New Olivine Reference Materials for Secondary Ion Mass Spectrometry Oxygen Isotope Measurements
Abstract
:1. Introduction
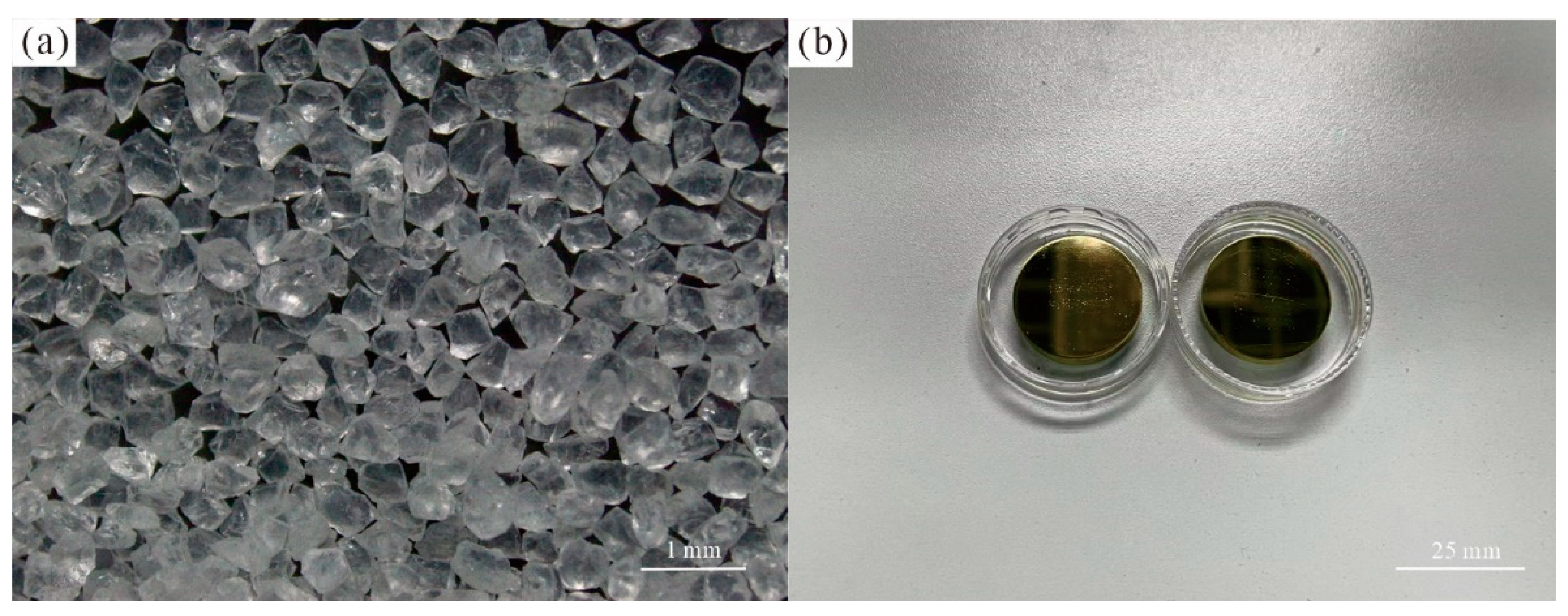
2. Materials and Methods
3. Results
3.1. Chemical Composition
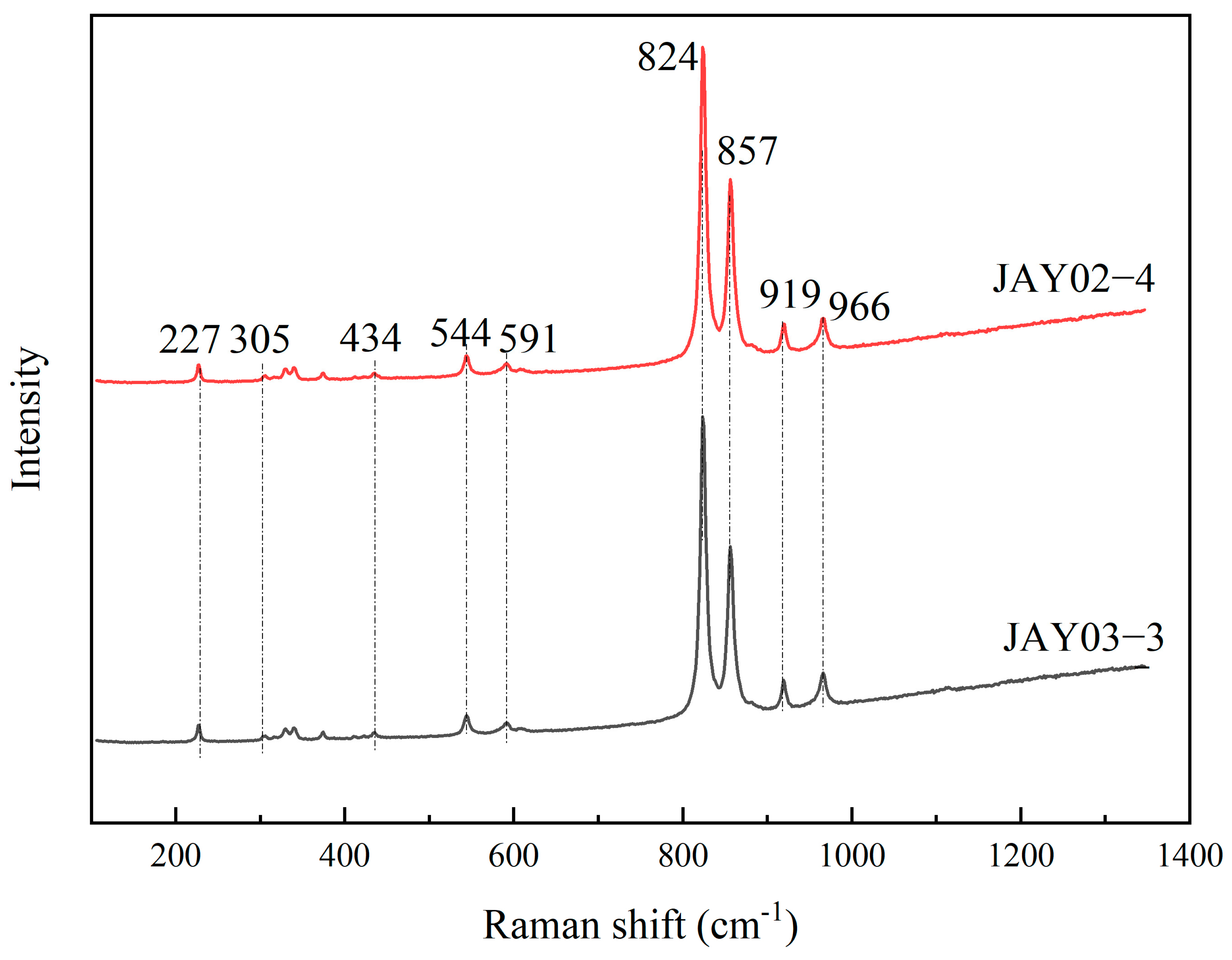
3.2. Raman Spectrum
3.3. Oxygen Isotope
4. Discussion
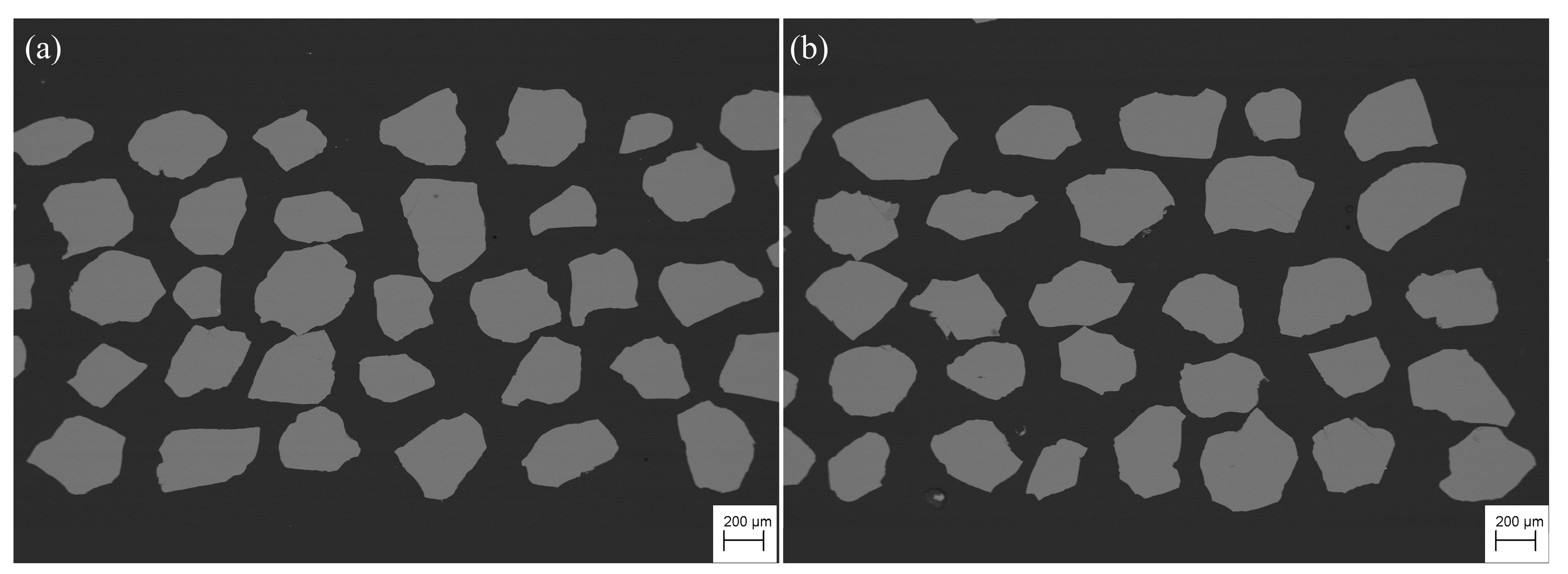
4.1. Homogeneity
4.2. Comparison with Other Reference Materials
5. Conclusions
- (1)
- The chemical compositions of the two olivine samples have extremely high Mg contents, with Fo values of 99.3 and 99.6, respectively. The Raman spectra of olivine have typical bands at approximately 227, 305, 434, 544, 591, 824, 857, 919, and 966 cm–1, with the characteristics of end-member forsterite. BSE images and Raman spectra show that olivine is homogeneous in chemical composition and free of inclusions.
- (2)
- The precision of SIMS analysis for sample JAY03-3 is 0.57‰ (2 s), and the precision of SIMS analysis for sample JAY02-4 is 0.70‰ (2 s). The SIMS oxygen isotope results suggest that the oxygen isotope compositions of the two olivine samples are homogeneous. These samples are suitable as standard materials for the SIMS oxygen isotope microanalysis of end-member forsterite. The recommended δ18O values of the two olivine materials are 16.37 ± 0.11‰ for sample JAY03-3 and 18.29 ± 0.14‰ for sample JAY02-4.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Hoog, J.C.M.; Gall, L.; Cornell, D.H. Trace-element geochemistry of mantle olivine and application to mantle petrogenesis and geothermobarometry. Chem. Geol. 2010, 270, 196–215. [Google Scholar] [CrossRef] [Green Version]
- Ferry, J.M.; Ushikubo, T.; Valley, J.W. Formation of Forsterite by Silicification of Dolomite during Contact Metamorphism. J. Petrol. 2011, 52, 1619–1640. [Google Scholar] [CrossRef] [Green Version]
- Nekrylov, N.; Plechov, P.Y.; Gritsenko, Y.D.; Portnyagin, M.V.; Shcherbakov, V.D.; Aydov, V.A.; Garbe-Schönberg, D. Major and trace element composition of olivine from magnesian skarns and silicate marbles. Am. Mineral. 2021, 106, 206–215. [Google Scholar] [CrossRef]
- Wang, J.; Su, B.-X.; Robinson, P.T.; Xiao, Y.; Bai, Y.; Liu, X.; Sakyi, P.A.; Jing, J.-J.; Chen, C.; Liang, Z.; et al. Trace elements in olivine: Proxies for petrogenesis, mineralization and discrimination of mafic-ultramafic rocks. Lithos 2021, 388, 106385. [Google Scholar] [CrossRef]
- Jiang, P.; Perfit, M.; Foster, D.A.; Trucco, A. Accurate analyses of key petrogenetic minor and trace elements in olivine by electron microprobe. Chem. Geol. 2022, 614, 121199. [Google Scholar] [CrossRef]
- Mourey, A.J.; Shea, T.; Lynn, K.J.; Lerner, A.H.; Lambart, S.; Costa, F.; Oalmann, J.; Lee, R.L.; Gansecki, C. Trace elements in olivine fingerprint the source of 2018 magmas and shed light on explosive-effusive eruption cycles at Kīlauea Volcano. Earth Planet. Sci. Lett. 2022, 595, 117769. [Google Scholar] [CrossRef]
- Demouchy, S.; Alard, O. Hydrogen, trace, and ultra-trace element distribution in natural olivines. Contrib. Mineral. Petrol. 2021, 176, 26. [Google Scholar] [CrossRef]
- Su, B.; Chen, Y.; Mao, Q.; Zhang, D.; Jia, L.-H.; Guo, S. Minor elements in olivine inspect the petrogenesis of orogenic peridotites. Lithos 2019, 344, 207–216. [Google Scholar] [CrossRef]
- Ferry, J.M.; Kitajima, K.; Strickland, A.; Valley, J.W. Ion microprobe survey of the grain-scale oxygen isotope geochemistry of minerals in metamorphic rocks. Geochim. Et Cosmochim. Acta 2014, 144, 403–433. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.-F.; Zou, H.; Xu, J.-F. Oxygen isotope heterogeneity of olivine crystals in orogenic peridotites from Songshugou, North Qinling Orogen: Petrogenesis and geodynamic implications. Am. Mineral. 2022, 107, 904–913. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Giuliani, A.; Cheng, Z.; Liu, B.; Kong, W. Geochemical and O–C–Sr–Nd Isotopic Constraints on the Petrogenetic Link between Aillikites and Carbonatites in the Tarim Large Igneous Province. J. Petrol. 2021, 62, egab017. [Google Scholar] [CrossRef]
- Günther, T.; Haase, K.M.; Junge, M.; Oberthür, T.; Woelki, D.; Krumm, S. Oxygen isotope and trace element compositions of platiniferous dunite pipes of the Bushveld Complex, South Africa—Signals from a recycled mantle component? Lithos 2018, 310, 332–341. [Google Scholar] [CrossRef]
- Eiler, J.M.; Graham, C.; Valley, J.W. SIMS analysis of oxygen isotopes: Matrix effects in complex minerals and glasses. Chem. Geol. 1997, 138, 221–244. [Google Scholar] [CrossRef]
- Valley, J.W.; Kita, N.T. In situ oxygen isotope geochemistry by ion microprobe. In Mineralogical Association of Canada, Short Course; Mineralogical Association of Canada: Toronto, ON, Canada, 2009; pp. 19–63. [Google Scholar]
- Vho, A.; Rubatto, D.; Putlitz, B.; Bouvier, A.S. New Reference Materials and Assessment of Matrix Effects for SIMS Measurements of Oxygen Isotopes in Garnet. Geostand. Geoanal. Res. 2020, 44, 459–471. [Google Scholar] [CrossRef]
- Tang, G.Q.; Su, B.X.; Li, Q.L.; Xia, X.P.; Jing, J.J.; Feng, L.J.; Martin, L.; Yang, Q.; Li, X.H. High-Mg# Olivine, Clinopyroxene and Orthopyroxene Reference Materials for In Situ Oxygen Isotope Determination. Geostand. Geoanal. Res. 2019, 43, 585–593. [Google Scholar] [CrossRef]
- Scicchitano, M.R.; Rubatto, D.; Hermann, J.; Majumdar, A.S.; Putnis, A. Oxygen isotope analysis of olivine by ion microprobe: Matrix effects and applications to a serpentinised dunite. Chem. Geol. 2018, 499, 126–137. [Google Scholar] [CrossRef]
- Page, F.Z.; Kita, N.T.; Valley, J.W. Ion microprobe analysis of oxygen isotopes in garnets of complex chemistry. Chem. Geol. 2010, 270, 9–19. [Google Scholar] [CrossRef]
- Wudarska, A.; Wiedenbeck, M.; Słaby, E.; Lempart-Drozd, M.; Harris, C.; Joachimski, M.M.; Lécuyer, C.; MacLeod, K.G.; Pack, A.; Vennemann, T.; et al. Inter-laboratory Characterisation of Apatite Reference Materials for Oxygen Isotope Analysis and Associated Methodological Considerations. Geostand. Geoanal. Res. 2022, 46, 277–306. [Google Scholar] [CrossRef]
- Seitz, S.; Baumgartner, L.P.; Bouvier, A.-S.; Putlitz, B.; Vennemann, T. Quartz Reference Materials for Oxygen Isotope Analysis by SIMS. Geostand. Geoanal. Res. 2017, 41, 69–75. [Google Scholar] [CrossRef]
- Siron, G.; Baumgartner, L.; Bouvier, A.-S.; Putlitz, B.; Vennemann, T. Biotite Reference Materials for Secondary Ion Mass Spectrometry 18O/16O Measurements. Geostand. Geoanal. Res. 2017, 41, 243–253. [Google Scholar] [CrossRef]
- Isa, J.; Kohl, I.E.; Liu, M.C.; Wasson, J.T.; Young, E.D.; McKeegan, K.D. Quantification of oxygen isotope SIMS matrix effects in olivine samples: Correlation with sputter rate. Chem. Geol. 2017, 458, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Fukuda, K.; Spicuzza, M.J.; Siron, G.; Heimann, A.; Hammerstrom, A.J.; Kita, N.T.; Ushikubo, T.; Valley, J.W. SIMS matrix effects in oxygen isotope analysis of olivine and pyroxene: Application to Acfer 094 chondrite chondrules and reconsideration of the primitive chondrule minerals (PCM) line. Chem. Geol. 2022, 608, 121016. [Google Scholar] [CrossRef]
- Peng, B.; He, M.; Yang, M.; Liu, X.; Sui, X.; Sun, K.; Wu, S. Petrogenesis of Jian forsterite jade solely composed of end-member forsterite (Fo 99.8): Constrained by trace element and oxygen isotope. Ore Geol. Rev. 2022, 150, 105167. [Google Scholar] [CrossRef]
- Wang, Y.; He, M.; Yan, W.; Yang, M.; Liu, X. Jianite: Massive Dunite Solely Made of Virtually Pure Forsterite from Ji’an County, Jilin Province, Northeast China. Minerals 2020, 10, 220. [Google Scholar] [CrossRef] [Green Version]
- Peng, B.; He, M.; Yang, M.; Wu, S.; Fan, J. Natural Forsterite Strongly Enriched in Boron: Crystal Structure and Spectroscopy. Crystals 2022, 12, 975. [Google Scholar] [CrossRef]
- Peres, P.; Kita, N.T.; Valley, J.W.; Fernandes, F.; Schuhmacher, M. New sample holder geometry for high precision isotope analyses. Surf. Interface Anal. 2013, 45, 553–556. [Google Scholar] [CrossRef]
- Li, X.-H.; Long, W.-G.; Li, Q.-L.; Liu, Y.; Zheng, Y.-F.; Yang, Y.-H.; Chamberlain, K.R.; Wan, D.-F.; Guo, C.-H.; Wang, X.-C.; et al. Penglai Zircon Megacrysts: A Potential New Working Reference Material for Microbeam Determination of Hf-O Isotopes and U-Pb Age. Geostand. Geoanal. Res. 2010, 34, 117–134. [Google Scholar] [CrossRef]
- Gong, B.; Zheng, Y.-F.; Chen, R.-X. TC/EA-MS online determination of hydrogen isotope composition and water concentration in eclogitic garnet. Phys. Chem. Miner. 2007, 34, 687–698. [Google Scholar] [CrossRef]
- Eiler, J.M.; Farley, K.A.; Valley, J.W.; Stolper, E.M.; Hauri, E.H.; Craig, H. Oxygen isotope evidence against bulk recycled sediment in the mantle sources of Pitcairn Island lavas. Nature 1995, 377, 138–141. [Google Scholar] [CrossRef]
- Baertschi, P. Absolute18O content of standard mean ocean water. Earth Planet. Sci. Lett. 1976, 31, 341–344. [Google Scholar] [CrossRef]
- Breitenfeld, L.; Dyar, M.; Carey, C.; Tague Jr, T.; Wang, P.; Mullen, T.; Parente, M. Predicting olivine composition using Raman spectroscopy through band shift and multivariate analyses. Am. Mineral. 2018, 103, 1827–1836. [Google Scholar] [CrossRef]
- Kuebler, K.E.; Jolliff, B.L.; Wang, A.; Haskin, L.A. Extracting olivine (Fo–Fa) compositions from Raman spectral peak positions. Geochim. Et Cosmochim. Acta 2006, 70, 6201–6222. [Google Scholar] [CrossRef]
- Guo, J.; Guo, F.; Yan Wang, C.; Li, C. Crustal recycling processes in generating the early Cretaceous Fangcheng basalts, North China Craton: New constraints from mineral chemistry, oxygen isotopes of olivine and whole-rock geochemistry. Lithos 2013, 170, 1–16. [Google Scholar] [CrossRef]
- Ling, X.; Li, Q.; Feng, L.; Zhang, D.; Liu, Y.; Tang, G.; Li, J.; Wu, S.; Huang, L.; Li, T.; et al. Beryl Reference Materials for In Situ Oxygen Isotope Determination. Crystals 2021, 11, 1322. [Google Scholar] [CrossRef]
- Wiedenbeck, M.; Trumbull, R.B.; Rosner, M.; Boyce, A.; Fournelle, J.H.; Franchi, I.A.; Halama, R.; Harris, C.; Lacey, J.H.; Marschall, H.; et al. Tourmaline Reference Materials for the In Situ Analysis of Oxygen and Lithium Isotope Ratio Compositions. Geostand. Geoanal. Res. 2020, 45, 97–119. [Google Scholar] [CrossRef]
- Li, Y.; Tang, G.-Q.; Liu, Y.; He, S.; Chen, B.; Li, Q.-L.; Li, X.-H. Revisiting apatite SIMS oxygen isotope analysis and Qinghu-AP reference material. Chem. Geol. 2021, 582, 120445. [Google Scholar] [CrossRef]
- Ávila, J.N.; Ireland, T.R.; Holden, P.; Lanc, P.; Latimore, A.; Schram, N.; Foster, J.; Williams, I.S.; Loiselle, L.; Fu, B. High-Precision, High-Accuracy Oxygen Isotope Measurements of Zircon Reference Materials with the SHRIMP-SI. Geostand. Geoanal. Res. 2019, 44, 85–102. [Google Scholar] [CrossRef]

| Sample | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MnO | NiO | MgO | CaO | Na2O | Total | Fo |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| JAY03-3-1 * | 42.32 | 0.00 | 0.02 | 0.08 | 0.76 | 0.06 | 0.00 | 57.12 | 0.01 | 0.01 | 100.37 | 99.26 |
| JAY03-3-2 * | 41.98 | 0.01 | 0.01 | 0.03 | 0.70 | 0.02 | 0.05 | 57.68 | 0.01 | 0.00 | 100.50 | 99.32 |
| JAY03-3-3 * | 42.01 | 0.04 | 0.02 | 0.09 | 0.76 | 0.00 | 0.00 | 57.53 | 0.01 | 0.00 | 100.43 | 99.27 |
| JAY03-3-4 * | 41.41 | 0.00 | 0.00 | 0.00 | 0.77 | 0.03 | 0.02 | 57.01 | 0.02 | 0.00 | 99.26 | 99.25 |
| mean | 41.93 | 0.01 | 0.01 | 0.05 | 0.75 | 0.03 | 0.02 | 57.33 | 0.01 | 0.00 | 100.14 | 99.28 |
| JAY02-4-1 | 43.34 | 0.00 | 0.00 | 0.00 | 0.35 | 0.03 | 0.03 | 56.01 | 0.02 | 0.04 | 99.82 | 99.65 |
| JAY02-4-2 | 42.61 | 0.07 | 0.02 | 0.00 | 0.38 | 0.08 | 0.02 | 56.20 | 0.01 | 0.04 | 99.41 | 99.62 |
| JAY02-4-3 | 41.99 | 0.04 | 0.02 | 0.00 | 0.35 | 0.05 | 0.06 | 56.69 | 0.00 | 0.01 | 99.21 | 99.66 |
| JAY02-4-4 | 42.15 | 0.00 | 0.03 | 0.00 | 0.40 | 0.06 | 0.02 | 56.73 | 0.01 | 0.03 | 99.43 | 99.61 |
| JAY02-4-5 | 42.31 | 0.02 | 0.02 | 0.01 | 0.35 | 0.01 | 0.06 | 56.90 | 0.02 | 0.03 | 99.74 | 99.65 |
| JAY02-4-6 | 42.26 | 0.00 | 0.00 | 0.00 | 0.35 | 0.00 | 0.06 | 56.96 | 0.02 | 0.00 | 99.65 | 99.66 |
| JAY02-4-7 | 41.91 | 0.00 | 0.03 | 0.00 | 0.35 | 0.05 | 0.05 | 57.08 | 0.01 | 0.01 | 99.48 | 99.66 |
| JAY02-4-8 | 41.04 | 0.00 | 0.00 | 0.01 | 0.42 | 0.02 | 0.00 | 57.13 | 0.01 | 0.01 | 98.65 | 99.59 |
| JAY02-4-9 | 41.78 | 0.00 | 0.00 | 0.03 | 0.41 | 0.00 | 0.00 | 57.38 | 0.00 | 0.02 | 99.60 | 99.60 |
| JAY02-4-10 | 42.16 | 0.00 | 0.00 | 0.00 | 0.36 | 0.00 | 0.06 | 56.88 | 0.02 | 0.00 | 99.48 | 99.64 |
| mean | 42.16 | 0.01 | 0.01 | 0.01 | 0.37 | 0.03 | 0.04 | 56.80 | 0.01 | 0.02 | 99.45 | 99.63 |
| Sample | δ18OVSMOW * (‰) | 1σ | n | |
|---|---|---|---|---|
| JAY02-4 | IGCAS | 18.29 | 0.14 | 2 |
| USTC | 18.28 | - | 1 | |
| mean | 18.29 | 0.14 | ||
| JAY03-3 | IGCAS | 16.57 | 0.11 | 2 |
| USTC | 16.17 | - | 1 | |
| mean | 16.37 | 0.11 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, B.; He, M.; Yang, M.; Shi, Y. New Olivine Reference Materials for Secondary Ion Mass Spectrometry Oxygen Isotope Measurements. Crystals 2023, 13, 987. https://doi.org/10.3390/cryst13070987
Peng B, He M, Yang M, Shi Y. New Olivine Reference Materials for Secondary Ion Mass Spectrometry Oxygen Isotope Measurements. Crystals. 2023; 13(7):987. https://doi.org/10.3390/cryst13070987
Chicago/Turabian StylePeng, Bijie, Mingyue He, Mei Yang, and Yujia Shi. 2023. "New Olivine Reference Materials for Secondary Ion Mass Spectrometry Oxygen Isotope Measurements" Crystals 13, no. 7: 987. https://doi.org/10.3390/cryst13070987




