Gallium-Modified Zinc Oxide Thin Films Prepared by Chemical Solution Deposition
Abstract
:1. Introduction
2. Experimental
2.1. Solution
2.2. Thin Films by Spin Coating
2.3. Structural and Microstructural Characterization of Thin Films
2.4. Electrical Resistivity of Thin Films
3. Results
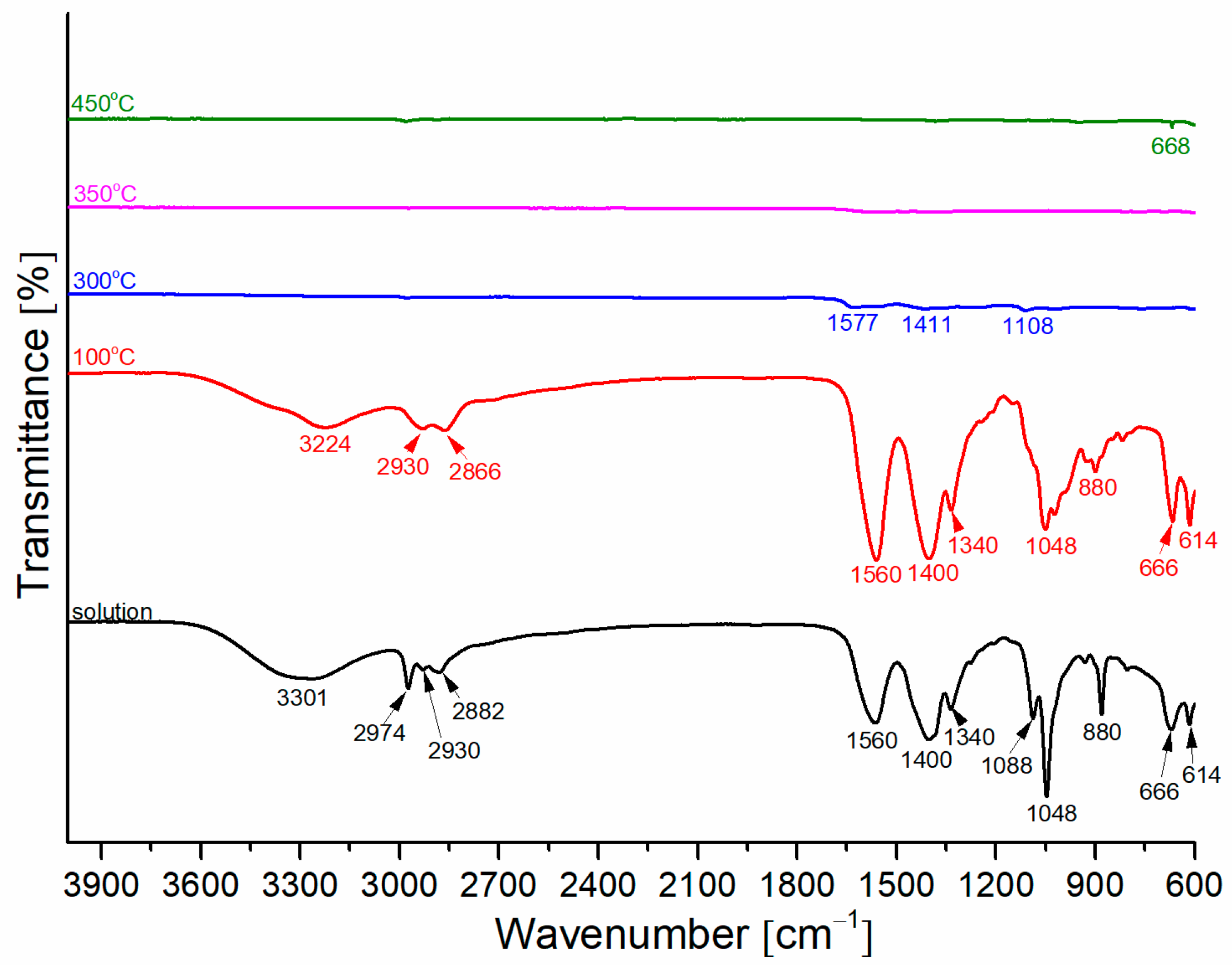
3.1. Precursor Solution
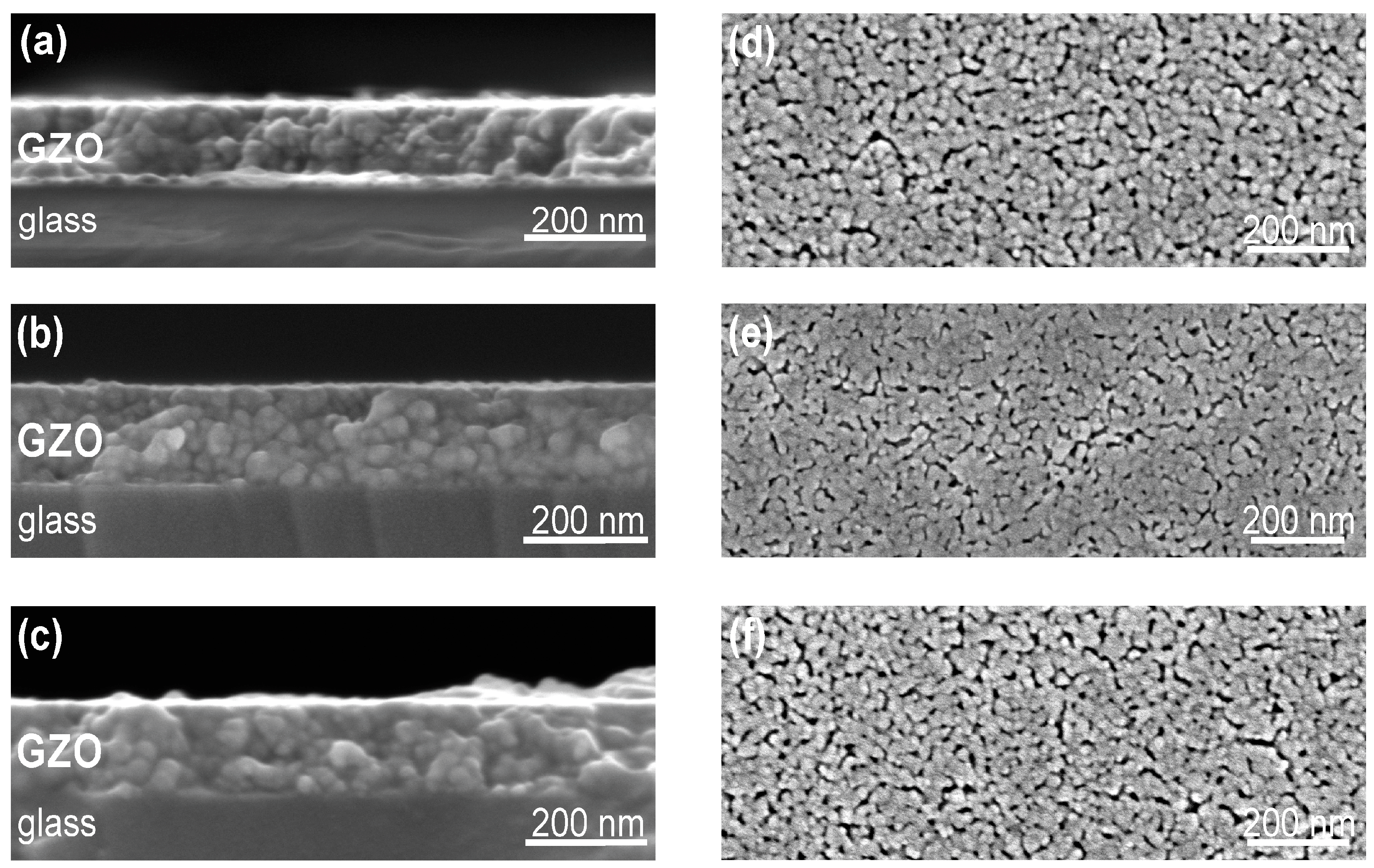
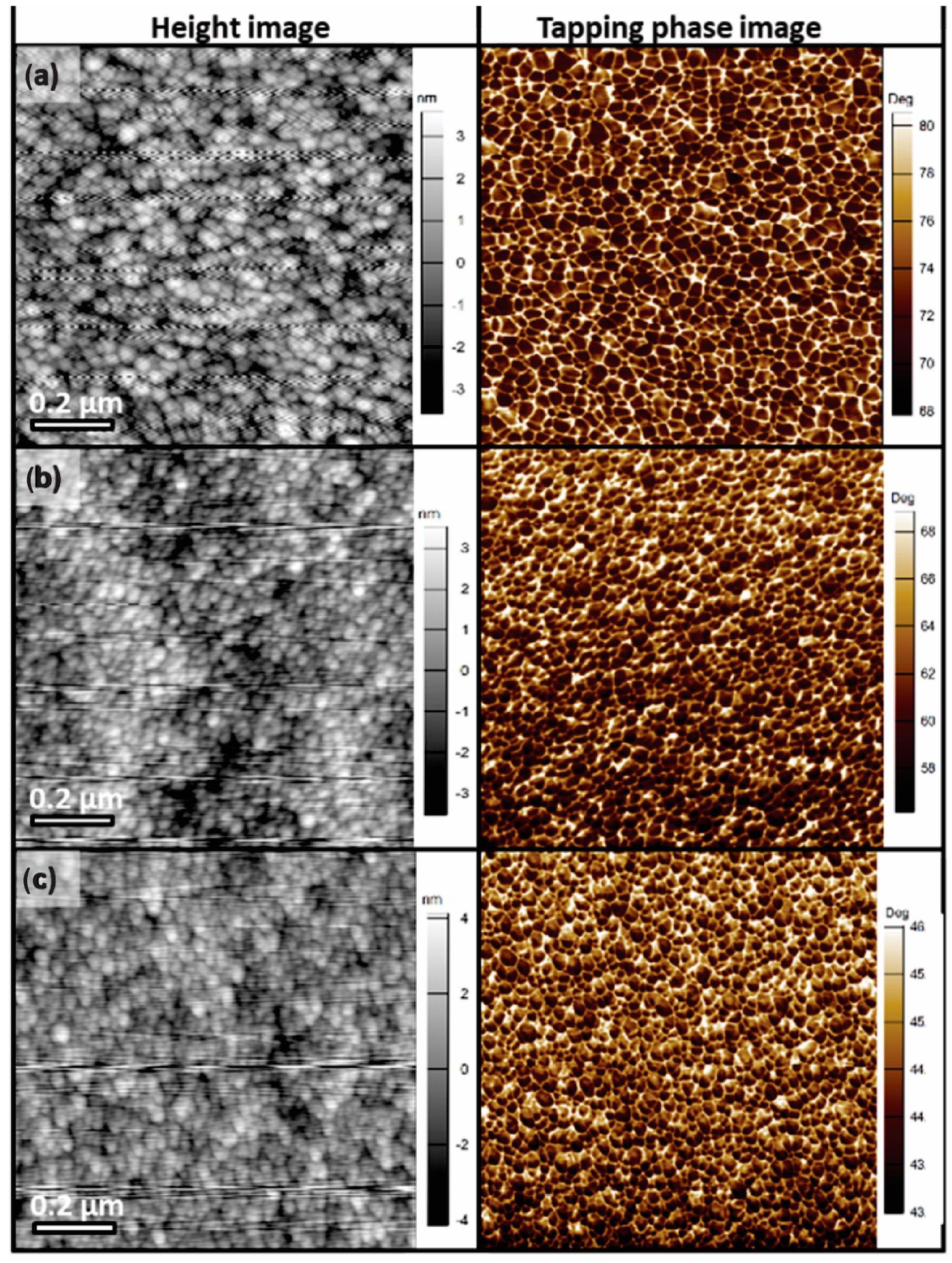
3.2. Microstructure and Electrical Properties of GZO Thin Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCluskey, M.D.; Jokela, S.J. Defects in ZnO. J. Appl. Phys. 2009, 106, 071101. [Google Scholar] [CrossRef] [Green Version]
- Van de Walle, C.G. Hydrogen as a Cause of Doping in Zinc Oxide. Phys. Rev. Lett. 2000, 85, 1012–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assadi, M.H.N.; Zheng, R.K.; Li, S.; Ringer, S.R. First-Principles Investigation of Electrical and Magnetic Properties of ZnO Based Diluted Magnetic Semiconductors Codoped with H. J. Appl. Phys. 2012, 111, 113901. [Google Scholar] [CrossRef]
- Cox, S.F.J.; Davis, E.A.; Cottrell, S.P.; King, P.J.C.; Lord, J.S.; Gil, J.M.; Alberto, H.V.; Vilão, R.C.; Piroto Duarte, J.; Ayres de Campos, N.; et al. Experimental Confirmation of the Predicted Shallow Donor Hydrogen State in Zinc Oxide. Phys. Rev. Lett. 2001, 86, 2601–2604. [Google Scholar] [CrossRef] [PubMed]
- Myong, S.Y.; Lim, K.S. Highly Stable and Textured Hydrogenated ZnO Thin Films. Appl. Phys. Lett. 2003, 82, 3026–3028. [Google Scholar] [CrossRef] [Green Version]
- Shannon, R.D.; Prewitt, C.T. Effective Ionic Radii in Oxides and Fluorides. Acta Crystallogr. B 1969, 25, 925–946. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zeng, H. ZnO-Based Transparent Conductive Thin Films: Doping, Performance, and Processing. J. Nanomater. 2013, 2013, 196521. [Google Scholar] [CrossRef] [Green Version]
- Norton, D.P.; Heo, Y.W.; Ivill, M.P.; Ip, K.; Pearton, S.J.; Chisholm, M.F.; Steiner, T. ZnO: Growth, Doping & Processing. Mater. Today 2004, 7, 34–40. [Google Scholar] [CrossRef]
- Sandeep, K.M.; Bhat, S.; Dharmaprakash, S.M.; Byrappa, K. Influence of Ga Doping Ratio on the Saturable Absorption Mechanism in Ga Doped ZnO Thin Solid Films Processed by Sol–Gel Spin Coating Technique. J. Phys. Appl. Phys. 2017, 50, 095105. [Google Scholar] [CrossRef]
- Look, D.C.; Leedy, K.D.; Vines, L.; Svensson, B.G.; Zubiaga, A.; Tuomisto, F.; Doutt, D.R.; Brillson, L.J. Self-Compensation in Semiconductors: The Zn Vacancy in Ga-Doped ZnO. Phys. Rev. B 2011, 84, 115202. [Google Scholar] [CrossRef] [Green Version]
- Winer, I.; Shter, G.E.; Mann-Lahav, M.; Grader, G.S. Effect of Solvents and Stabilizers on Sol–Gel Deposition of Ga-Doped Zinc Oxide TCO Films. J. Mater. Res. 2011, 26, 1309–1315. [Google Scholar] [CrossRef]
- Miranda, G.G.; de Sousa e Silva, R.L.; dos Santos Pessoni, H.V.; Franco, A. Raman Spectroscopy Study of Ga-Doped ZnO Ceramics: An Estimative of the Structural Disorder Degree. Phys. B Condens. Matter 2021, 606, 412726. [Google Scholar] [CrossRef]
- Ponja, S.D.; Sathasivam, S.; Parkin, I.P.; Carmalt, C.J. Highly Conductive and Transparent Gallium Doped Zinc Oxide Thin Films via Chemical Vapor Deposition. Sci. Rep. 2020, 10, 638. [Google Scholar] [CrossRef] [Green Version]
- Nayak, P.K.; Kim, J.; Chung, S.; Jeong, J.; Lee, C.; Hong, Y. Spin-Coated Ga-Doped ZnO Transparent Conducting Thin Films for Organic Light-Emitting Diodes. J. Phys. Appl. Phys. 2009, 42, 139801. [Google Scholar] [CrossRef]
- Yoon, M.H.; Lee, S.H.; Park, H.L.; Kim, H.K.; Jang, M.S. Solid Solubility Limits of Ga and Al in ZnO. J. Mater. Sci. Lett. 2002, 21, 1703–1704. [Google Scholar] [CrossRef]
- Bernardo, M.S.; Villanueva, P.G.; Jardiel, T.; Calatayud, D.G.; Peiteado, M.; Caballero, A.C. Ga-Doped ZnO Self-Assembled Nanostructures Obtained by Microwave-Assisted Hydrothermal Synthesis: Effect on Morphology and Optical Properties. J. Alloys Compd. 2017, 722, 920–927. [Google Scholar] [CrossRef]
- Sbeta, M.; Serin, T.; Yildiz, A. Determination of the Critical Carrier Concentration for the Metal–Insulator Transition in Ga-Doped ZnO. J. Mater. Sci. Mater. Electron. 2018, 29, 14111–14115. [Google Scholar] [CrossRef]
- Appani, S.K.; Singh, D.; Nandi, R.; Sutar, D.S.; Major, S.S. Influence of Oxygen Partial Pressure on the Strain Behaviour of Reactively Co-Sputtered Ga Doped ZnO Thin Films. Thin Solid Films 2023, 764, 139624. [Google Scholar] [CrossRef]
- Horng, R.-H.; Ou, S.-L.; Huang, C.-Y.; Ravadgar, P.; Wu, C.-I. Effects of Ga Concentration and Rapid Thermal Annealing on the Structural, Optoelectronic and Photoluminescence Properties of Ga-Doped ZnO Thin Films. Thin Solid Films 2016, 605, 30–36. [Google Scholar] [CrossRef]
- Rao, P.T.; Kumar, C.S.M. Resistivity Stability of Ga Doped ZnO Thin Films with Heat Treatment in Air and Oxygen Atmospheres. J. Cryst. Process Technol. 2012, 2, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Jun, M.-C.; Park, S.-U.; Koh, J.-H. Comparative Studies of Al-Doped ZnO and Ga-Doped ZnO Transparent Conducting Oxide Thin Films. Nanoscale Res. Lett. 2012, 7, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayami, R.; Endo, N.; Abe, T.; Miyase, Y.; Sagawa, T.; Yamamoto, K.; Tsukada, S.; Gunji, T. Zinc–Diethanolamine Complex: Synthesis, Characterization, and Formation Mechanism of Zinc Oxide via Thermal Decomposition. J. Sol-Gel Sci. Technol. 2018, 87, 743–748. [Google Scholar] [CrossRef]
- Serrao, F.J.; Dharmaprakash, S.M. Structural, Optical and Electrical Properties of Sol–Gel Prepared Ga:ZnO Thin Film. Mater. Res. Innov. 2016, 20, 470–474. [Google Scholar] [CrossRef]
- Nakagawara, O.; Kishimoto, Y.; Seto, H.; Koshido, Y.; Yoshino, Y.; Makino, T. Moisture-Resistant ZnO Transparent Conductive Films with Ga Heavy Doping. Appl. Phys. Lett. 2006, 89, 091904. [Google Scholar] [CrossRef]
- Akazawa, H. Degradation of Transparent Conductive Properties of Undoped ZnO and Ga-Doped ZnO Films Left in Atmospheric Ambient for Several Years and Trials to Recover Initial Conductance. J. Vac. Sci. Technol. Vac. Surf. Films 2014, 32, 021512. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Nakagawara, O.; Seto, H.; Koshido, Y.; Yoshino, Y. Improvement in Moisture Durability of ZnO Transparent Conductive Films with Ga Heavy Doping Process. Vacuum 2009, 83, 544–547. [Google Scholar] [CrossRef]
- Sato, Y.; Makino, H.; Yamamoto, N.; Yamamoto, T. Structural, Electrical and Moisture Resistance Properties of Ga-Doped ZnO Films. Thin Solid Films 2011, 520, 1395–1399. [Google Scholar] [CrossRef]
- Barasheed, A.Z.; Kumar, S.R.S.; Alshareef, H.N. Temperature Dependent Thermoelectric Properties of Chemically Derived Gallium Zinc Oxide Thin Films. J. Mater. Chem. C 2013, 1, 4122. [Google Scholar] [CrossRef]
- Schroder, D.K. Semiconductor Material and Device Characterization, 3rd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. i–xv. [Google Scholar]
- Znaidi, L.; Soler Illia, G.J.A.A.; Benyahia, S.; Sanchez, C.; Kanaev, A.V. Oriented ZnO Thin Films Synthesis by Sol–Gel Process for Laser Application. Thin Solid Films 2003, 428, 257–262. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, W.; Yu, J.; Zhi, Y.; Li, H.; Hu, Z.-Y.; Sang, X.; Guo, E.; Tang, W.; Wu, Z. One-Step Growth of Amorphous/Crystalline Ga2O3 Phase Junctions for High-Performance Solar-Blind Photodetection. ACS Appl. Mater. Interfaces 2019, 11, 45922–45929. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, Z.; Li, W.; Qiu, J. Preparation of ZnO Porous Thin Films by Sol–Gel Method Using PEG Template. Mater. Lett. 2005, 59, 3620–3625. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Ghaffarkani, M.; Ameri, M.; Safari, N.; Mohajerani, E. Solvent Selection for Fabrication of Low Temperature ZnO Electron Transport Layer in Perovskite Solar Cells. Opt. Mater. 2020, 106, 109977. [Google Scholar] [CrossRef]
- Socrates, G. Infrared Characteristic Group Frequencies Tables and Charts, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 1994. [Google Scholar]
- Oba, F.; Togo, A.; Tanaka, I.; Paier, J.; Kresse, G. Defect Energetics in ZnO: A Hybrid Hartree-Fock Density Functional Study. Phys. Rev. B 2008, 77, 245202. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.S.; Mamat, M.H.; Md. Sin, N.D.; Malek, M.F.; Zoolfakar, A.S.; Suriani, A.B.; Mohamed, A.; Ahmad, M.K.; Rusop, M. Fabrication of Hierarchical Sn-Doped ZnO Nanorod Arrays through Sonicated Sol−gel Immersion for Room Temperature, Resistive-Type Humidity Sensor Applications. Ceram. Int. 2016, 42, 9785–9795. [Google Scholar] [CrossRef]
- Lai, C.; Wang, X.; Zhao, Y.; Fong, H.; Zhu, Z. Effects of Humidity on the Ultraviolet Nanosensors of Aligned Electrospun ZnO Nanofibers. RSC Adv. 2013, 3, 6640. [Google Scholar] [CrossRef]
- Ghosh, M.; Ningthoujam, R.S.; Vatsa, R.K.; Das, D.; Nataraju, V.; Gadkari, S.C.; Gupta, S.K.; Bahadur, D. Role of Ambient Air on Photoluminescence and Electrical Conductivity of Assembly of ZnO Nanoparticles. J. Appl. Phys. 2011, 110, 054309. [Google Scholar] [CrossRef] [Green Version]
- Kushwaha, A.; Aslam, M. Hydrogen-Incorporated ZnO Nanowire Films: Stable and High Electrical Conductivity. J. Phys. Appl. Phys. 2013, 46, 485104. [Google Scholar] [CrossRef]



| Composition | Solvent | Annealing Conditions | Thickness [nm] | Resistivity [Ωcm] | Reference |
|---|---|---|---|---|---|
| GZO 1% Ga | 2-propanol | 580 °C, 60 min, air | 550 550 | / | Winer et al. [11] |
| 450 °C, 60 min, H2/N2 | 0.0068 | ||||
| GZO 1.5% Ga | 2-methoxyethanol | 650 °C, 90 min, air | 150 | 0.0495 | Jun et al. [21] |
| GZO 2% Ga | 2-methoxyethanol | 600 °C, 60 min, air | 300 300 | 0.960 | Nayak et al. [14] |
| 500 °C, 45 min, H2/N2 | 0.0033 | ||||
| GZO 2% Ga | ethanol | 500 °C, 60 min, air | 460 | 100 | Sbeta et al. [17] |
| 660 | 200 | ||||
| GZO 3% Ga | 2-methoxyethanol | 500 °C, 60 min, air | / | 0.00223 | Serrao et al. [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanoska, I.; Kmet, B.; Uršič, H.; Kuscer, D. Gallium-Modified Zinc Oxide Thin Films Prepared by Chemical Solution Deposition. Crystals 2023, 13, 1030. https://doi.org/10.3390/cryst13071030
Stojanoska I, Kmet B, Uršič H, Kuscer D. Gallium-Modified Zinc Oxide Thin Films Prepared by Chemical Solution Deposition. Crystals. 2023; 13(7):1030. https://doi.org/10.3390/cryst13071030
Chicago/Turabian StyleStojanoska, Izabela, Brigita Kmet, Hana Uršič, and Danjela Kuscer. 2023. "Gallium-Modified Zinc Oxide Thin Films Prepared by Chemical Solution Deposition" Crystals 13, no. 7: 1030. https://doi.org/10.3390/cryst13071030





