Synthesis of Peptides from Glycine on Anatases with Different Crystal Facets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
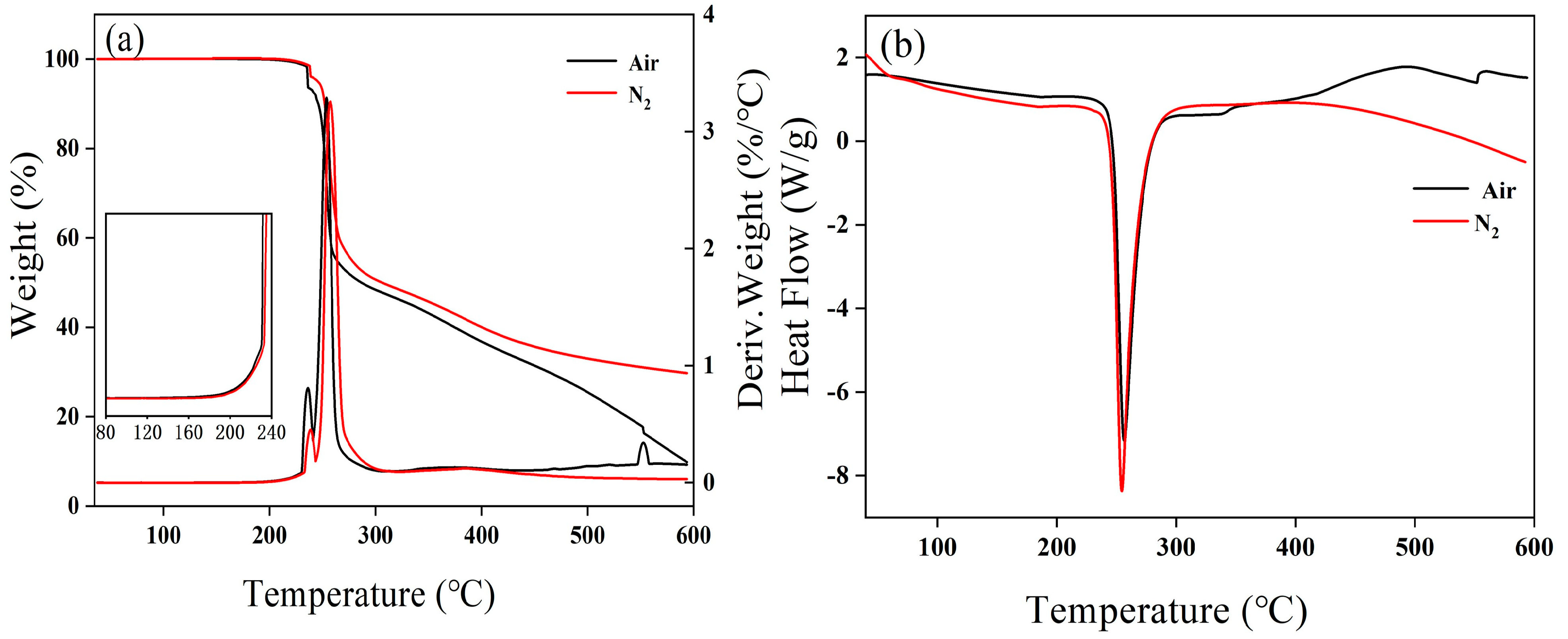
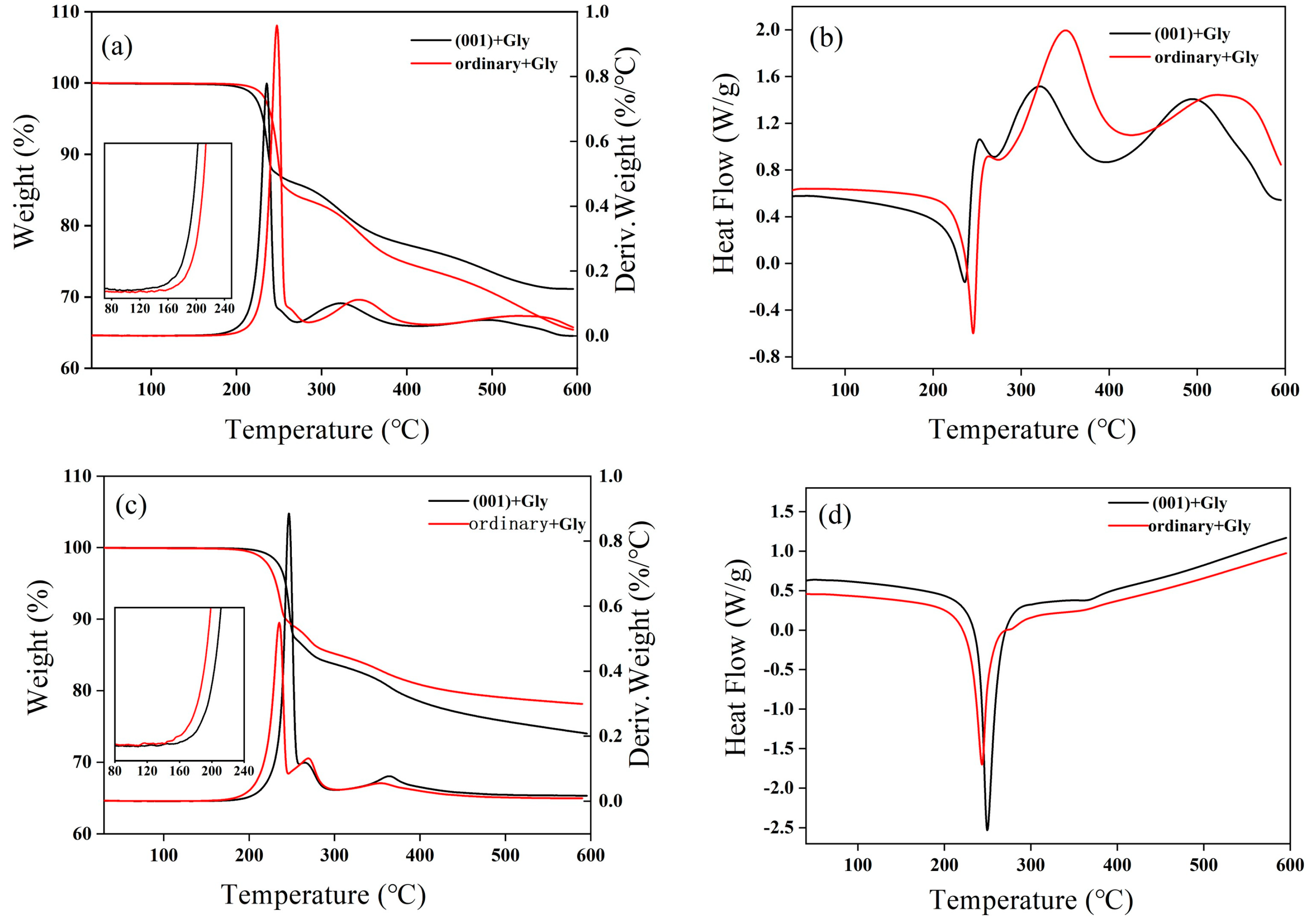
2.2. Thermal Analysis Experiments
2.3. Heating Experiment of Glycine on the Surface of Anatases
2.4. Illumination Experiment for Condensation of Glycine on the Surface of Anatases
2.5. HPLC Analysis
2.6. H-NMR Analysis
2.7. FTIR Analysis
2.8. Simulated Caculation
3. Results and Discussion
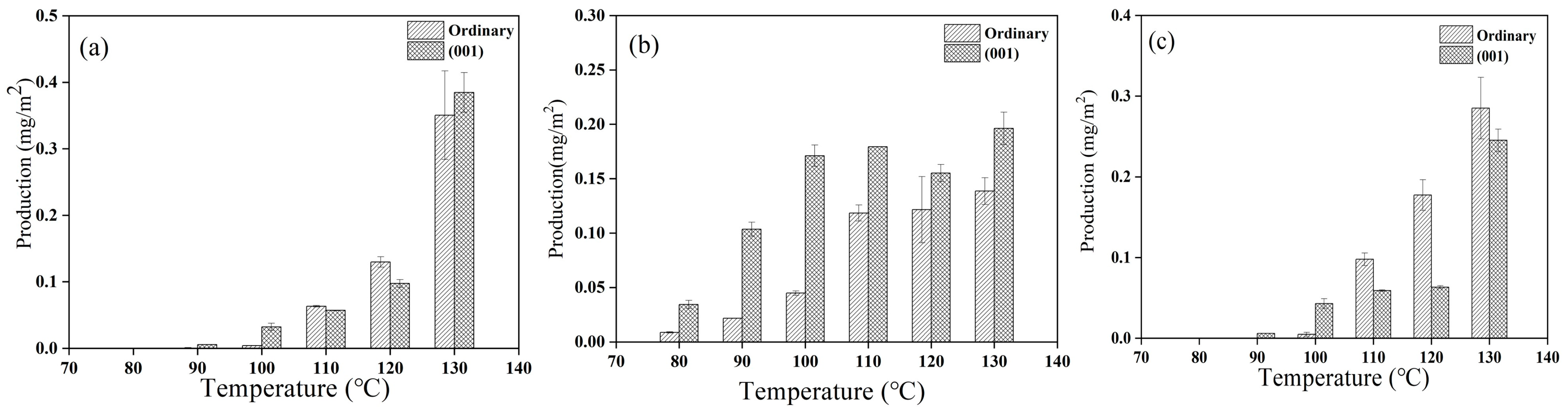
3.1. Effect of Temperature on the Condensation of Glycine on the Surface of Anatases with Different Crystal Facets
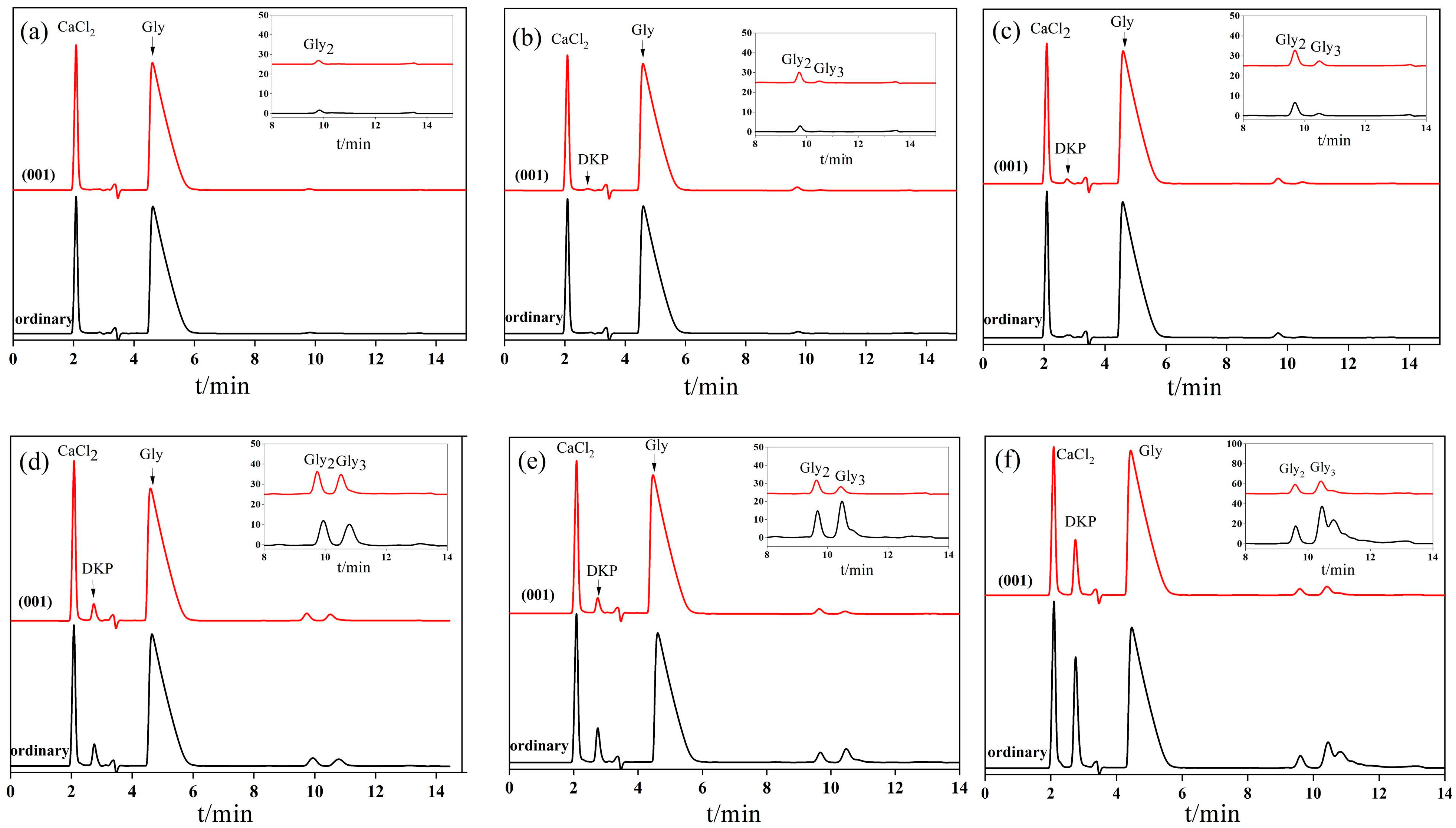
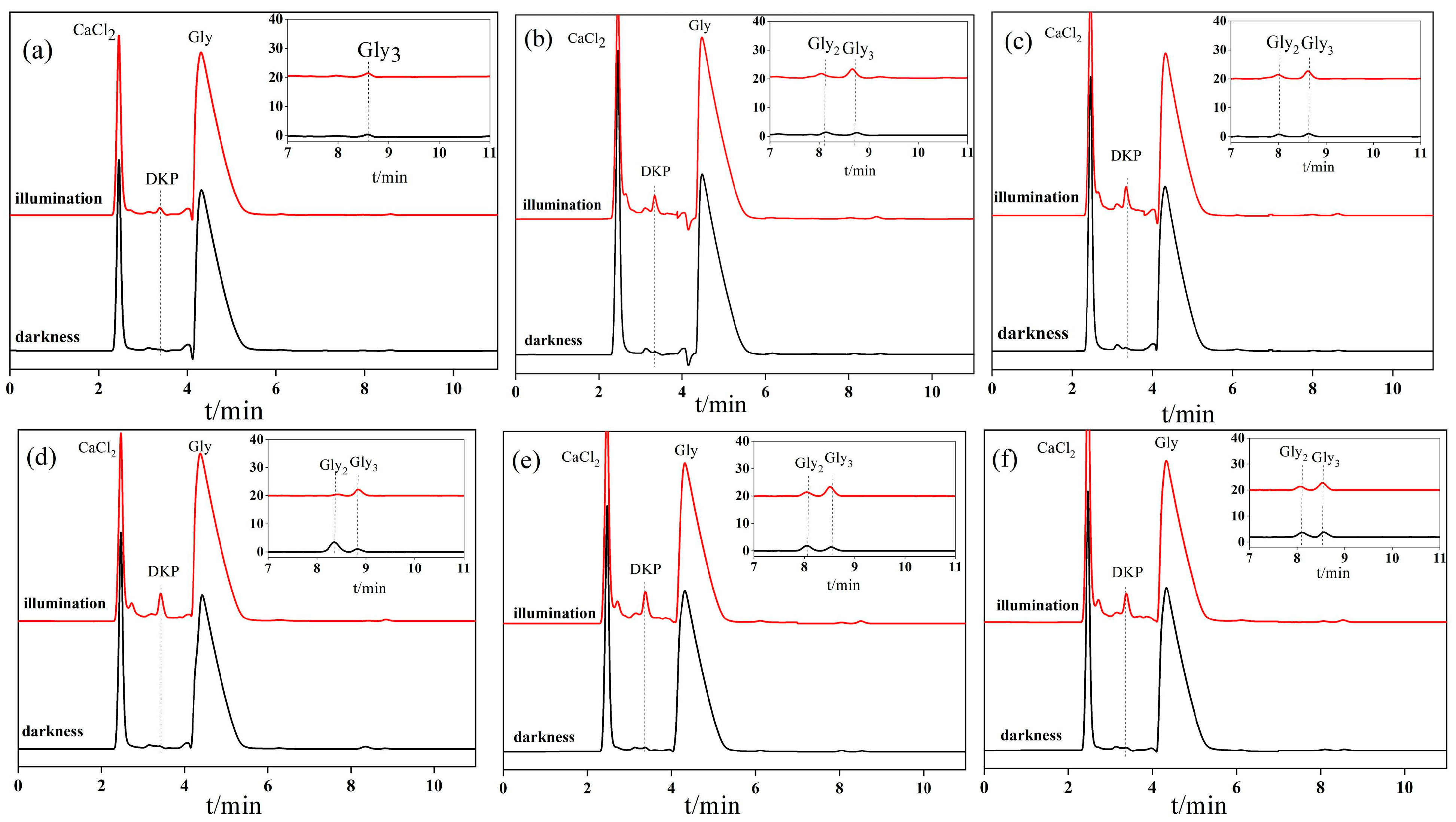
3.2. Glycine Condensation Products Catalyzed on the Surface of Anatases with Different Crystal Facets
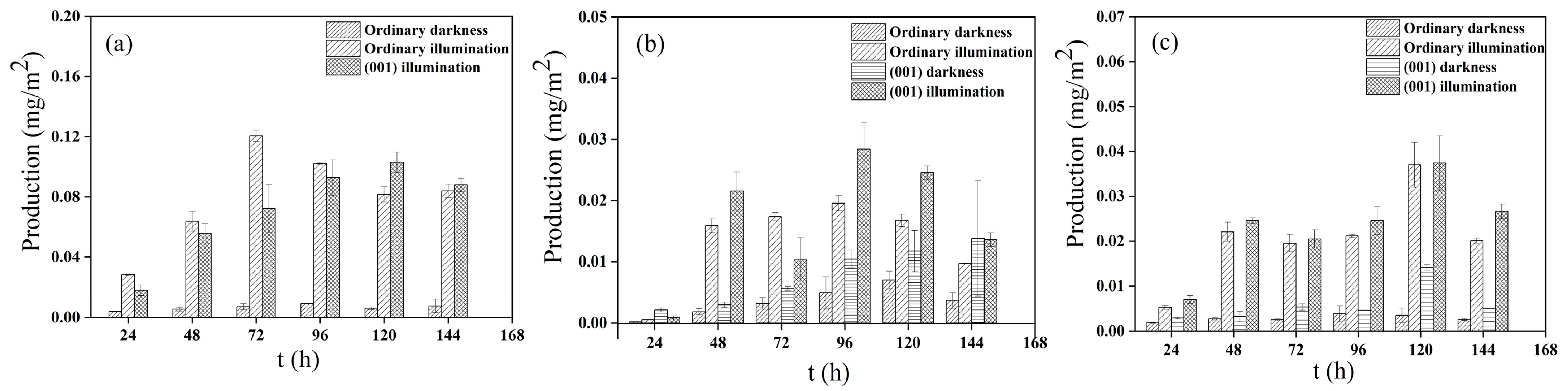
3.3. Analysis of Glycine Condensation Products Catalyzed on the Surface of Anatases with Different Crystal Facets under Light Irradiation
3.4. Identification of Peptides Condensed from Glycine though NMR
3.5. Analysis of Peptides Condensed from Glycine though FTIR
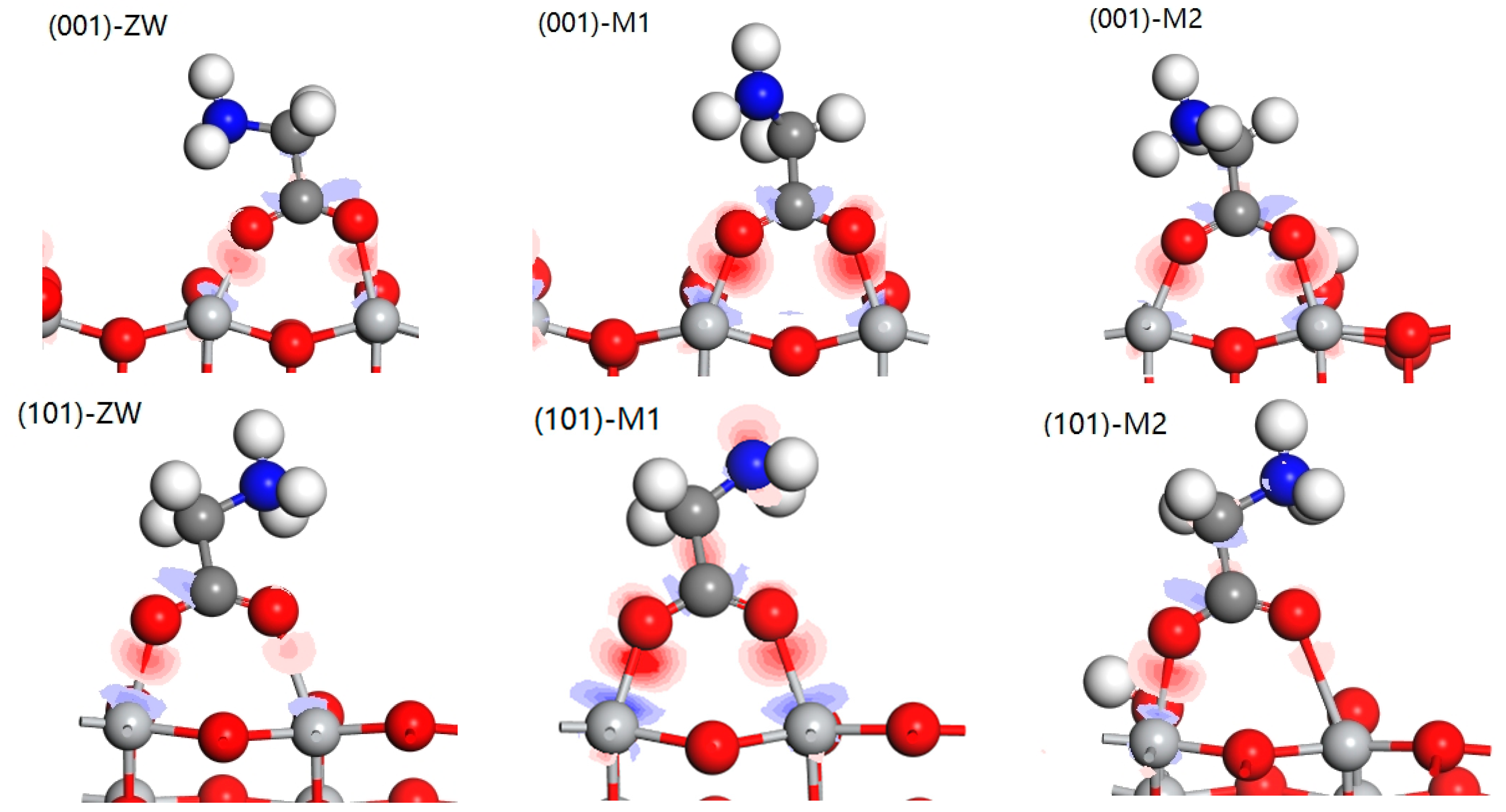
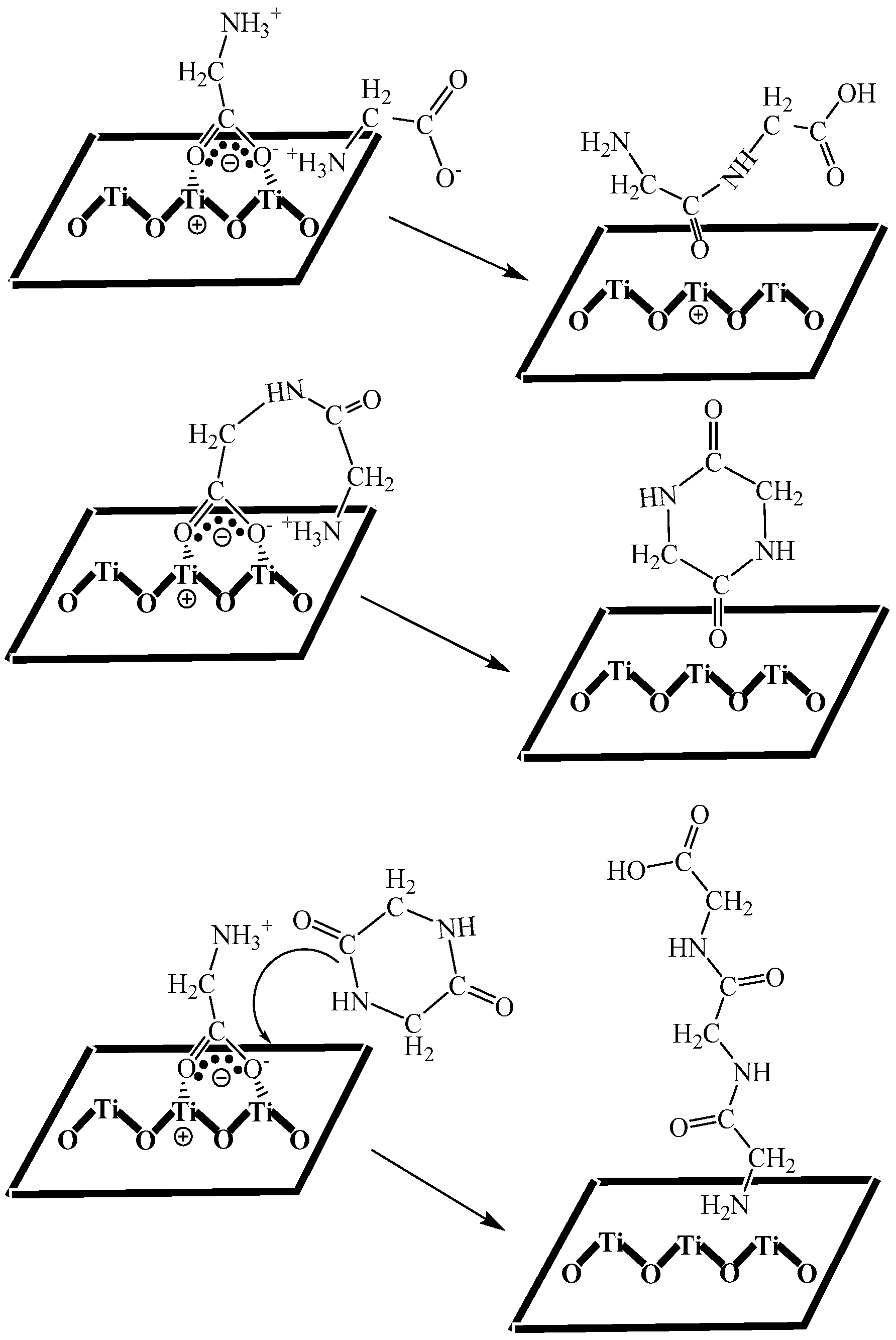
3.6. Mechanism Analysis of Glycine Condensation on the Surface of Anatases with Different Crystal Facets through Simulated Calculation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, A.; Li, Y.; LI, Y.; Ding, H.; Wang, C. The role of mineral photoelectron energy in the origin and evolution of early life on the Earth. Geol. Rev. 2023, 69, 234–246. [Google Scholar]
- El Samrout, O.; Fabbiani, M.; Berlier, G.; Lambert, J.F.; Martra, G. Emergence of Order in Origin-of-Life Scenarios on Mineral Surfaces: Polyglycine Chains on Silica. Langmuir 2022, 38, 15516–15525. [Google Scholar] [CrossRef]
- Hu, Q. Adsorption, Transformation and Condensation of Amino Acids at Fldspar Surfaces: Exploring the Roles of Minerals in the Origin of Life. Master’s Thesis, Southwest University, Chongqing, China, 2021. [Google Scholar]
- Jonsson, C.M.; Jonsson, C.L.; Sverjensky, D.A.; Cleaves, H.J.; Hazen, R.M. Attachment of l-glutamate to rutile (α-TiO2): A potentiometric, adsorption, and surface complexation study. Langmuir 2009, 25, 12127–12135. [Google Scholar] [CrossRef]
- Ustunol, I.B.; Gonzalez-Pech, N.I.; Grassian, V.H. pH-dependent adsorption of α-amino acids, lysine, glutamic acid, serine and glycine, on TiO2 nanoparticle surfaces. J. Colloid Interface Sci. 2019, 554, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.D. The Physical Basis of Life; Routledge and Paul: London, UK, 1951. [Google Scholar]
- Goldschmidt, V.M. Geochemical aspects of the origin of complex organic molecules on the Earth, as precursors to organic life. New Biol. 1952, 12, 97–105. [Google Scholar]
- Liu, Z.; Zhong, X.; Liu, Y.; Rao, H.; Wei, H.; Hu, W.; Liu, M. Adsorption and Mechanism of Glycine on the Anatase with Exposed (001) and (101) Facets. Minerals 2022, 12, 798. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, M.; Liu, Y.; Wei, H.; Zhang, W.; Nie, X.; Dong, F. Adsorption and thermal polymerization of glycine on surface of anatase. Acta Petrol. Mineral. 2018, 37, 505–511. [Google Scholar]
- Wu, J. Condensation of Amino Acids on Hydroxyapatite. Master’s Thesis, Zhejiang University, Hangzhou, China, 2010. [Google Scholar]
- Huber, C.; Eisenreich, W.; Hecht, S.; Wachtershauser, G. A possible primordial peptide cycle. Science 2003, 301, 938–940. [Google Scholar] [CrossRef]
- Chaudhuri, P.; Canuto, S. An ab initio study of the peptide bond formation between alanine and glycine: Electron correlation effects on the structure and binding energy. J. Mol. Struct. THEOCHEM 2002, 577, 267–279. [Google Scholar] [CrossRef]
- Chien, C.Y.; Yu, S.S. Ester-mediated peptide formation promoted by deep eutectic solvents: A facile pathway to proto-peptides. Chem. Commun. 2020, 56, 11949–11952. [Google Scholar] [CrossRef]
- Rimola, A.; Fabbiani, M.; Sodupe, M.; Ugliengo, P.; Martra, G. How does silica catalyze the amide bond formation under dry conditions? Role of specific surface silanol pairs. ACS Catal. 2018, 8, 4558–4568. [Google Scholar] [CrossRef]
- Rimola, A.; Tosoni, S.; Sodupe, M.; Ugliengo, P. Peptide bond formation activated by the interplay of lewis and brønsted catalysts. Chem. Phys. Lett. 2005, 408, 295–301. [Google Scholar] [CrossRef]
- Pantaleone, S.; Ugliengo, P.; Sodupe, M.; Rimola, A. When the surface matters: Prebiotic peptide-bond formation on the TiO2 (101) anatase surface through periodic DFT-D2 simulations. Chem. Eur. J. 2018, 24, 16292–16301. [Google Scholar] [CrossRef] [PubMed]
- El Samrout, O.; Berlier, G.; Lambert, J.F.; Martra, G. Polypeptide Chain Growth Mechanisms and Secondary Structure Formation in Glycine Gas-Phase Deposition on Silica Surfaces. J. Phys. Chem. B 2023, 127, 673–684. [Google Scholar] [CrossRef]
- de Castro Silva, F.; Lima, L.C.B.; Silva-Filho, E.C.; Fonseca, M.G.; Lambert, J.F.; Jaber, M. A comparative study of alanine adsorption and condensation to peptides in two clay minerals. Appl. Clay Sci. 2020, 192, 105617. [Google Scholar] [CrossRef]
- Zhang, Y. Regulation of Amino Acids on the Exposed Facet, Morphology and Crystal Phase of TiO2. Master’s Thesis, Changsha University of Science & Technology, Changsha, China, 2019. [Google Scholar]
- Pantaleone, S.; Rimola, A.; Canonical, M.S. Deprotonated or Zwitterionic? A Computational Study on Amino Acid Interaction with the TiO2 (101) Anatase Surface. J. Phys. Chem. C 2017, 121, 14156–14165. [Google Scholar] [CrossRef]
- Fleming, G.J.; Adib, K.; Rodriguez, J.A.; Barteau, M.A.; White, J.M.; Idriss, H. The adsorption and reactions of the amino acid proline on rutile TiO2 (110) surfaces. Surf. Sci. 2008, 602, 2029–2038. [Google Scholar]
- Thomas, A.G.; Flavell, W.R.; Chatwin, C.P.; Kumarasinghe, A.R.; Rayner, S.M.; Kirkham, P.F.; Tsoutsou, D.; Johal, T.K.; Patel, S. Adsorption of phenylalanine on single crystal rutile TiO2 (110) surface. Surf. Sci. 2007, 601, 3828–3832. [Google Scholar] [CrossRef]
- Tonner, R. Adsorption of proline and glycine on the TiO2 (110) surface: A density functional theory study. ChemPhysChem 2010, 11, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- YazdanYar, A.; Aschauer, U.; Bowen, P. Adsorption Free Energy of Single Amino Acids at the Rutile (110)/Water Interface Studied by Well-Tempered Metadynamics. J. Phys. Chem. C 2018, 122, 11355–11363. [Google Scholar] [CrossRef]
- Li, A.; Li, Z.; Xing, J.; Zhang, L.; Qin, X. Chemical Characterization of Different Vinegars by NMR-Based Metabolomic Approach. Food Sci. 2013, 34, 247–253. [Google Scholar]
- Hao, J.; Guo, J.; Xie, F.; Xia, Q.; Xie, J. Study on the mechanism of glycine pyrolysis to hydrocyanic acid. In Proceedings of the 2012 Annual Conference of the Chinese Tobacco Society, Chongqing, China, 11 September 2012. [Google Scholar]
- Meng, M.; Xia, L.; Guo, L. Adsorption and Thermal Condensation of Glycine on Kaolinite. Acta Phys.-Chim. Sin. 2007, 23, 32–36. [Google Scholar] [CrossRef]
- Bujdák, J.; Rode, B.M. Silica, alumina and clay catalyzed peptide bond formation: Enhanced efficiency of alumina catalyst. Orig. Life Evol. Biosph. 1999, 29, 451–461. [Google Scholar] [CrossRef]
- Shanker, U.; Bhushan, B.; Bhattacharjee, G. Oligomerization of glycine and alanine catalyzed by iron oxides: Implications for prebiotic chemistry. Orig. Life Evol. Biosph. 2012, 42, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Marshall-Bowman, K.; Ohara, S.; Sverjensky, D.A.; Hazen, R.M.; Cleaves, H.J. Catalytic peptide hydrolysis by mineral surface: Implications for prebiotic chemistry. Geochim. Cosmochim. Acta 2010, 74, 5852–5861. [Google Scholar] [CrossRef]
- Feng, J.; Pan, Y.; Ou, Y.; Li, W.; Deng, Z. Determination of the Configuration of a Compound Containing Labile Protons in Water Solution with NMR Techniques. J. Instrum. Anal. 2005, 24, 1–5. [Google Scholar]
- Kromhout, R.A.; Moulton, W.G. Nuclear magnetic resonance: Structure of the amino group I. J. Chem. Phys. 1955, 23, 1673–1679. [Google Scholar] [CrossRef]
- Kong, X. Synthesis and Characterization of Amino Acids Derived Chiral Poly (amide-imids)s Bearing Dipeptide in the Main Chain. Master’s Thesis, Qilu University of Technology, Jinan, China, 2015. [Google Scholar]
- Hori, S.S.; Yamauchi, K.; Kuroki, S.; Ando, I. Proton NMR Chemical Shift Behavior of Hydrogen-Bonded Amide Proton of Glycine-Containing Peptides and Polypeptides as Studied by ab initio MO Calculation. Int. J. Mol. Sci. 2002, 3, 907–913. [Google Scholar] [CrossRef]
- Hu, Z.; Luo, Y.; Zhang, C. The Investigation of Interaction on Peptide with Silver(I) Ion Using NMR Spectra. Chin. J. Spectrosc. Lab. 1999, 16, 27–33. [Google Scholar]
- Qin, H.; Zhao, T. Studies on the I Dentifi Cation of Traditional Chinese Herbal Medicines By 1HNMR. Acta Pharm. Sin. 1999, 34, 58–62. [Google Scholar]
- Max, J.J.; Trudel, M.; Chapados, C. Infrared titration of aqueous glycine. Appl. Spectrosc. 1998, 52, 226–233. [Google Scholar] [CrossRef]
- Ramachandran, E.; Baskaran, K.; Natarajan, S. XRD, thermal, FTIR and SEM studies on gel grown γ-glycine crystals. Cryst. Res. Technol. J. Exp. Ind. Crystallogr. 2007, 42, 73–77. [Google Scholar] [CrossRef]
- Larbot, A.; Laaziz, I.; Marignan, J.; Quinson, J.F. Porous texture of a titanium oxide gel: Evolution as a function of medium used. J. Non-Cryst. Solids 1992, 147, 157–161. [Google Scholar] [CrossRef]
- Bujdák, J.; Rode, B.M. The effect of smectite composition on the catalysis of peptide bond formation. J. Mol. Evol. 1996, 43, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Rimola, A.; Sodupe, M.; Ugliengo, P. Aluminosilicate surfaces as promoters for peptide bond formation: An assessment of Bernal’s hypothesis by ab Initio methods. J. Am. Chem. Soc. 2007, 129, 8333–8344. [Google Scholar] [CrossRef]
- Xia, L. Studies on the Adsorption and Thermal Condensation of Glycine on Kaolinite and Montmorillonite. Master’s Thesis, Tianjin University, Tianjin, China, 2007. [Google Scholar]
- Jonsson, C.M.; Jonsson, C.L.; Estrada, C.; Sverjensky, D.A.; Cleaves II, H.J.; Hazen, R.M. Adsorption of l-aspartate to rutile (α-TiO2): Experimental and theoretical surface complexation studies. Geochim. Cosmochim. Acta 2010, 74, 2356–2367. [Google Scholar] [CrossRef]
- Han, X.; Wang, X.; Xie, S.; Kuang, Q.; Ouyang, J.; Xie, Z.; Zheng, L. Carbonate ions-assisted syntheses of anatase TiO2 nanoparticles exposed with high energy (001) facets. Rsc Adv. 2012, 2, 3251–3253. [Google Scholar] [CrossRef]
- Gong, X.Q.; Selloni, A. Role of steps in the reactivity of the anatase TiO2 (101) surface. J. Catal. 2007, 249, 134–139. [Google Scholar] [CrossRef]
- Barcaro, G.; Sementa, L.; Carravetta, V.; Yano, T.; Hara, M.; Monti, S. Experimental and theoretical elucidation of catalytic pathways in TiO2-initiated prebiotic polymerization. Chem. Chem. Phys. 2019, 21, 5435–5447. [Google Scholar] [CrossRef]
- Schmidt, M. X-ray photoelectron spectroscopy studies on adsorption of amino acids from aqueous solutions onto oxidised titanium surfaces. Arch. Orthop. Trauma Surg. 2001, 12, 403–410. [Google Scholar] [CrossRef]




| Production | Standard Curve | R2 |
|---|---|---|
| DKP | y = 252.20x + 0.3087 | 0.9987 |
| Gly2 | y = 117.01x + 0.0692 | 0.9992 |
| Gly3 | y = 220.07x + 0.10329 | 0.9958 |
| Type of Vibration | Absorption Peak Displacement (cm−1) |
|---|---|
| υas (NH3+) | 3171.3 |
| υas (CH2) | 2917.3 |
| υas (COO–) | 1613.3 |
| δs (NH3+) | 1521.7 |
| ρw (CH2) | 1333.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhong, X.; Wu, H.; Liu, Z.; Nie, X.; Zhou, L.; Wei, H.; Hu, W.; Liu, M. Synthesis of Peptides from Glycine on Anatases with Different Crystal Facets. Crystals 2023, 13, 1113. https://doi.org/10.3390/cryst13071113
Chen J, Zhong X, Wu H, Liu Z, Nie X, Zhou L, Wei H, Hu W, Liu M. Synthesis of Peptides from Glycine on Anatases with Different Crystal Facets. Crystals. 2023; 13(7):1113. https://doi.org/10.3390/cryst13071113
Chicago/Turabian StyleChen, Jingping, Xiaomei Zhong, Haiyan Wu, Zeling Liu, Xiaoqin Nie, Lei Zhou, Hongfu Wei, Wenyuan Hu, and Mingxue Liu. 2023. "Synthesis of Peptides from Glycine on Anatases with Different Crystal Facets" Crystals 13, no. 7: 1113. https://doi.org/10.3390/cryst13071113
APA StyleChen, J., Zhong, X., Wu, H., Liu, Z., Nie, X., Zhou, L., Wei, H., Hu, W., & Liu, M. (2023). Synthesis of Peptides from Glycine on Anatases with Different Crystal Facets. Crystals, 13(7), 1113. https://doi.org/10.3390/cryst13071113






