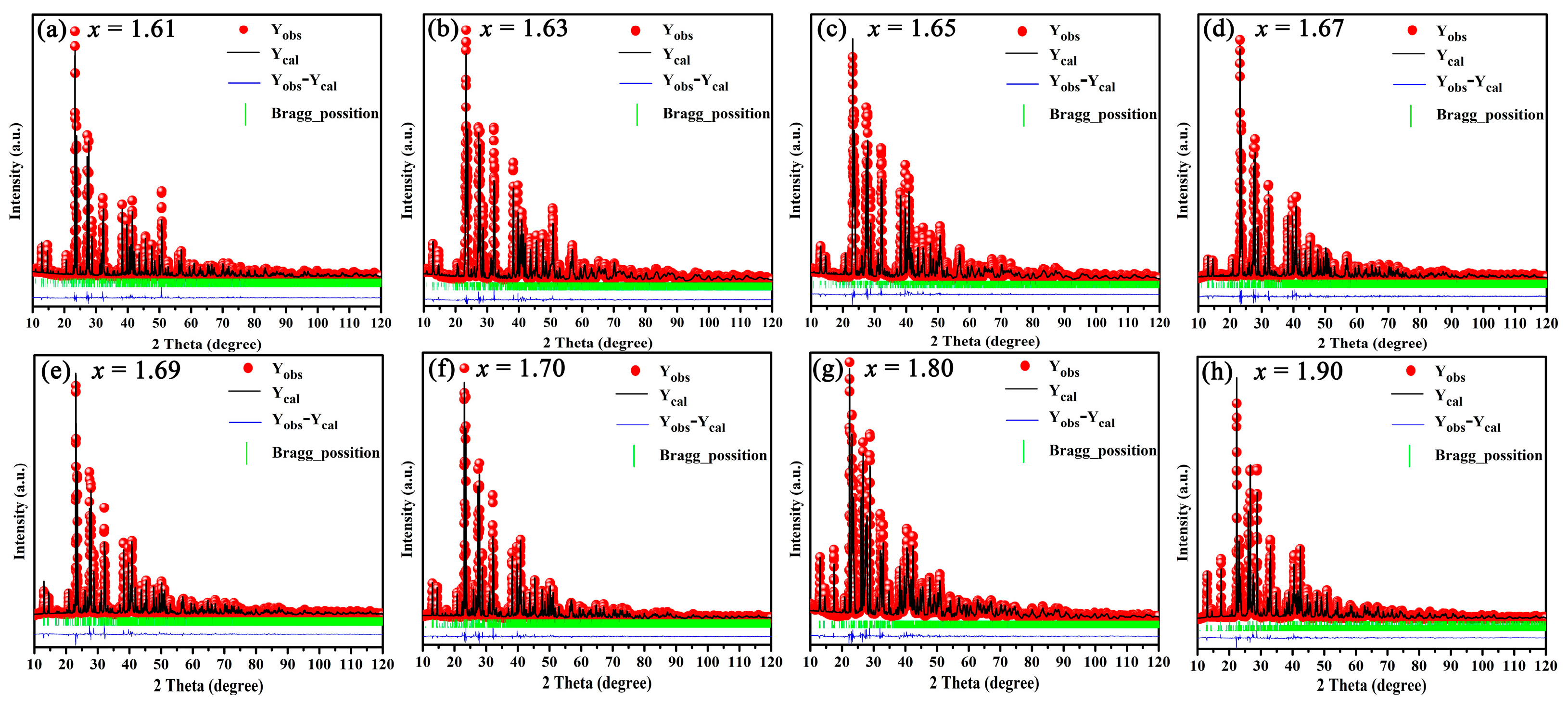
The Rietveld refinement of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics was conducted for quantitative analysis via phase composition. The results of the Rietveld refinement are shown in Table 1, and the calculated XRD patterns of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics matched the measured XRD patterns well (Figure 2). Therefore, the fitting content of phase compositions was accurate. As shown in Table 1, the Ba5Si8O21 main phase and Ba3Si5O13 second phase existed in BaSixO1+2x (1.61 ≤ x ≤ 1.64) ceramics. The main phase in BaSixO1+2x (1.65 ≤ x ≤ 1.67) ceramics changed from the Ba5Si8O21 to Ba3Si5O13 phase. Moreover, the Ba5Si8O21 second phase changed to the BaSi2O5 phase in the BaSixO1+2x (x = 1.68) ceramics. The BaSixO1+2x (x = 1.68) ceramics with 98.5 wt% Ba3Si5O13 and 1.85 wt% BaSi2O5 was synthesised, and the BaSi2O5-Ba3Si5O13 system could be obtained in BaSixO1+2x (1.68 ≤ x ≤ 1.69) ceramics. With the further increase in x, the other Ba5Si8O21 second phase appeared in BaSixO1+2x (1.7 ≤ x ≤ 1.90) ceramics, and the content of the BaSi2O5 phase increased gradually. Lastly, the results of the Rietveld refinement indicated that the coexistence of the Ba5Si8O21 and Ba3Si5O13 phases were obtained in BaSixO1+2x (1.61 ≤ x ≤ 1.67) ceramics (Ba5Si8O21–Ba3Si5O13 system), and the Ba3Si5O13 single-phase ceramics could be synthesised in BaSixO1+2x (1.67 < x < 1.68) ceramics. The phase compositions of the BaSixO1+2x ceramics was significantly affected by the ratio of Ba:Si.

Table 1.
The lattice parameters and Rietveld discrepancy factors of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics sintered at their densification temperatures.
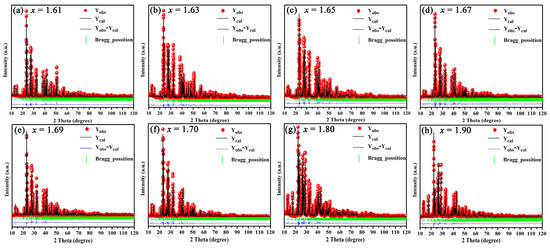
Figure 2.
XRD patterns of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics after whole XRD pattern fitting.
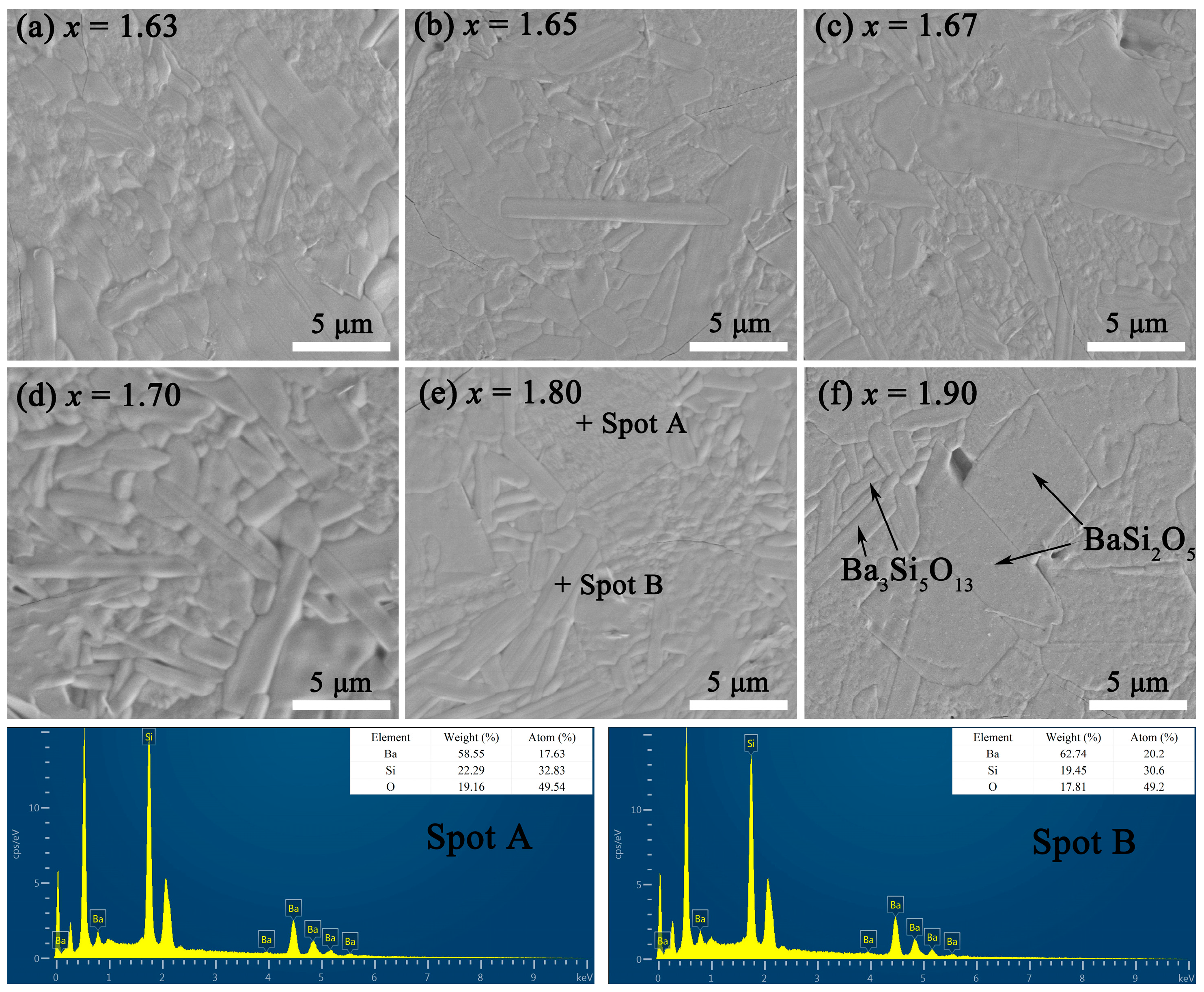
As shown in Figure 3 and S1, the thermally etched SEM and EDS map scanning images of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics were obtained. The dense and smooth microstructures of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics were observed. Only the grains of barium silicates were observed in BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics. However, the similar monoclinic structure and elemental composition caused difficulty in distinguishing the grains of Ba5Si8O21 and Ba3Si5O13 based on Figure 3 and S1. In Figure 3e, the EDS results of Spots A and B indicated that the strip-shaped (Spot B) and large (Spot A) grains were Ba3Si5O13 and BaSi2O5, respectively. The Ba3Si5O13 second phase can be observed in Figure 3f. With the increase in x, the content of strip-shaped grains decreased gradually and the average grain sizes increased, which implies that the content of BaSi2O5 rose gradually.
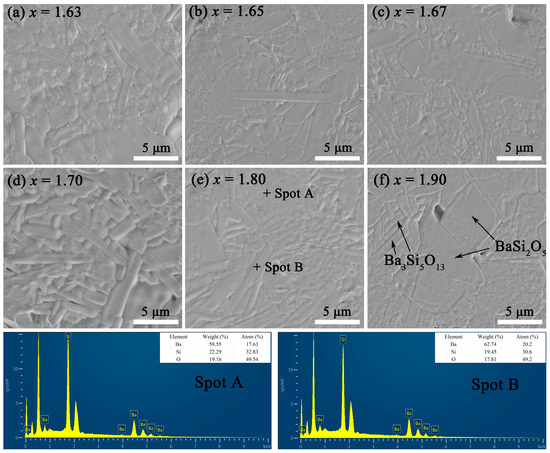
Figure 3.
SEM and EDS images of thermally etched BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics.
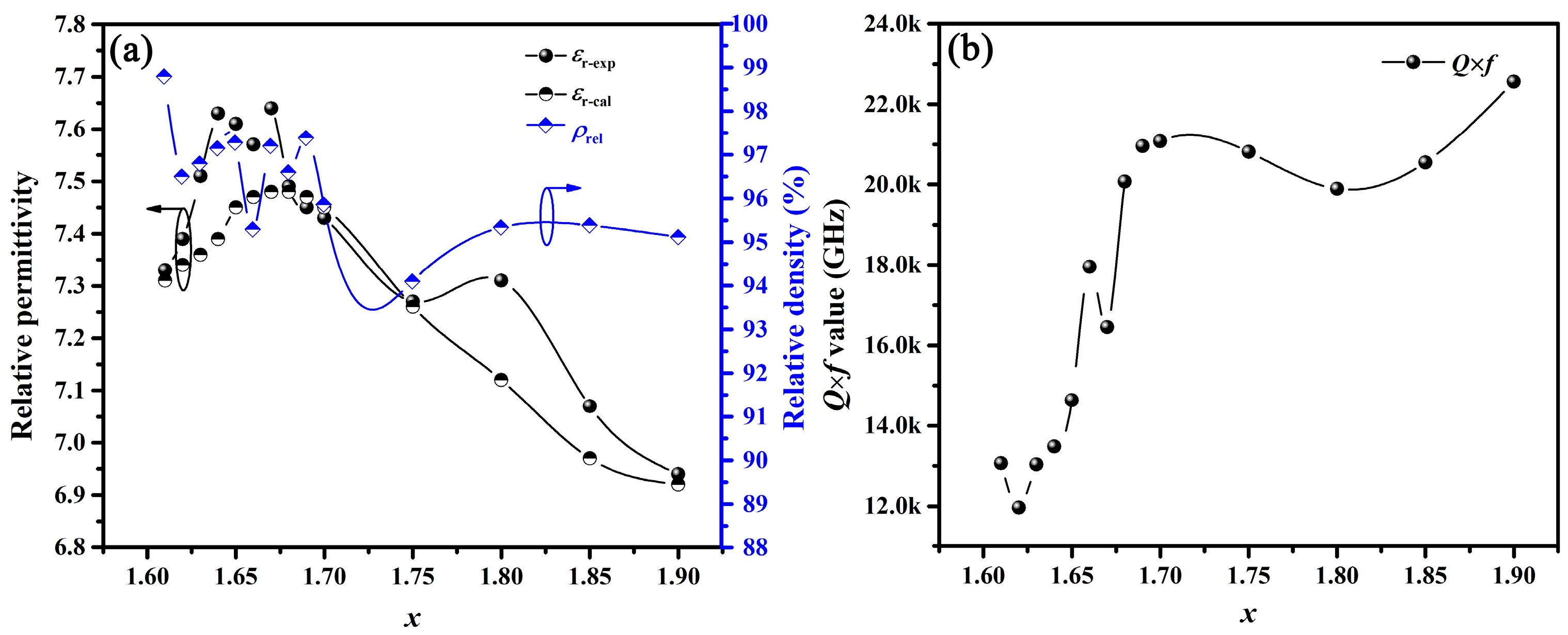
The microwave dielectric properties of the BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics sintered at the optimal temperature were obtained, as shown in Table S1 (see Supplementary materials) and Figure 4 and Figure 5. As shown in Figure 4a, the relative density (ρrel) and experimental relative permittivity (εr-exp) of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics fluctuated with the increase in x, and all BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics exhibited high ρrel (>95%) except th BaSixO1+2x (x = 1.75) ceramics. Therefore, ρrel might have little effect on themicrowave dielectric properties of BaSixO1+2x ceramics. In the BaSixO1+2x (1.61 ≤ x ≤ 1.67) ceramics, the BaSixO1+2x (x = 1.67) ceramics with a 91.27 wt% Ba3Si5O13 phase exhibited the largest εr-exp value, and a low εr-exp (7.33) was obtained in BaSixO1+2x (x = 1.61) ceramics with a 95.87 wt% Ba5Si8O21 phase, which was attributed to the low εr-exp value (7.3) of Ba5Si8O21 ceramics [9]. In BaSixO1+2x (1.68 ≤ x ≤ 1.90) ceramics, εr-exp decreased from 7.49 to 6.94, and the BaSixO1+2x (x = 1.90) ceramics with a 71.12 wt% BaSi2O5 phase exhibited the lowest εr-exp value. For multi-phase ceramics, εr could be calculated using the Lichtenecker empirical rule [26,27,28].
where εr-cal represents the calculated relative permittivity; V1, V2 and V3 are the volume fraction of phases 1, 2 and 3; and εr-1, εr-2 and εr-3 are the εr-exp values of Ba5Si8O21, Ba3Si5O13 and BaSi2O5 ceramics [9], respectively. The εr-cal of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics increased initially and then decreased, and the variation tendency of the εr-exp values was similar to εr-cal with x (Figure 4a). This finding implies that phase compositions significantly affected the εr-exp values for BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics. Moreover, the BaSixO1+2x (x = 1.66) ceramics exhibited lower εr-exp (7.57) than BaSixO1+2x (x = 1.64, 1.65, 1.67) ceramics, which was attributed to their low ρrel. The BaSixO1+2x (x = 1.80) ceramics exhibited larger ρrel and εr-exp than BaSixO1+2x (x = 1.75) ceramics. Thus, the effect of ρrel on the εr-exp of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics cannot be neglected.
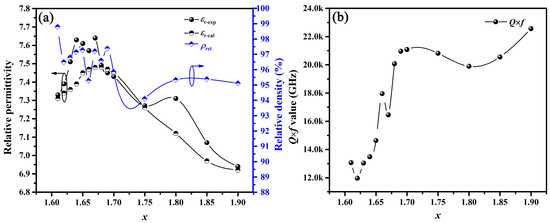
Figure 4.
(a) Variation in εr-exp, ρrel and εr-cal of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics with x; (b) variation in Q × f values of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics with x.
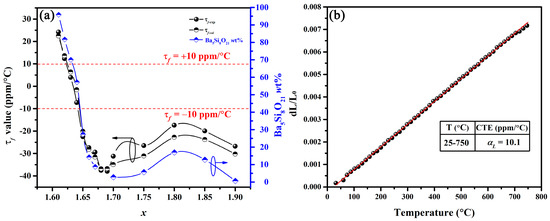
Figure 5.
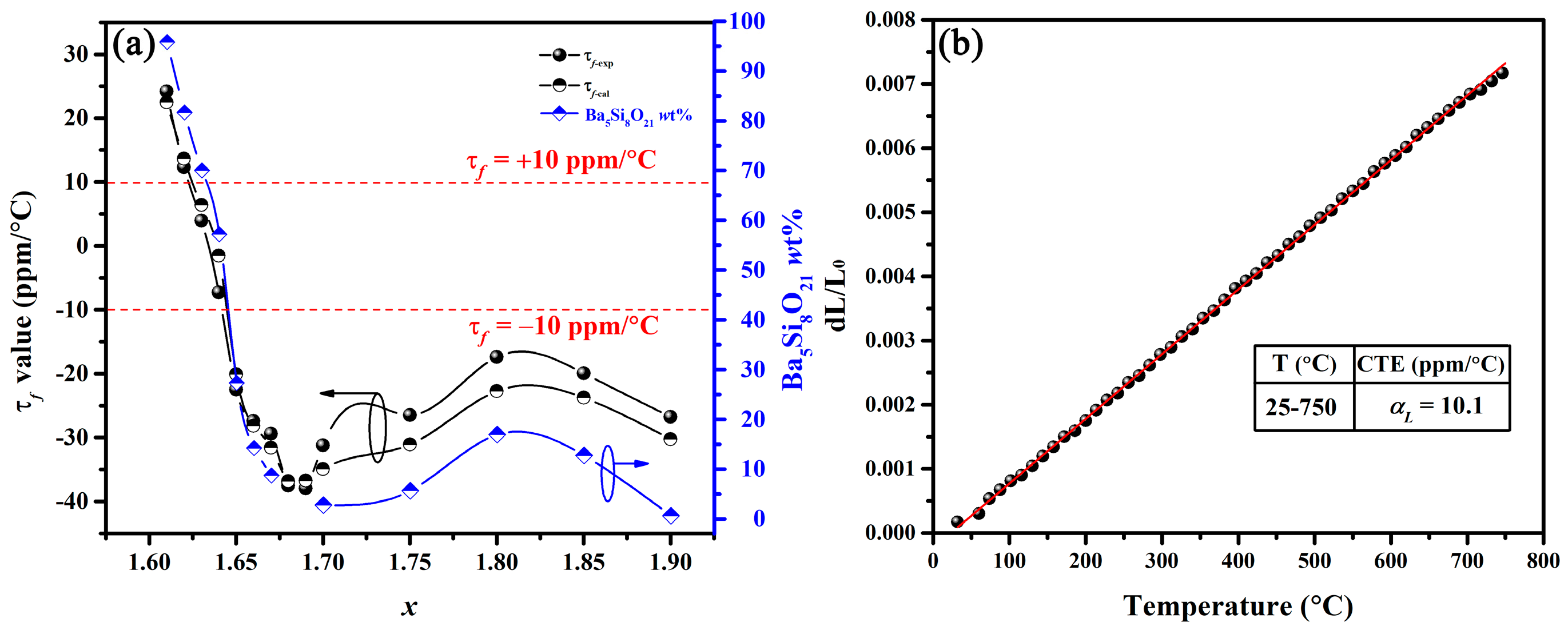
(a) Variation in τf-exp, τf-cal and Ba5Si8O21 wt% of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics with x; (b) thermal expansion of BaSixO1+2x (x = 1.63) ceramics.
Figure 4b shows the Q × f values of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics. The BaSixO1+2x (1.68 ≤ x ≤ 1.90) ceramics exhibited higher Q × f values than BaSixO1+2x (1.61 ≤ x ≤ 1.67) ceramics, and the BaSixO1+2x (x = 1.90) ceramics with 71.12 wt% BaSi2O5 phase exhibited high Q × f value (22,563 GHz), which was attributed to the high Q × f value of BaSi2O5 ceramics [9]. Moreover, the low Q × f value of BaSixO1+2x (x = 1.80) ceramics was obtained at 1.68 ≤ x ≤ 1.90. This result indicates that the largest harmful inner stress at the two-phase interface was obtained in BaSixO1+2x (x = 1.80) ceramics with 41.79 wt% BaSi2O5 phase, 41.34 wt% Ba3Si5O13 phase and 16.87 wt% Ba5Si8O21 phase. The inner stress at the two-phase interface could not be neglected in multiphase BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics. The BaSixO1+2x (x = 1.61) ceramics with minimal Ba3Si5O13 phase (4.13 wt%) exhibited lower Q × f value (13,062 GHz) than Ba5Si8O21 single-phase ceramics (16,700 GHz) due to the existence of inner stress at the two-phase interface. The Q × f value of BaSixO1+2x (x = 1.68) ceramics with a 98.15 wt% Ba3Si5O13 phase was higher than that of BaSixO1+2x (1.61 ≤ x ≤ 1.67) ceramics, which means that Ba3Si5O13 ceramics might exhibit higher Q × f value than Ba5Si8O21 ceramics.
The τf-exp values of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics were obtained, as shown in Figure 5a. At 1.61 ≤ x ≤ 1.67 (Ba5Si8O21-Ba3Si5O13 system), the τf-exp values changed from +24.20 ppm/°C to −29.40 ppm/°C, and the BaSixO1+2x (x = 1.63 and 1.64) ceramics exhibited near-zero τf-exp values. At 1.68 ≤ x ≤ 1.90, the τf-exp values of the BaSixO1+2x ceramics fluctuated with x, and the small |τf-exp| value was obtained at x = 1.80. For multi-phase ceramics, τf-cal could be calculated using Lichtenecker’s empirical rule [26,27,28].
where τf-cal represents the calculated τf; V1, V2 and V3 are the volume fractions of phases 1, 2 and 3, respectively; and τf1, τf2 and τf3 are the τf values of Ba5Si8O21, Ba3Si5O13 and BaSi2O5 ceramics [9], respectively. τf-cal agreed well with the τf-exp in BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics, and τf-exp, τf-cal and content of Ba5Si8O21 phase of the BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics with x displayed a similar trend. Therefore, the content of the Ba5Si8O21 phase significantly affected the τf-exp of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics. At 1.65 ≤ x ≤ 1.75, the BaSixO1+2x ceramics with the Ba3Si5O13 main phase exhibited negative τf-exp values. Therefore, the present study confirmed the negative τf value of Ba3Si5O13 ceramics, which was controversial in a previous work [9]. Ba5Si8O21, as a new τf compensator, successfully adjusted the negative τf-exp value of Ba3Si5O13 to near zero (+3.95 ppm/°C and −7.25 ppm/°C) in the BaSixO1+2x (x = 1.63, 1.64) ceramics.
In accordance with Formula (3) [29,30], the τf related to αL and τε and αL can be seen in Figure 5b.
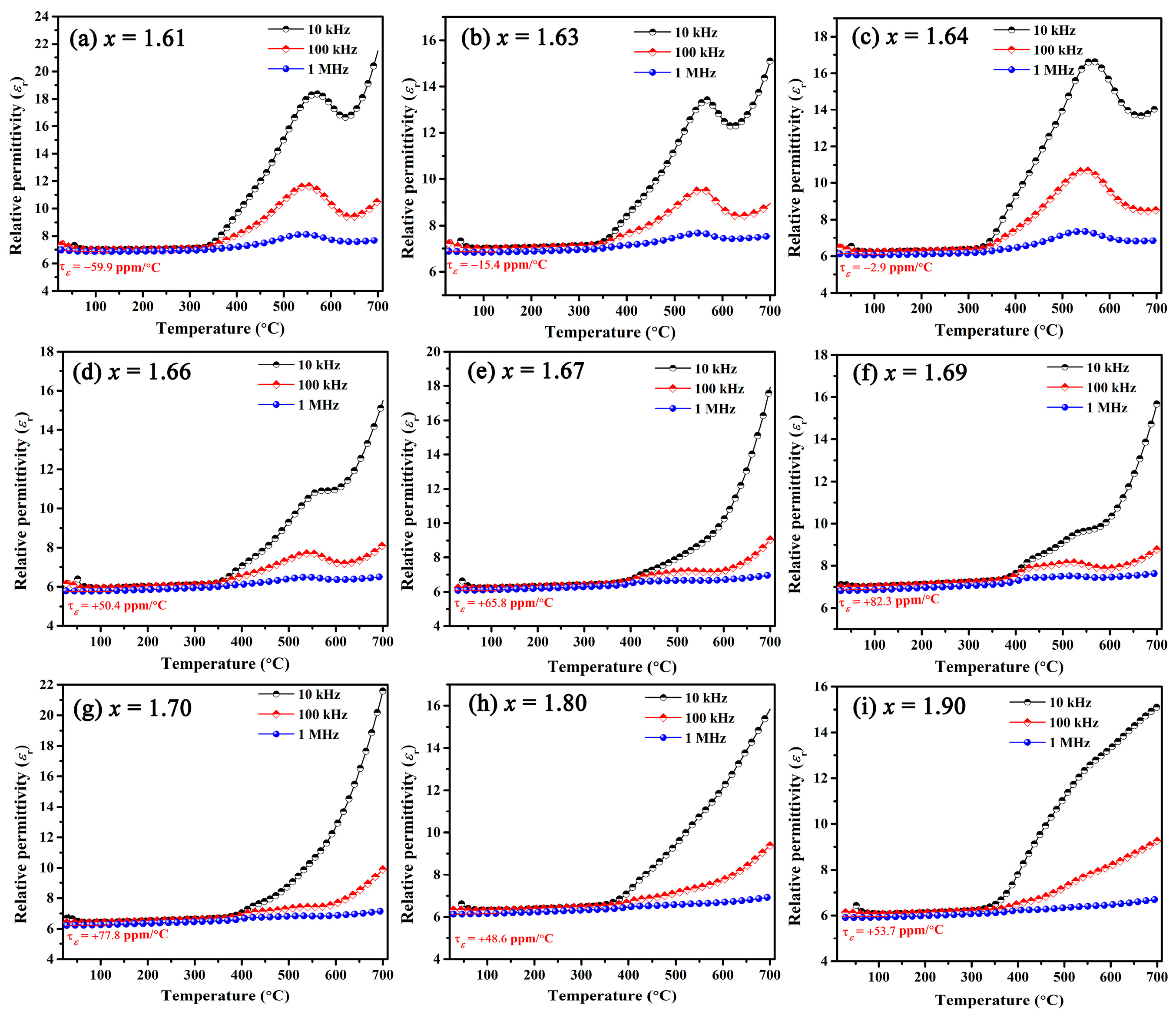
with the increase in measured temperature, the length of BaSixO1+2x (x = 1.63) ceramics increased gradually, and the average linear thermal expansion coefficient (CTE in Figure 5b) at 25–750 °C is 10.1 ppm/°C. The dependence of εr on temperature for BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics is shown in Figure 6, and the fitting τε values at 30–125 °C and 1 MHz were obtained. The negative τε values were obtained in BaSixO1+2x (1.61 ≤ x ≤ 1.64) ceramics, and BaSixO1+2x (1.66 ≤ x ≤ 1.90) ceramics exhibited positive τε values. At 1.61 ≤ x ≤ 1.64, the negative τε values increased gradually to near zero, and BaSixO1+2x (x = 1.64) ceramics exhibited a near-zero τε value (−2.9 ppm/°C). At 1.66 ≤ x ≤ 1.90, the small τε value was obtained in BaSixO1+2x (x = 1.80) ceramics. According to the τf–εr relationship, a similar variation in the τε and τf values of the BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics was observed as x increased, which indicates that the measured τf-exp values using the Hakki–Coleman method were exacted. The measured αL (10.1 ppm/°C) and τε (−15.4 ppm/°C) values of BaSixO1+2x (x = 1.63) ceramics also confirmed their near-zero τf-exp value.
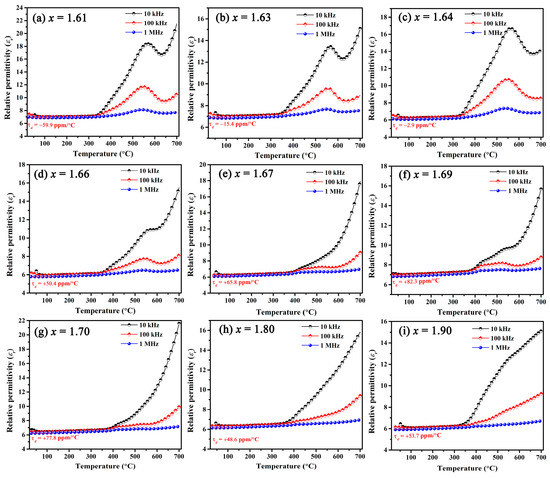
Figure 6.
Temperature and frequency dependences of relative permittivity for BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics.
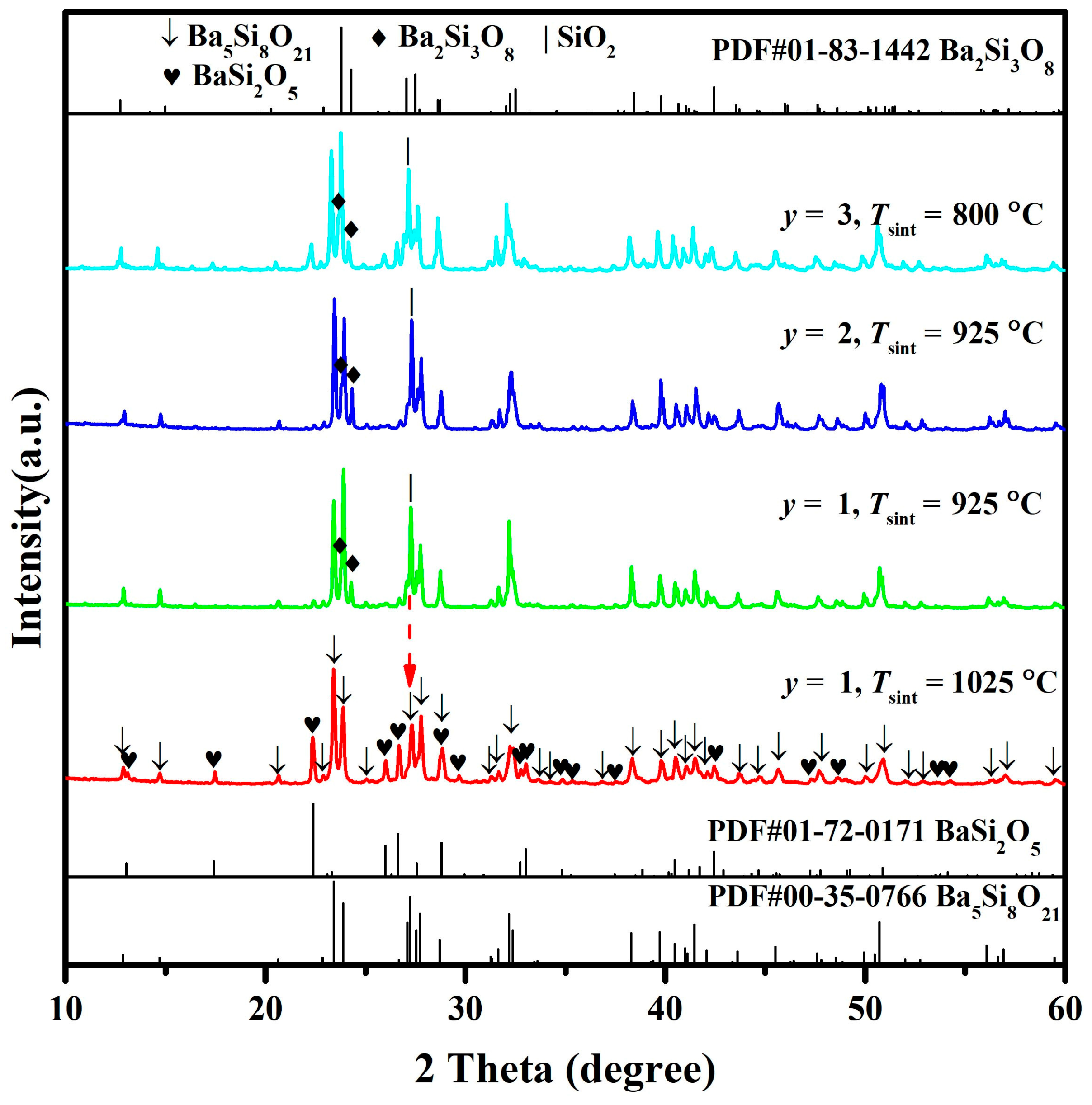
At high Tsint (≥1300 °C), the phase compositions of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics were revealed in this work. However, the phase compositions of barium silicates were sensitive to Tsint, and the complex phase transformation of barium silicates existed with the change in Tsint, especially Ba5Si8O21, Ba3Si5O13 and BaSi2O5 ceramics [9,10]. Thus, the phase transformation of BaSixO1+2x ceramics with the change in Tsint must be clarified. The BaSixO1+2x (x = 1.63) ceramics sintered at 1300 °C presented the coexistence of Ba5Si8O21 and Ba3Si5O13 phases and exhibited good microwave dielectric properties. As shown in Figure 7, the XRD patterns of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics were obtained. The coexistence of Ba5Si8O21 and BaSi2O5 phases was obtained in BaSi1.63O4.26-1 wt% LiF ceramics sintered at 1025 °C, and the Ba3Si5O13 second phase of BaSi1.63O4.26 ceramics sintered at 1300 °C was inhibited. However, the new Ba2Si3O8 and SiO2 second phases appeared in BaSi1.63O4.26-1 wt% LiF ceramics when Tsint = 925 °C, which implies that the Ba2Si3O8 and SiO2 phase can transform into BaSi2O5 phases at Tsint = 1025 °C. With the increase in the content of LiF, the Tsint of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics decreased gradually. The coexistence of Ba5Si8O21, Ba2Si3O8, BaSi2O5 and SiO2 phases was observed in BaSi1.63O4.26-y wt% LiF (y = 2, 3) ceramics sintered at 925 and 800 °C.
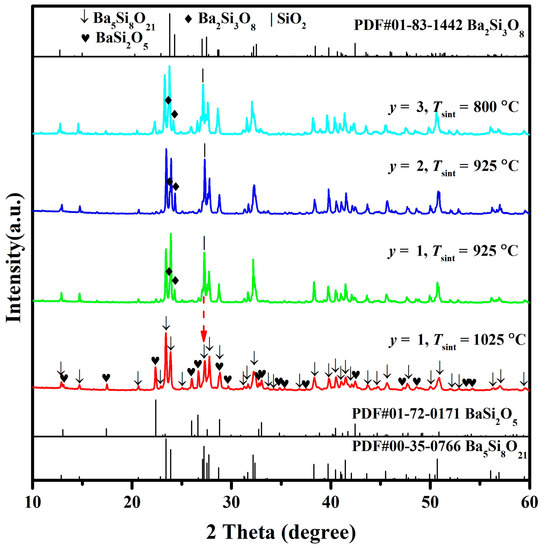
Figure 7.
XRD patterns of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics at different sintering temperatures.
Figure S2 and Table 2 show the results of Rietveld refinement. The calculated XRD patterns of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics matched the measured XRD patterns well (Figure S2). The phase compositions of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics were observed, as shown in Table 2. The BaSi1.63O4.26-1 wt% LiF ceramics with 74.39 wt% Ba5Si8O21 and 25.61 wt% BaSi2O5 phases was obtained. The decrease in Tsint induced the appearance of the Ba2Si3O8 second phase, and 84.51 wt% and 68.69 wt% Ba5Si8O21 phases were obtained in BaSi1.63O4.26-y wt% LiF (y = 2, 3) ceramics. The Ba3Si5O13 phase could not be obtained in BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics with low Tsint (≤1025 °C), which means that Ba3Si5O13 phase can only be synthesised at high Tsint (≥1300 °C). The Ba5Si8O21, Ba2Si3O8 and BaSi2O5 phases can be synthesised at low Tsint (≤925 °C). In summary, the Ba5Si8O21 phase was the most stable phase of barium silicates, and the phase compositions of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics were related to Tsint.

Table 2.
The lattice parameters and Rietveld discrepancy factors of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics sintered at their densification temperatures.
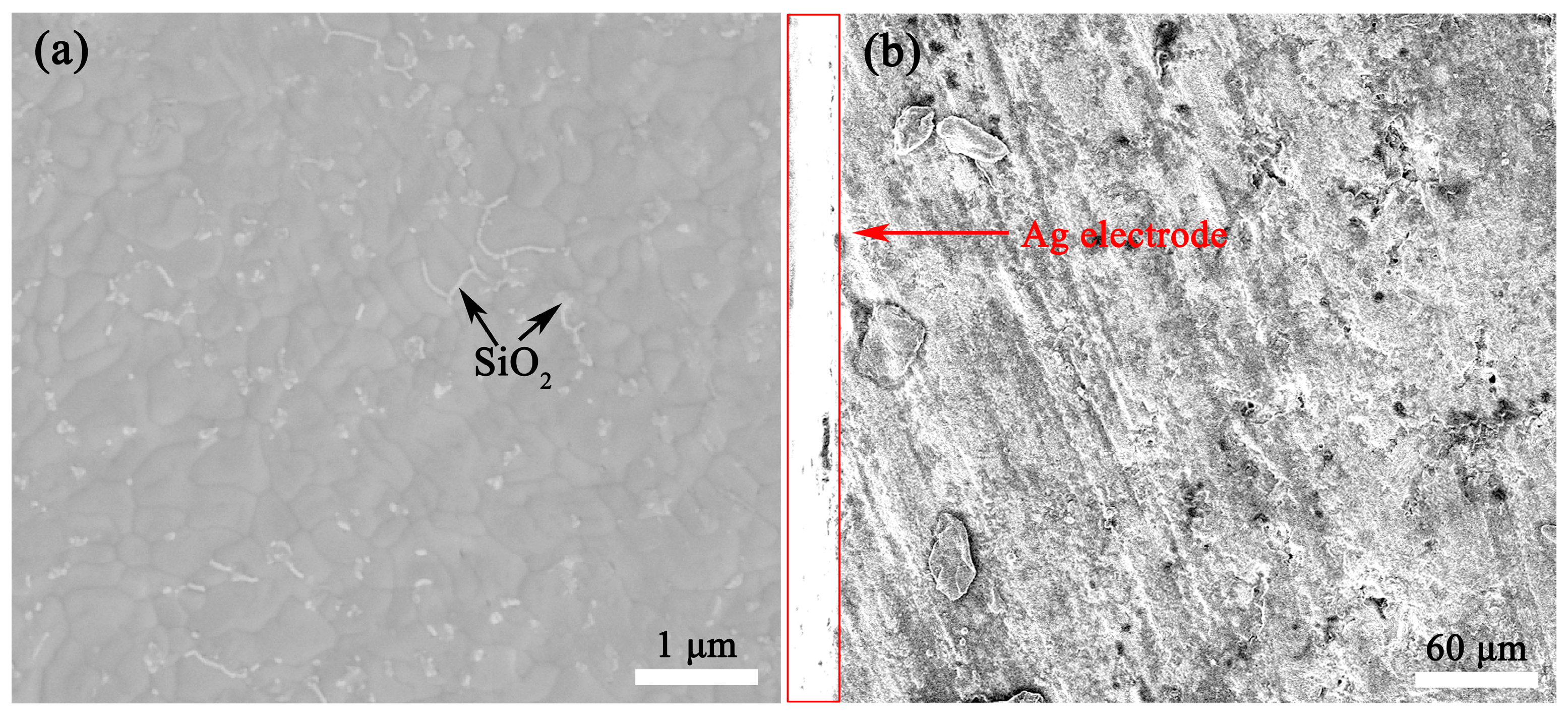
The thermally etched SEM images of BaSi1.63O4.26-3 wt% LiF and BaSi1.63O4.26-3 wt% LiF-Ag electrode ceramics were obtained, as shown in Figure 8. Dense and smooth microstructures were observed in BaSi1.63O4.26-3 wt% LiF ceramics. The evident small SiO2 second phase existed at the grain boundary, as shown in Figure 8 and S3. No LiF or liquid phases existed, and complex phase compositions could not be clarified via SEM images. As shown in Figure 8b, the surface of BaSi1.63O4.26-3 wt% LiF ceramics could be well combined with Ag electrodes, which indicates that it is well able be co-fired with Ag electrodes at 800 °C.
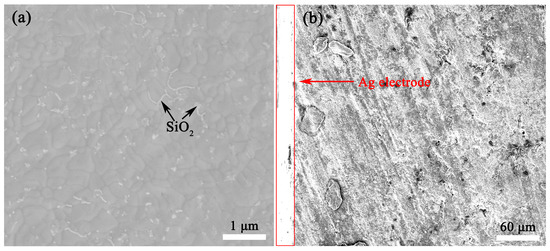
Figure 8.
SEM images of the thermally etched (a) BaSi1.63O4.26-3 wt% LiF ceramics and (b) BaSi1.63O4.26-3 wt% LiF-Ag electrode ceramics.
The evolution of the phase compositions and sintering characteristic of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics on microwave dielectric properties was investigated. As shown in Table 3, with the addition of a LiF sintering aid, the optimal Tsint of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics were able to be reduced to less than 1025 °C. At the optimal sintering temperatures, the ρrel of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics decreased from 96.5% (y = 1) to 94.4% (y = 3), and the addition of a LiF sintering aid [31] and the decrease in sintering temperature caused a reduction in ρrel. The εr-exp and Q × f values decreased gradually, and the τf-exp values fluctuated with x. The decrease in εr-exp and Q × f values was mainly attributed to the decline in ρrel. According to Formula (2), the τf-cal values of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics were calculated, and similar variations in τf-exp, τf-cal and the content of the Ba5Si8O21 phase of the BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics were observed as y increased. The τf-exp values of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics were significantly affected by the content of the Ba5Si8O21 phase, and the near-zero τf-exp value (+5.75 ppm/°C) was obtained in BaSi1.63O4.26-3 wt% LiF ceramics with 68.69 wt% Ba5Si8O21, 15.55 wt% Ba2Si3O8, 13.70 wt% BaSi2O5 and 2.06 wt% SiO2 phases.

Table 3.
The optimal sintering temperature, relative density and microwave dielectric properties of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics.
4. Conclusions
BaSixO1+2x (1.61 ≤ x ≤ 1.90) and BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics were synthesised, and the evolution of their phase compositions was investigated. The two-phase system of Ba5Si8O21 and Ba3Si5O13 was obtained at 1.61 ≤ x ≤ 1.67, and no other second phase existed. At 1.67 ≤ x ≤ 1.69, the content of Ba3Si5O13 phase was more than 90 wt%. The Ba5Si8O21 second phase could be inhibited in BaSixO1+2x (1.68 ≤ x ≤ 1.69) ceramics, and the other BaSi2O5 phase was induced. At 1.70 ≤ x ≤ 1.90, the excessive Si element content of BaSixO1+2x ceramics caused the appearance of the Ba5Si8O21 second phase, and the coexistence of BaSi2O5, Ba5Si8O21 and Ba3Si5O13 phases was observed. Therefore, the phase compositions of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics sintered at high temperature (≥1300 °C) were significantly controlled by the ratio of Ba:Si. The εr-exp and Q × f values of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics were mainly affected by the phase compositions and ρrel, and the τf-exp values were controlled via the content of the Ba5Si8O21 phase with a positive τf value. The near-zero τf-exp value (+3.95 ppm/°C) was obtained at x = 1.63. Moreover, the LiF sintering aids were able to reduce the sintering temperature of BaSi1.63O4.26 ceramics, and the low sintering temperature (800 °C) was obtained in BaSi1.63O4.26-y wt% LiF (y = 3) ceramics. When sintering temperature was less than 1025 °C, the Ba3Si5O13 phase of BaSi1.63O4.26 ceramics was inhibited and the Ba2Si3O8, BaSi2O5 and SiO2 appeared. The content of phases could be affected by the sintering temperature, which shows that the phase compositions of BaSixO1+2x ceramics were related to their sintering temperature. The development of microwave dielectric properties of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics strongly relied on their phase compositions, and microwave dielectric properties (εr = 7.10, Q × f = 12,463 GHz, and τf = +5.75 ppm/°C) were obtained in BaSi1.63O4.26-3 wt% LiF ceramics.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cryst13060870/s1, Figure S1: EDS map scanning images of the thermally etched BaSixO1+2x (x = 1.67 and 1.80) ceramics; Figure S2: XRD patterns of BaSi1.63O4.26-y wt% LiF (1 ≤ y ≤ 3) ceramics after whole XRD pattern fitting; Figure S3: EDS map scanning images of the thermally etched BaSi1.63O4.26-3 wt% LiF ceramics; Table S1: Optimal sintered temperature (Tsint), relative density (ρrel) and microwave dielectric properties of BaSixO1+2x (1.61 ≤ x ≤ 1.90) ceramics.
Author Contributions
Conceptualization, T.W. and Y.L.; methodology, Z.Z.; software, X.L. (Xiaotian Liu); validation, X.L. (Xiaoxiao Li); formal analysis, T.W.; investigation, K.D.; resources, K.D.; data curation, T.W.; writing—original draft preparation, K.D.; writing—review and editing, K.D. and W.L.; visualization, Y.L.; supervision, C.Y.; project administration, Y.C.; funding acquisition, W.L. and S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Natural Science Foundation of China grant number 52072133.
Data Availability Statement
Data is unavailable due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, D.; Pang, L.X.; Wang, D.W.; Li, C.; Jin, B.B.; Reaney, I.M. High permittivity and low loss microwave dielectrics suitable for 5G resonators and low temperature co-fired ceramic architecture. J. Mater. Chem. C 2017, 5, 10094–10098. [Google Scholar] [CrossRef]
- Wu, F.F.; Zhou, D.; Du, C.; Jin, B.B.; Li, C.; Qi, Z.M. Design of a Sub-6 GHz dielectric resonator antenna with novel temperature-stabilized (Sm1−xBix)NbO4 (x = 0–0.15) microwave dielectric ceramics. ACS Appl. Mater. Inter. 2022, 14, 7030–7038. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.Z.; Yu, Z.Z.; Shu, L.L.; Liu, L.J.; Chen, Y.; Li, C.C. A low-firing melilite ceramic Ba2CuGe2O7 and compositional modulation on microwave dielectric properties through Mg substitution. J. Adv. Ceram. 2021, 10, 108–119. [Google Scholar] [CrossRef]
- Tian, H.R.; Zheng, J.J.; Liu, L.T.; Wu, H.T.; Kimura, H.; Lu, Y.Z. Structure characteristics and microwave dielectric properties of Pr2(Zr1−xTix)3(MoO4)9 solid solution ceramic with a stable temperature coefficient. J. Mater. Sci. Technol. 2022, 116, 121–129. [Google Scholar] [CrossRef]
- Liu, L.T.; Chen, Y.G.; Feng, Z.B.; Wu, H.T.; Zhang, X.Y. Crystal structure, infrared spectra, and microwave dielectric properties of the EuNbO4 ceramic. Ceram Int. 2021, 47, 4321–4326. [Google Scholar] [CrossRef]
- Grebenschikov, R.G.; Toropov, N.A. New data on the barium oxide-silica phase diagram. Izvest. Akad. Nauk. SSSR Ser. Chem. 1962, 11, 545–553. [Google Scholar]
- Zhang, R.L.; Maeda, T.; Maruta, R.; Kusaka, S.; Ding, B.J.; Murai, K.I. Luminescence enhancement of Eu2+, Ce3+ co-doped Ba3Si5O13-δNδ phosphors. J. Solid State Chem. 2010, 183, 620–623. [Google Scholar] [CrossRef]
- Gorelova, L.A.; Bubnova, R.S.; Krivovichev, S.V.; Krzhizhanovskaya, M.G.; Filatov, S.K. Thermal expansion and structural complexity of Ba silicates with tetrahedrally coordinated Si atoms. J. Solid State Chem. 2016, 235, 76–84. [Google Scholar] [CrossRef]
- Lei, W.; Zou, Z.Y.; Chen, Z.H.; Ullah, B.; Zeb, A.; Lan, X.K. Controllable τf value of barium silicate microwave dielectric ceramics with different Ba/Si ratios. J. Am. Ceram. Soc. 2018, 101, 25–30. [Google Scholar] [CrossRef]
- Wu, P.; Yang, H.Y.; Yang, H.C.; Gui, L.; Wang, Y.C.; Liu, Q. Synthesis of a low-firing BaSi2O5 microwave dielectric ceramics with low dielectric constant. Ceram. Int. 2022, 48, 17289–17297. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.N.; Ding, S.H.; Song, T.X.; Yin, Z.Z.; Dan, J.Y. Crystal structure and microwave dielectric properties of Li-modified BaSi2O5 ceramics. J. Mater. Res. Technol. 2023, 22, 2792–2805. [Google Scholar] [CrossRef]
- Song, X.Q.; Du, K.; Zou, Z.Y.; Chen, Z.H.; Lu, W.Z.; Wang, S.H. Temperature-stable BaAl2Si2O8–Ba5Si8O21-based low-permittivity microwave dielectric ceramics for LTCC applications. Ceram. Int. 2017, 43, 14453–14456. [Google Scholar] [CrossRef]
- Joseph, T.; Sebastian, M.T. Microwave dielectric properties of alkaline earth orthosilicates M2SiO4 (M = Ba, Sr, Ca). Mater. Lett. 2011, 65, 891–893. [Google Scholar] [CrossRef]
- Wang, D.W.; Ding, L.F.; Heng, B.; Zhu, H.K.; Ding, X.F.; Wang, Z.F. Temperature-stable crystal structure and microwave dielectric properties of BaSi2O5-Ba3V2O8 composite ceramics. J. Alloys Compd. 2022, 927, 167096–167104. [Google Scholar] [CrossRef]
- Du, K.; Song, X.Q.; Zou, Z.Y.; Fan, J.; Lu, W.Z.; Lei, W. Improved microwave dielectric properties of novel low-permittivity Sn-doped Ca2HfSi4O12 ceramics. Mater. Res. Bull. 2020, 129, 110887. [Google Scholar] [CrossRef]
- Wu, S.P.; Jiang, C.; Mei, Y.X.; Tu, W.P. Synthesis and microwave dielectric properties of Sm2SiO5 ceramics. J. Am Ceram. Soc. 2012, 95, 37–40. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, S.; Jiang, C.; Li, J. Microwave dielectric properties of SnO2-doped CaSiO3 ceramics. Ceram. Int. 2013, 39, 2223–2229. [Google Scholar] [CrossRef]
- Du, K.; Guo, Y.B.; Wang, F.; Fan, J.; Wang, X.C.; Lu, W.Z. Optimized sintering behavior and microwave dielectric properties of Ca1+2xSnSi2x+yO3+6x+2y by composition modulation. J. Am. Ceram. Soc. 2021, 104, 974–984. [Google Scholar] [CrossRef]
- Du, K.; Yin, C.Z.; Guo, Y.B.; Zhang, C.; Wang, X.C.; Lu, W.Z. Phase transition, Infrared spectra, and microwave dielectric properties of temperature-stable CaSnSi1-xGexO5 ceramics. Ceram. Int. 2021, 47, 24781–24792. [Google Scholar] [CrossRef]
- Qin, J.C.; Liu, Z.F.; Ma, M.S.; Li, Y.X. Machine learning approaches for permittivity prediction and rational design of microwave dielectric ceramics. J. Mater. 2021, 7, 1284–1293. [Google Scholar] [CrossRef]
- Qin, J.C.; Liu, Z.F.; Ma, M.S.; Liu, F.; Qi, Z.M.; Li, Y.X. Structure and microwave dielectric properties of gillespite-type ACuSi4O10 (A = Ca, Sr, Ba) ceramics and quantitative prediction of the Q×f value via machine learning. ACS Appl. Mater. Interfaces 2021, 13, 17817–17826. [Google Scholar] [CrossRef] [PubMed]
- Kamutzki, F.; Schneider, S.; Barowski, J.; Gurlo, A.; Hanaor, D.A.H. Silicate dielectric ceramics for millimetre wave applications. J. Eur. Ceram. Soc. 2021, 41, 3879–3894. [Google Scholar] [CrossRef]
- Yusa, H.; Sata, N.; Ohishi, Y. Rhombohedral (9R) and hexagonal (6H) perovskites in barium silicates under high pressure. Am. Miner. 2007, 92, 648–654. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Hakki, B.W.; Coleman, P.D. A dielectric resonant method of measuring inductive capacitance in the millimeter range. IEEE Trans. Microw. Theory Technol. 1960, 8, 402–410. [Google Scholar] [CrossRef]
- Paladino, A.E. Temperature-compensated MgTi2O5-TiO2 dielectrics. J. Am. Ceram. Soc. 1971, 54, 168–169. [Google Scholar] [CrossRef]
- Fukuda, K.; Kitoh, R.; Awai, I. Microwave characteristics of TiO2-Bi2O3 dielectric resonator. Jpn. J. Appl. Phys. 1993, 32, 4584–4588. [Google Scholar] [CrossRef]
- He, H.; Xu, Y. A unified equation for predicting the dielectric constant of a two phase composite. Appl. Phys. Lett. 2014, 104, 062906. [Google Scholar] [CrossRef]
- Zhou, D.; Guo, H.H.; Fu, M.S.; Yao, X.G.; Lin, H.X.; Liu, W.F. Anomalous dielectric behaviour at the monoclinic to tetragonal phase transition in La(Nb0.9V0.1)O4. Inorg. Chem. Front. 2021, 8, 156–163. [Google Scholar]
- Du, K.; Yin, C.Z.; Guo, Y.B.; Wang, X.C.; Lu, W.Z.; Lei, W. Phase transition and permittivity stability against temperature of CaSn1-xTixGeO5 ceramics. J. Eur. Ceram. Soc. 2022, 42, 147–153. [Google Scholar] [CrossRef]
- Song, X.Q.; Du, K.; Li, J.; Lan, X.K.; Lu, W.Z.; Wang, X.H. Low-fired fluoride microwave dielectric ceramics with low dielectric loss. Ceram. Int. 2019, 45, 279–286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).