Base-Controlled Regiospecific Mono-Benzylation/Allylation and Diallylation of 4-Aryl-5-indolyl-1,2,4-triazole-3-thione: Thio-Aza Allyl Rearrangement
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedures
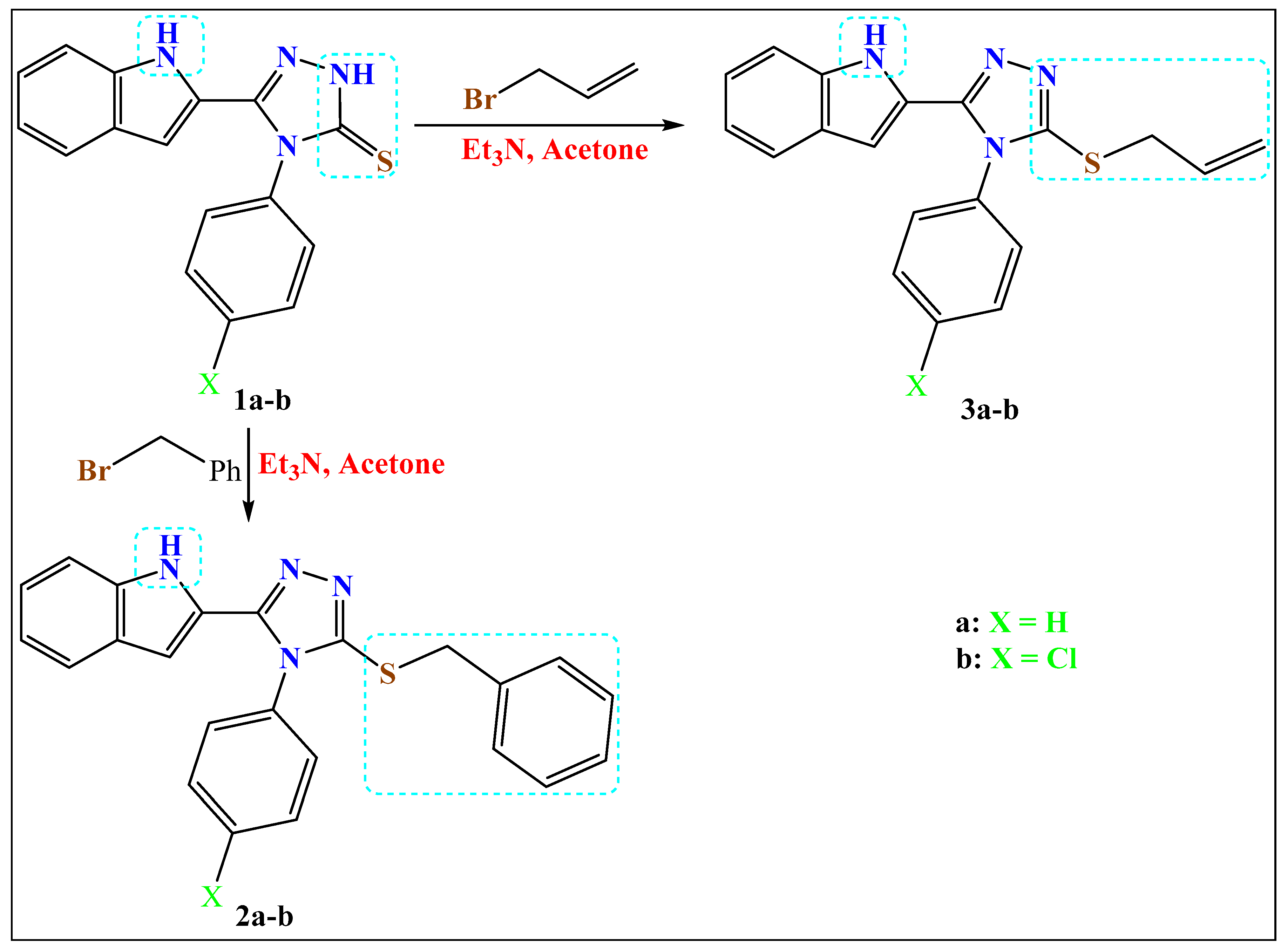
2.1.1. Method a: Synthesis of the S-Alkylated Products
- 3-(Benzylsulfanyl)-4-phenyl-5-(1H-indol-2-yl)-1,2,4-triazole (2a)
- 3-(Benzylsulfanyl)-4-(4-chlorophenyl)-5-(1H-indol-2-yl)-1,2,4-triazole (2b)
- 3-(All-1-ylsulfanyl)-4-phenyl-5-(1H-indol-2-yl)-1,2,4-triazole (3a)
- 3-(All-1-ylsulfanyl)-4-(4-chlorophenyl)-5-(1H-indol-2-yl)-1,2,4-triazole (3b)
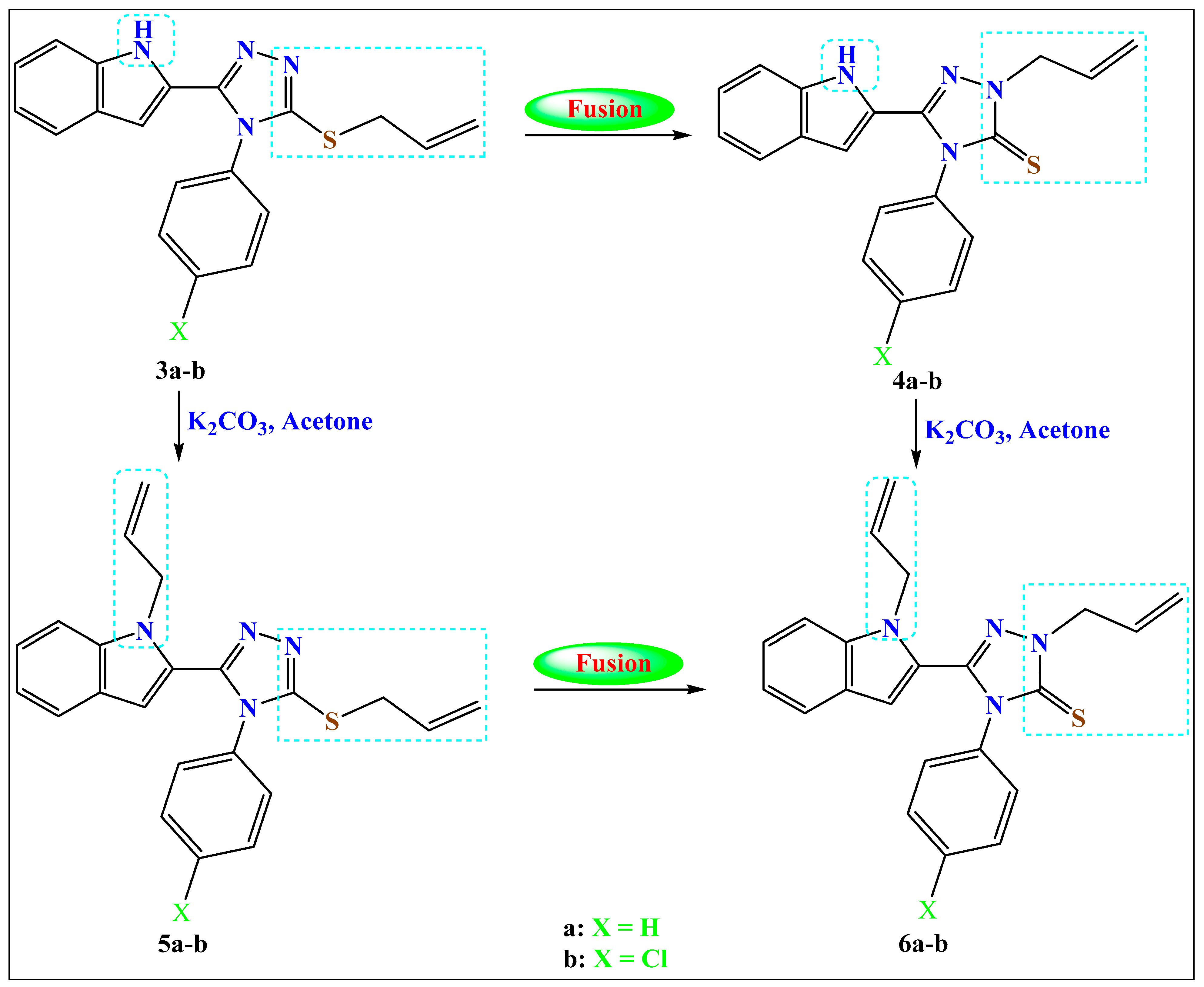
2.1.2. Method b: Fusion of the Allyl-sulfanyl Isomers (Syhthesis of the N-Allylated 4a–b and N-,N-Diallylated Compounds 6a–b)
- 2-(All-1-yl)-4-phenyl-5-(1H-indol-2-yl)-3-thioxo-1,2,4-triazole (4a)
- 2-(All-1-yl)-4-(4-chlorophenyl)-5-(1H-indol-2-yl)-3-thioxo-1,2,4-triazole (4b)
2.1.3. Method c: Synthesis of S,N-Diallylated Compounds 5a–b and N,N-Diallylated Compounds 6a–b
- 3-(All-1-ylsulfanyl)-5-((1-all-1-yl)-indol-2-yl)-4-phenyl-1,2,4-triazole (5a)
- 3-(All-1-ylsulfanyl)-5-((1-all-1-yl)-indol-2-yl)-4-(4-chlorophenyl)-1,2,4-triazole (5b)
- 2-(All-1-yl)-5-((1-all-1-yl)-indol-2-yl)-4-phenyl-3-thioxo-1,2,4-triazole (6a)
- 2-(All-1-yl)-5-((1-all-1-yl)-indol-2-yl)-4-(4-chlorophenyl)-3-thioxo-1,2,4-triazole (6b)
2.2. Crystal Structure Determination
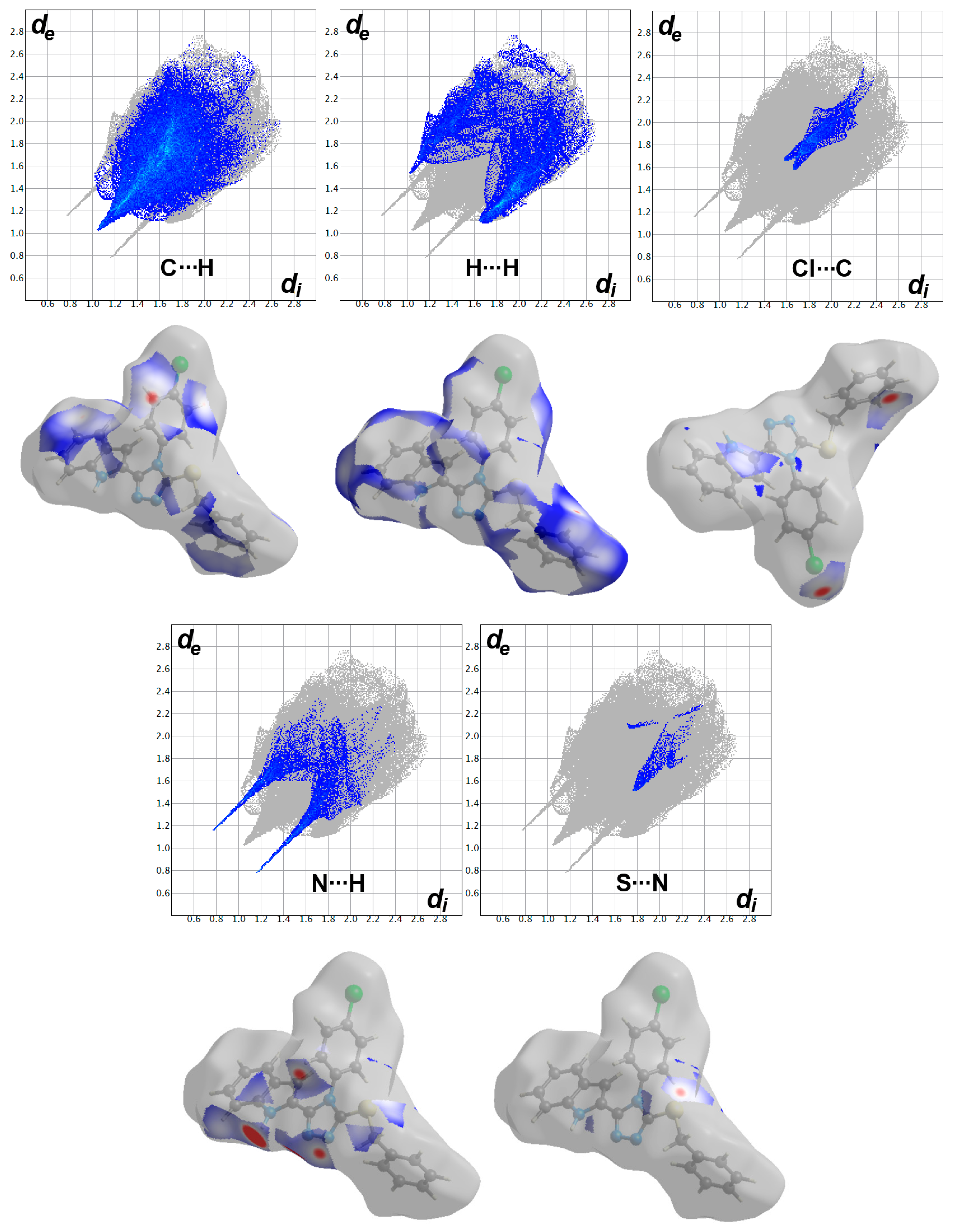
2.3. Hirshfeld Surface Analysis
3. Results and Discussion
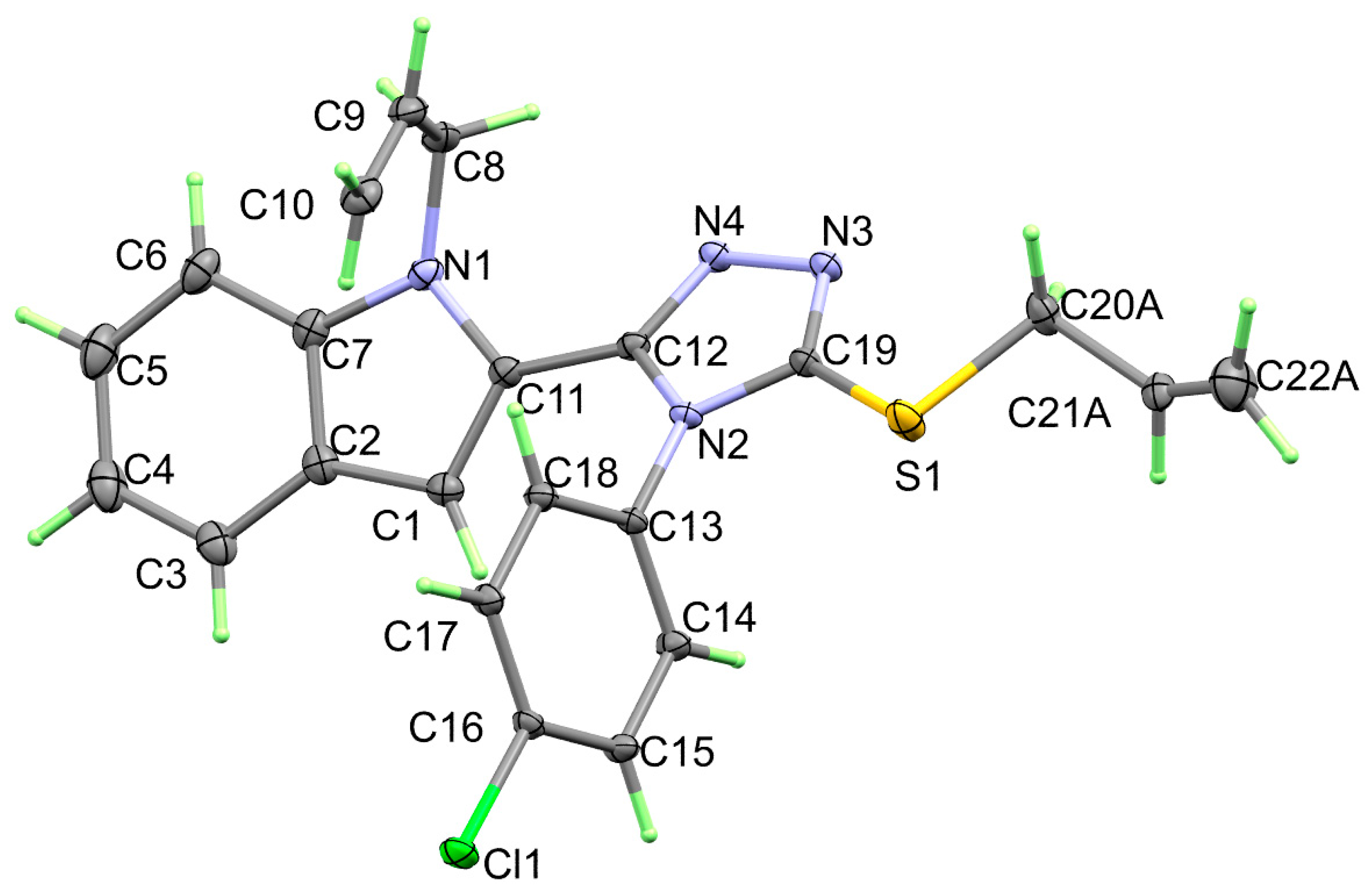
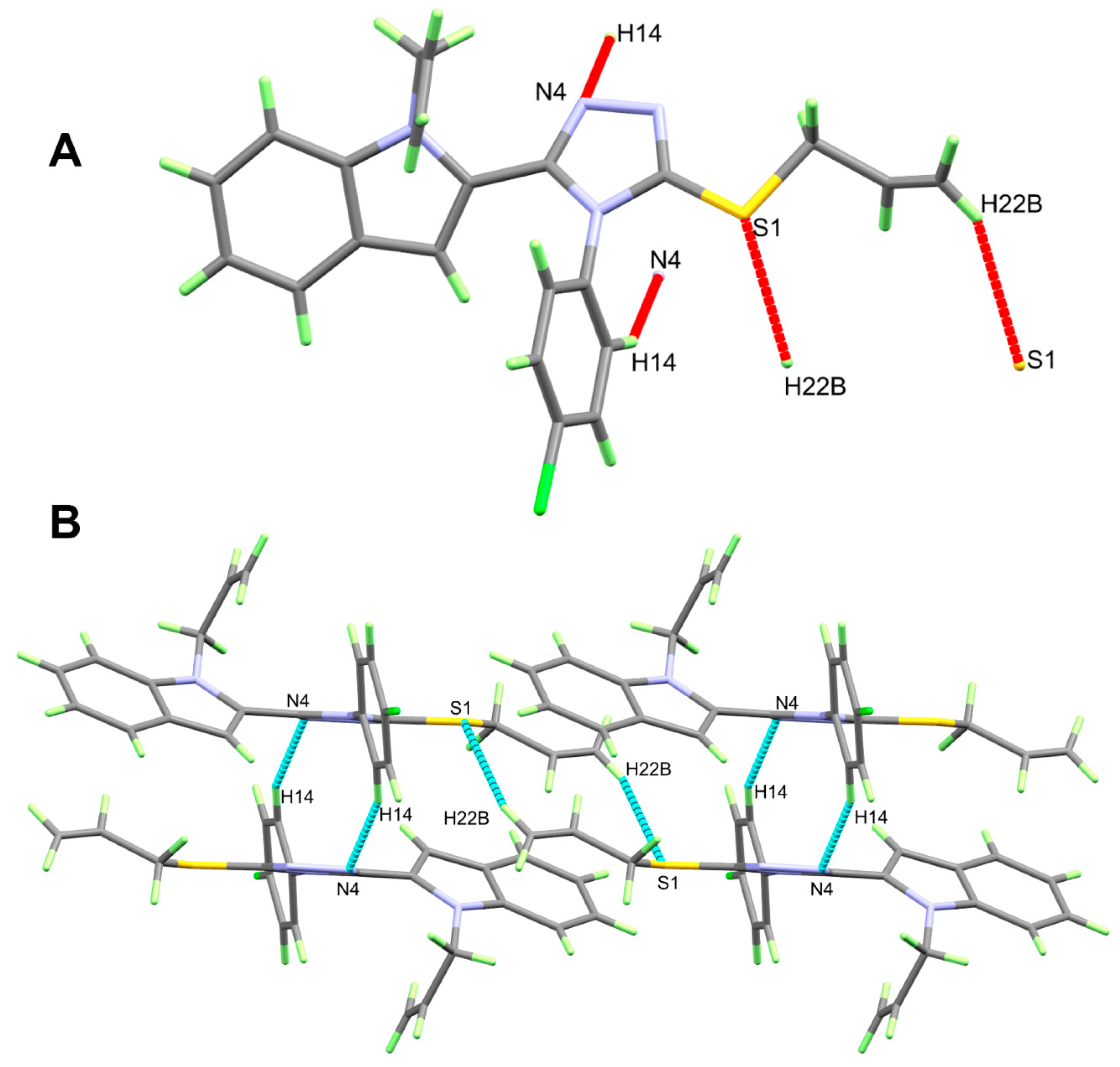
3.1. Crystal Structure Description
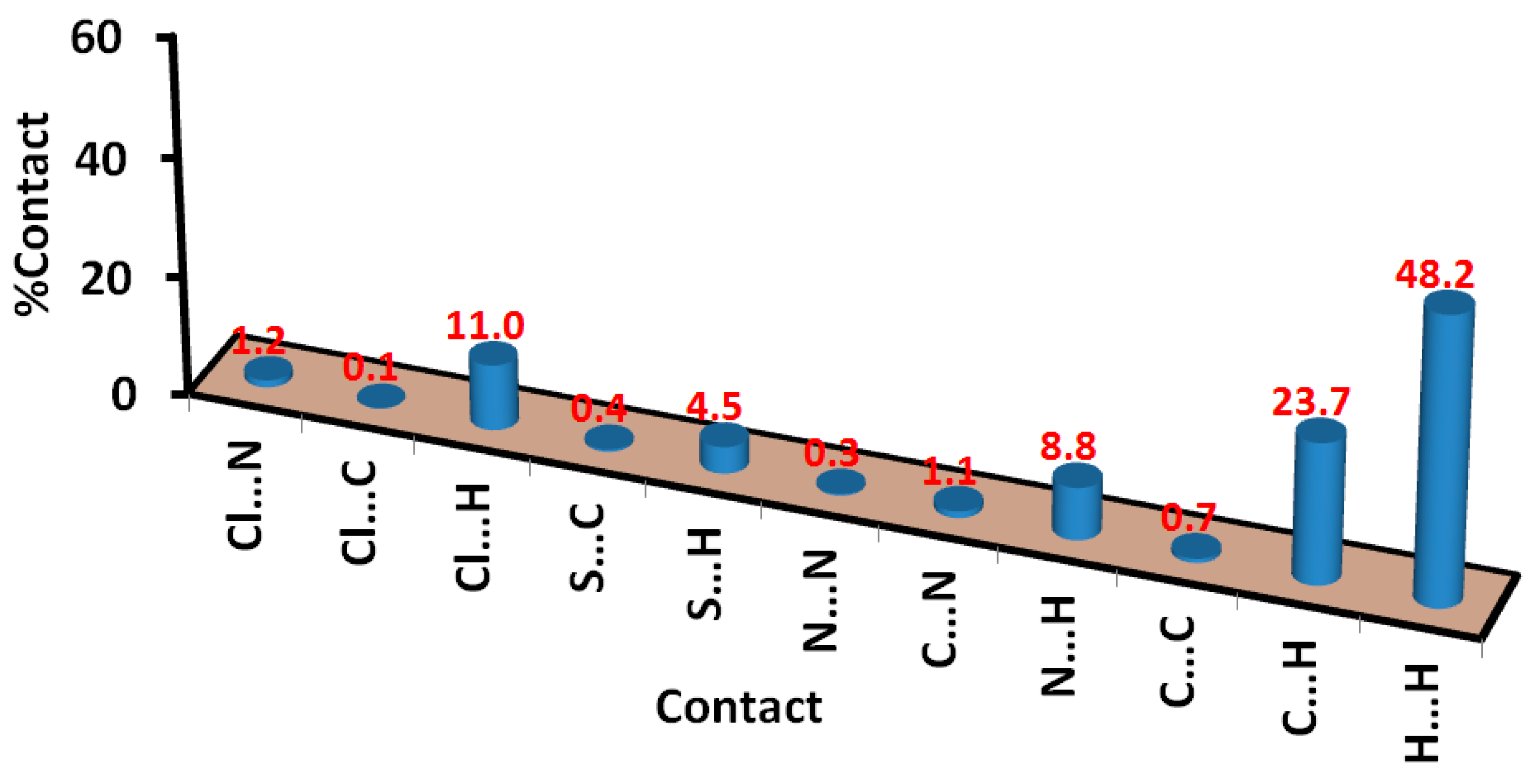
3.2. Hirshfeld Surface Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elshaier, Y.A.; Nemr, M.T.; Al Refaey, M.; Fadaly, W.A.; Barakat, A. Chemistry of 2-vinylindoles: Synthesis and applications. New J. Chem. 2022, 46, 13383–13400. [Google Scholar] [CrossRef]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef]
- Hong, C.; Firestone, G.L.; Bjeldanes, L.F. Bcl-2 family-mediated apoptotic effects of 3, 3′-diindolylmethane (DIM) in human breast cancer cells. Biochem. Pharmacol. 2002, 63, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and cytotoxicity of novel 2, 2′-bis-and 2, 2′, 2″-tris-indolylmethanes-based bengacarboline analogs. Arch. Pharm. Res. 2012, 35, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K.W.; Li, Y.; Wang, Z.; Sarkar, S.H.; Sarkar, F.H. Gene expression profiling revealed survivin as a target of 3, 3′-diindolylmethane-induced cell growth inhibition and apoptosis in breast cancer cells. Cancer Res. 2006, 66, 4952–4960. [Google Scholar] [CrossRef]
- Yoo, M.; Choi, S.-U.; Choi, K.Y.; Yon, G.H.; Chae, J.-C.; Kim, D.; Zylstra, G.; Kim, E. Trisindoline synthesis and anticancer activity. Biochem. Biophys. Res. Commun. 2008, 376, 96–99. [Google Scholar] [CrossRef]
- Safe, S.; Papineni, S.; Chintharlapalli, S. Cancer chemotherapy with indole-3-carbinol, bis (3′-indolyl) methane and synthetic analogs. Cancer Lett. 2008, 269, 326–338. [Google Scholar] [CrossRef]
- Foderaro, T.A.; Barrows, L.R.; Lassota, P.; Ireland, C.M. Bengacarboline, a New β-Carboline from a Marine Ascidian Didemnum sp. J. Org. Chem. 1997, 62, 6064–6065. [Google Scholar] [CrossRef]
- Nagarsenkar, A.; Prajapti, S.K.; Guggilapu, S.D.; Birineni, S.; Kotapalli, S.S.; Ummanni, R.; Babu, B.N. Investigation of triazole-linked indole and oxindole glycoconjugates as potential anticancer agents: Novel Akt/PKB signaling pathway inhibitors. Med. Chem. Commun. 2016, 7, 646–653. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Bednarczyk-Cwynar, B.; Vasylenko, O.; Kazakova, O.; Zimenkovsky, B.; Zaprutko, L.; Lesyk, R. Synthesis of new potential anticancer agents based on 4-thiazolidinone and oleanane scaffolds. Med. Chem. Res. 2012, 21, 3568–3580. [Google Scholar] [CrossRef]
- Pingaew, R.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and structure–activity relationship of mono-indole-, bis-indole-, and tris-indole-based sulfonamides as potential anticancer agents. Mol. Divers. 2013, 17, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.J.; Bao, C.J.; Tao, X.; Wang, J.; Atoyan, R.; Qu, H.; Wang, D.G.; Yin, L.; Samson, M.; Forrester, J.; et al. CUDC-101, a multitargeted inhibitor of histone deacetylase, epidermal growth factor receptor, and human epidermal growth factor receptor 2, exerts potent anticancer activity. Cancer Res. 2010, 70, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Al-Quawasmeh, R.A.; Huesca, M.; Nedunuri, V.; Peralta, R.; Wright, J.; Lee, Y.; Young, A. Potent antimicrobial activity of 3-(4, 5-diaryl-1H-imidazol-2-yl)-1H-indole derivatives against methicillin-resistant Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2010, 20, 3518–3520. [Google Scholar] [CrossRef]
- Mascal, M.; Modes, K.V.; Durmus, A. Concise photochemical synthesis of the antimalarial indole alkaloid decursivine. Angew. Chem. Int. Ed. Engl. 2011, 50, 4445–4446. [Google Scholar] [CrossRef] [PubMed]
- Sechi, M.; Derudas, M.; Dallocchio, R.; Dessi, A.; Bacchi, A.; Sannia, L.; Carta, F.; Palomba, M.; Ragab, O.; Chan, C.; et al. Design and synthesis of novel indole β-diketo acid derivatives as HIV-1 integrase inhibitors. J. Med. Chem. 2014, 47, 5298–5310. [Google Scholar] [CrossRef] [PubMed]
- Mandour, A.H.; El-Sawy, E.R.; Shaker, K.H.; Mustafa, M.A. Synthesis, anti-inflammatory, analgesic and anticonvulsant activities of 1, 8-dihydro-1-ary1-8-alkyl pyrazolo (3, 4-b) indoles. Acta Pharm. 2010, 60, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Narayana, B.; Ashalatha, B.V.; Vijayaraj, K.K.; Fernandes, J.; Sarojini, B.K. Synthesis of some new biologically active 1, 3, 4-oxadiazolyl nitroindoles and a modified Fischer indole synthesis of ethyl nitro indole-2-carboxylates. Bioorg. Med. Chem. 2005, 13, 4638–4644. [Google Scholar] [CrossRef]
- Ty, N.; Dupeyre, G.; Chabot, G.G.; Seguin, J.; Tillequin, F.; Scherman, D.; Michel, S.; Cachet, X. Synthesis and biological evaluation of new disubstituted analogues of 6-methoxy-3-(3′, 4′, 5′-trimethoxybenzoyl)-1H-indole (BPR0L075), as potential antivascular agents. Bioorg. Med. Chem. 2008, 16, 7494–7503. [Google Scholar] [CrossRef]
- Islam, M.S.; Barakat, A.; Al-Majid, A.M.; Ali, M.; Yousuf, S.; Choudhary, M.I.; Khalil, R.; Ul-Haq, Z. Catalytic asymmetric synthesis of indole derivatives as novel α-glucosidase inhibitors in vitro. Bioorg. Chem. 2018, 79, 350–354. [Google Scholar] [CrossRef]
- Bi, W.; Bi, Y.; Xue, P.; Zhang, Y.; Gao, X.; Wang, Z.; Li, M.; Baudy-Floc’h, M.; Ngerebara, N.; Gibson, K.M.; et al. Synthesis and characterization of novel indole derivatives reveal improved therapeutic agents for treatment of ischemia/reperfusion (I/R) injury. J. Med. Chem. 2010, 53, 6763–6767. [Google Scholar] [CrossRef]
- Boraei, A.T.; Ghabbour, H.A.; Gomaa, M.S.; El Ashry, E.S.H.; Barakat, A. Synthesis and anti-proliferative assessment of triazolo-thiadiazepine and triazolo-thiadiazine scaffolds. Molecules 2019, 24, 4471. [Google Scholar] [CrossRef] [PubMed]
- Boraei, A.T.A.; Gomaa, M.S.; El Sayed, E.S.H.; Duerkop, A. Design, selective alkylation and X-ray crystal structure determination of dihydro-indolyl-1,2,4-triazole-3-thione and its 3-benzylsulfanyl analogue as potent anticancer agents. Eur. J. Med. Chem. 2017, 125, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Boraei, A.T.; Ashour, H.K.; El Sayed, H.; Abdelmoaty, N.; El-Falouji, A.I.; Gomaa, M.S. Design and synthesis of new phthalazine-based derivatives as potential EGFR inhibitors for the treatment of hepatocellular carcinoma. Bioorg. Chem. 2019, 85, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Boraei, A.T.; Singh, P.K.; Sechi, M.; Satta, S. Discovery of novel functionalized 1, 2, 4-triazoles as PARP-1 inhibitors in breast cancer: Design, synthesis and antitumor activity evaluation. Eur. J. Med. Chem. 2019, 182, 111621. [Google Scholar] [CrossRef]
- Kaur, R.; Dwivedi, A.R.; Kumar, B.; Kumar, V. Recent developments on 1, 2, 4-triazole nucleus in anticancer compounds: A review. Anti-Cancer Agent. Med. Chem. 2016, 16, 465–489. [Google Scholar] [CrossRef]
- Ayati, A.; Emami, S.; Foroumadi, A. The importance of triazole scaffold in the development of anticonvulsant agents. Eur. J. Med. Chem. 2016, 109, 380–392. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, S.A.; Budagumpi, S.; Nagaraja, B.M. Triazole: A Promising Antitubercular Agent. Chem. Biol. Drug Des. 2015, 86, 410–423. [Google Scholar] [CrossRef]
- Kucukguzel, S.G.; Cikla-Suzgun, P. Recent advances bioactive 1, 2, 4-triazole-3-thiones. Eur. J. Med. Chem. 2015, 97, 830–870. [Google Scholar] [CrossRef]
- Guo, L.; Wu, M.; Leng, S.; Qiang, Y.; Zheng, X. Synergistic effect of purpald with tartaric acid on the corrosion inhibition of mild steel: From electrochemical to theoretical insights. Prot. Met. Phys. Chem. Surf. 2018, 54, 917–925. [Google Scholar] [CrossRef]
- Yan, D.; Xiang, Y.; Li, K.; Chen, Y.; Yang, Z.; Guo, D. Synthesis, characterization and properties of 1, 2, 4-triazolo [3–b][1,3,4] thiadiazole derivatives and their europium complexes. J. Mol. Struct. 2014, 1074, 487–495. [Google Scholar] [CrossRef]
- Hamdy, R.; Ziedan, N.; Ali, S.; El-Sadek, M.; Lashin, E.; Brancale, A.; Jones, A.T.; Westwell, A.D. Synthesis and evaluation of 3-(benzylthio)-5-(1H-indol-3-yl)-1,2,4-triazol-4-amines as Bcl-2 inhibitory anticancer agents. Bioorg. Med. Chem. Lett. 2013, 23, 2391–2394. [Google Scholar] [CrossRef] [PubMed]
- Altowyan, M.S.; Haukka, M.; Soliman, S.M.; Barakat, A.; Boraei, A.T.A.; Aboelmagd, A. Stereoselective Synthesis of New 4-Aryl-5-indolyl-1,2,4-triazole S- and N-β-Galactosides: Characterizations, X-ray Crystal Structure and Hirshfeld Surface Analysis. Crystals 2023, 13, 797. [Google Scholar] [CrossRef]
- Rikagu Oxford Diffraction. CrysAlisPro; Rikagu Oxford Diffraction Inc.: Yarnton, UK, 2020. [Google Scholar]
- Sheldrick, G.M. Shelxt–integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17. University of Western Australia. 2017. Available online: https://crystalexplorer.net/ (accessed on 30 July 2020).





| 5b | 2b | |
|---|---|---|
| CCDC no. | 2,264,129 | 2,264,130 |
| empirical formula | C22H19ClN4S | C23H17ClN4S |
| fw | 406.92 | 416.92 |
| temp (K) | 120 (2) | 120 (2) |
| λ (Å) | 0.71073 | 1.54184 |
| cryst syst | Monoclinic | Triclinic |
| space group | P21/c | |
| a (Å) | 11.0242 (3) | 12.1537 (5) |
| b (Å) | 16.5074 (4) | 12.8679 (5) |
| c (Å) | 11.0507 (2) | 15.0994 (5) |
| α (deg) | 90 | 70.272 (3) |
| β (deg) | 97.688 (2) | 69.417 (3) |
| γ (deg) | 90 | 67.402 (4) |
| V (Å3) | 1992.94(8) | 1984.17 (15) |
| Z | 4 | 4 |
| ρcalc (mg/m3) | 1.356 | 1.396 |
| μ (Mo Kα) (mm−1) | 0.312 | 2.818 |
| No. reflns. | 23,307 | 53,703 |
| Completeness to theta = 25.242° | 100% | |
| Completeness to theta = 67.684° | 100% | |
| Unique reflns. | 6619 | 8329 |
| GOOF (F2) | 1.037 | 1.034 |
| Rint | 0.0475 | 0.0342 |
| R1a (I ≥ 2σ) | 0.0461 | 0.0296 |
| wR2b (I ≥ 2σ) | 0.1106 | 0.0747 |
| Bond | Length/Å | Bond | Length/Å |
|---|---|---|---|
| 5b | 2b | ||
| Cl(1)-C(16) | 1.7389 (13) | Cl(1)-C(13) | 1.7325 (13) |
| S(1)-C(19) | 1.7418 (16) | S(1)-C(16) | 1.7460 (14) |
| S(1)-C(20B) | 1.8204 (16) | S(1)-C(17) | 1.8318 (14) |
| S(1)-C(20A) | 1.8204 (16) | N(1)-C(1) | 1.3716 (18) |
| N(1)-C(7) | 1.386 (2) | N(1)-C(8) | 1.3788 (17) |
| N(1)-C(11) | 1.3938 (19) | N(2)-C(16) | 1.3740 (17) |
| N(1)-C(8) | 1.457 (2) | N(2)-C(9) | 1.3755 (17) |
| N(2)-C(19) | 1.3732 (18) | N(2)-C(10) | 1.4372 (16) |
| N(2)-C(12) | 1.3802 (18) | N(3)-C(16) | 1.3096 (17) |
| N(2)-C(13) | 1.4364 (17) | N(3)-N(4) | 1.3989 (16) |
| N(3)-C(19) | 1.3124 (18) | N(4)-C(9) | 1.3131 (17) |
| N(3)-N(4) | 1.4027 (18) | Cl(1B)-C(13B) | 1.7373 (13) |
| N(4)-C(12) | 1.3190 (17) |
| D-H…A | d(D-H) | d(H…A) | d(D…A) | <(DHA) | Symm. Code |
|---|---|---|---|---|---|
| 5b | |||||
| C14-H14…N4 | 0.95 | 2.58 | 3.456 (2) | 154 | 1 − x, 1 − y, 1 − z |
| C22A-H22B…S1 | 0.95 | 2.87 | 3.741 (6) | 153 | 2 − x, 1 − y, 1 − z |
| 2b | |||||
| C17-H17B…N3 | 0.99 | 2.55 | 3.025 (2) | 109 | |
| C19-H19…N3 | 0.95 | 2.6 | 3.388 (2) | 140 | |
| N1-H1…N4B | 0.86 (2) | 2.08 (2) | 2.913 (2) | 164.1 (18) | |
| N1B-H1B…N4 | 0.88 (2) | 0.88 (2) | 2.920 (2) | 163.1 (18) | |
| C11-H11…N3 | 0.95 | 2.59 | 3.440 (2) | 149 | 1 − x, 1 − y, 1 − z |
| C15B--H15B…N1B | 0.95 | 2.53 | 3.396 (2) | 152 | −x, 2 − y, 1 − z |
| Contact | Distance | Contact | Distance |
|---|---|---|---|
| H8B…H22D | 2.084 | H20A…C1 | 2.599 |
| H22D…C8 | 2.696 | H17…C19 | 2.657 |
| H22D…C10 | 2.607 | H21B…C5 | 2.747 |
| H5…C17 | 2.762 | H14…N4 | 2.458 |
| H20C…C2 | 2.580 | S1…H22B | 2.754 |
| H20C…C1 | 2.531 | Cl1…N3 | 3.149 |
| Contact | A | B |
|---|---|---|
| Cl…S | 0.0 | 0.3 |
| Cl…N | 0.3 | 0.4 |
| Cl…C | 4.4 | 2.3 |
| Cl…H | 8.5 | 10.6 |
| S…N | 1.3 | 0.9 |
| S…C | 0.5 | 1.8 |
| S…H | 5.8 | 3.2 |
| N…N | 0.2 | 0.0 |
| C…N | 1.7 | 1.5 |
| N…H | 9.0 | 9.3 |
| C…C | 2.7 | 4.2 |
| C…H | 22.1 | 26.8 |
| H…H | 43.6 | 38.7 |
| Contact | Distance | Contact | Distance |
|---|---|---|---|
| S1…N4B | 3.289 | H23…H17C | 2.121 |
| N4B…H1 | 1.933 | H19…H2B | 2.075 |
| N3…H11 | 2.479 | C14…H11B | 2.762 |
| Cl1…C21 | 3.246 | C19…H12B | 2.756 |
| H23…H17C | 2.121 | C19B…H5 | 2.776 |
| H19…H2B | 2.075 | C11B…H15 | 2.567 |
| H21…H5B | 2.122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, E.E.; Youssef, M.F.; Boraei, A.T.A.; Haukka, M.; Soliman, S.M.; Barakat, A.; Sarhan, A.A.M. Base-Controlled Regiospecific Mono-Benzylation/Allylation and Diallylation of 4-Aryl-5-indolyl-1,2,4-triazole-3-thione: Thio-Aza Allyl Rearrangement. Crystals 2023, 13, 992. https://doi.org/10.3390/cryst13070992
Salama EE, Youssef MF, Boraei ATA, Haukka M, Soliman SM, Barakat A, Sarhan AAM. Base-Controlled Regiospecific Mono-Benzylation/Allylation and Diallylation of 4-Aryl-5-indolyl-1,2,4-triazole-3-thione: Thio-Aza Allyl Rearrangement. Crystals. 2023; 13(7):992. https://doi.org/10.3390/cryst13070992
Chicago/Turabian StyleSalama, Eid E., Mohamed F. Youssef, Ahmed T. A. Boraei, Matti Haukka, Saied M. Soliman, Assem Barakat, and Ahmed A. M. Sarhan. 2023. "Base-Controlled Regiospecific Mono-Benzylation/Allylation and Diallylation of 4-Aryl-5-indolyl-1,2,4-triazole-3-thione: Thio-Aza Allyl Rearrangement" Crystals 13, no. 7: 992. https://doi.org/10.3390/cryst13070992
APA StyleSalama, E. E., Youssef, M. F., Boraei, A. T. A., Haukka, M., Soliman, S. M., Barakat, A., & Sarhan, A. A. M. (2023). Base-Controlled Regiospecific Mono-Benzylation/Allylation and Diallylation of 4-Aryl-5-indolyl-1,2,4-triazole-3-thione: Thio-Aza Allyl Rearrangement. Crystals, 13(7), 992. https://doi.org/10.3390/cryst13070992








