Thermally Tunable Structural Color Based on Patterned Ultra-Thin Asymmetric Fabry–Perot Cavity with Phase-Change Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metasurface Structure
2.2. Color Calculation
2.3. Key Parameters of Color
3. Results and Discussion
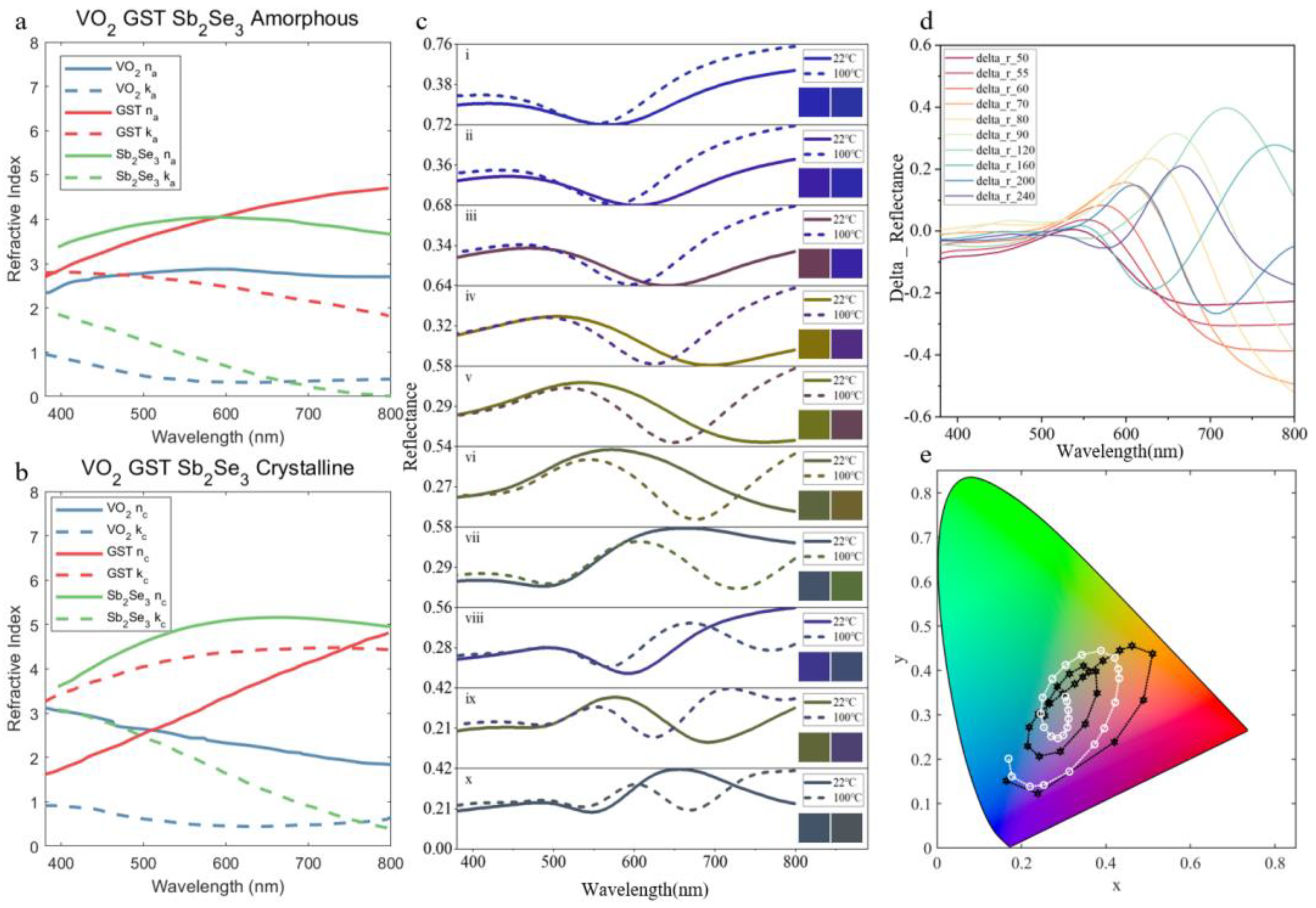
3.1. The Influence of Temperature on Structural Color
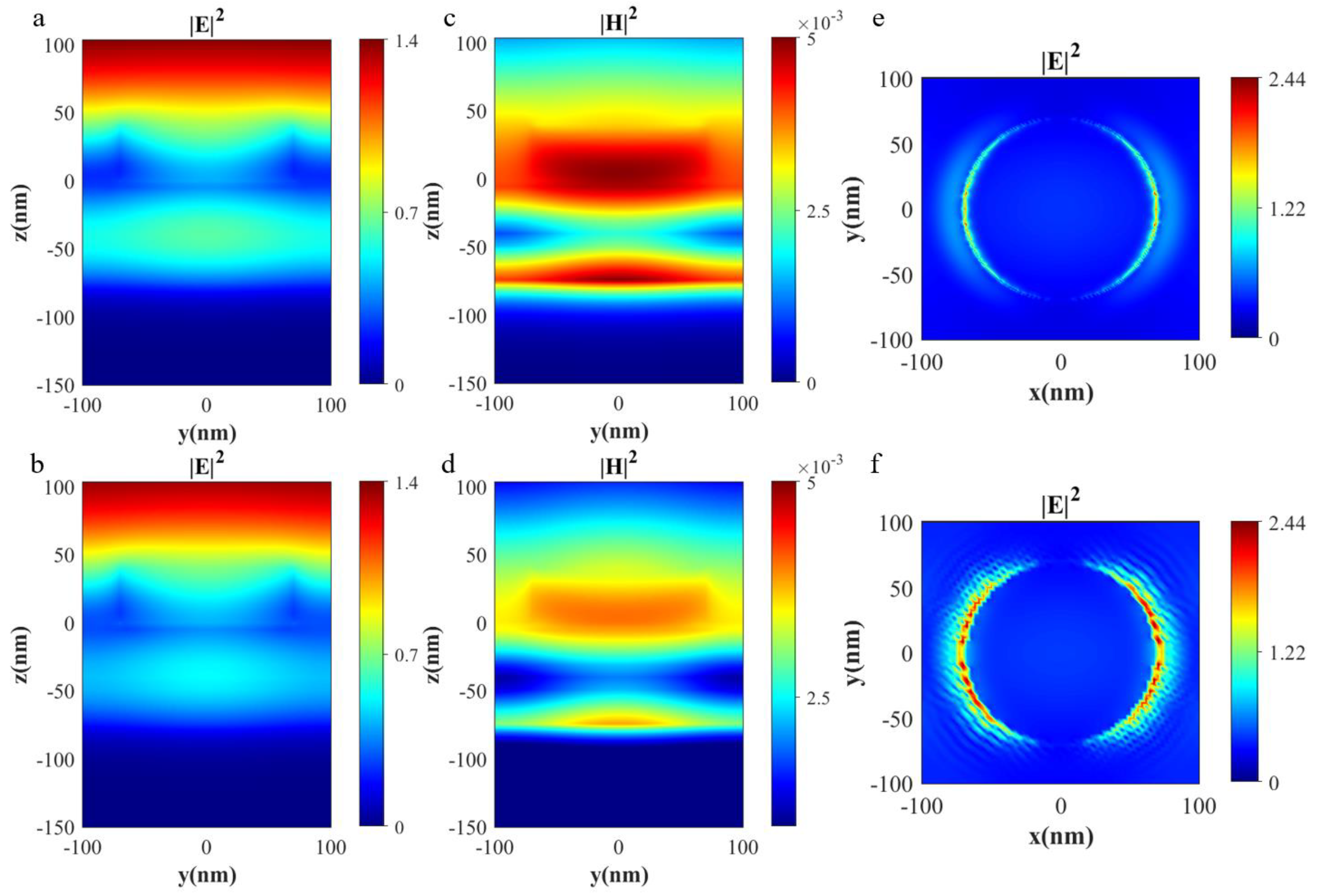
3.2. Physical Mechanism Investigation
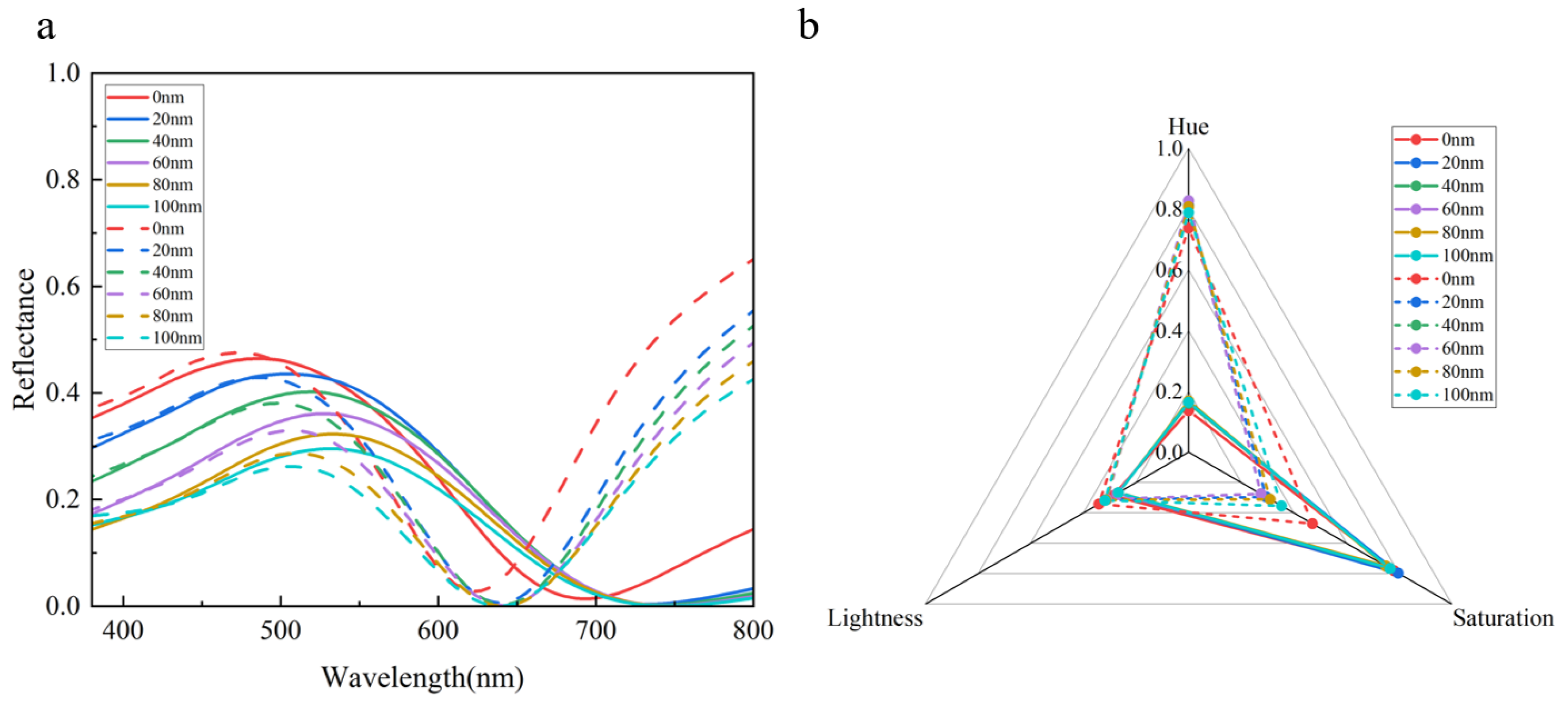
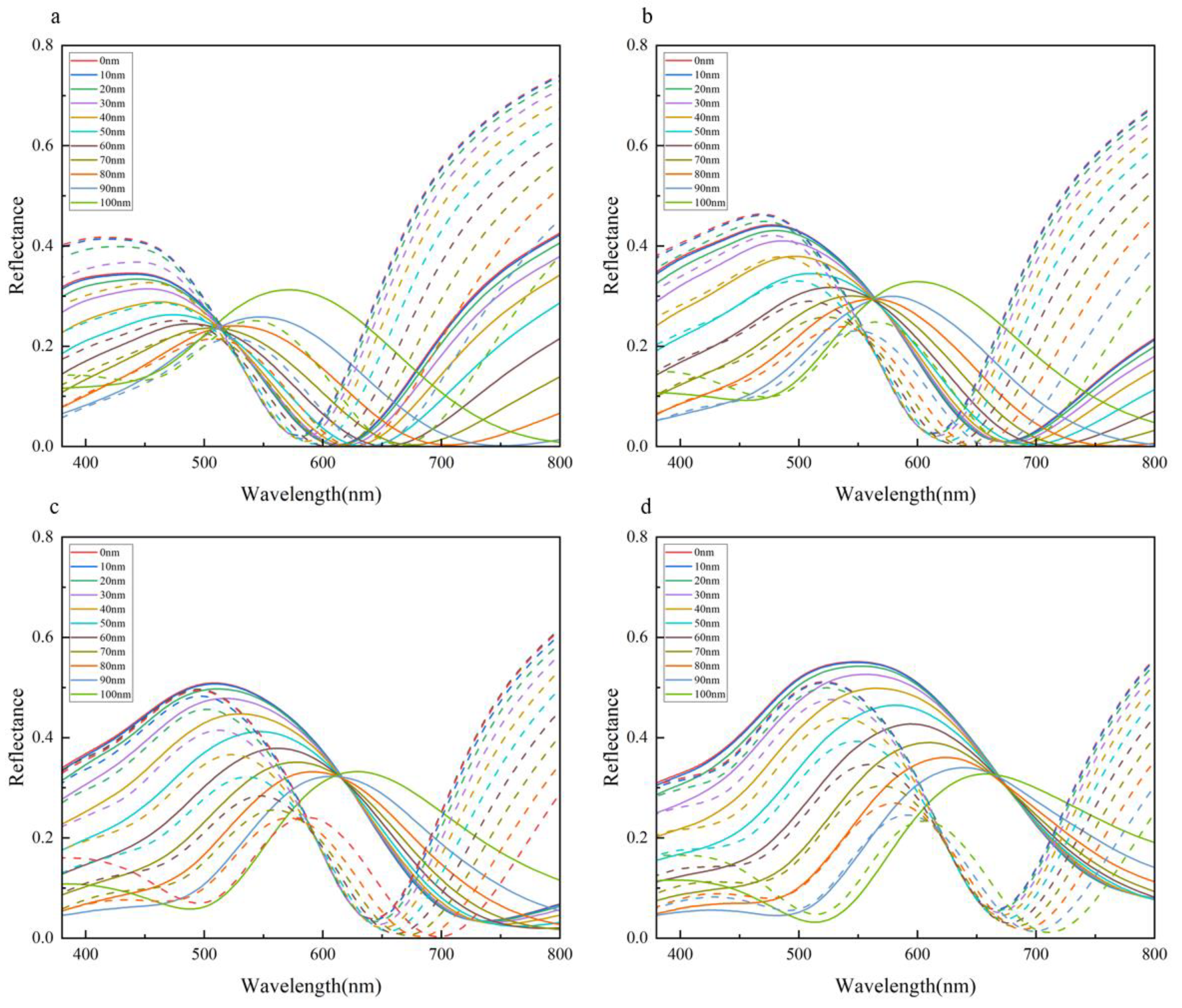
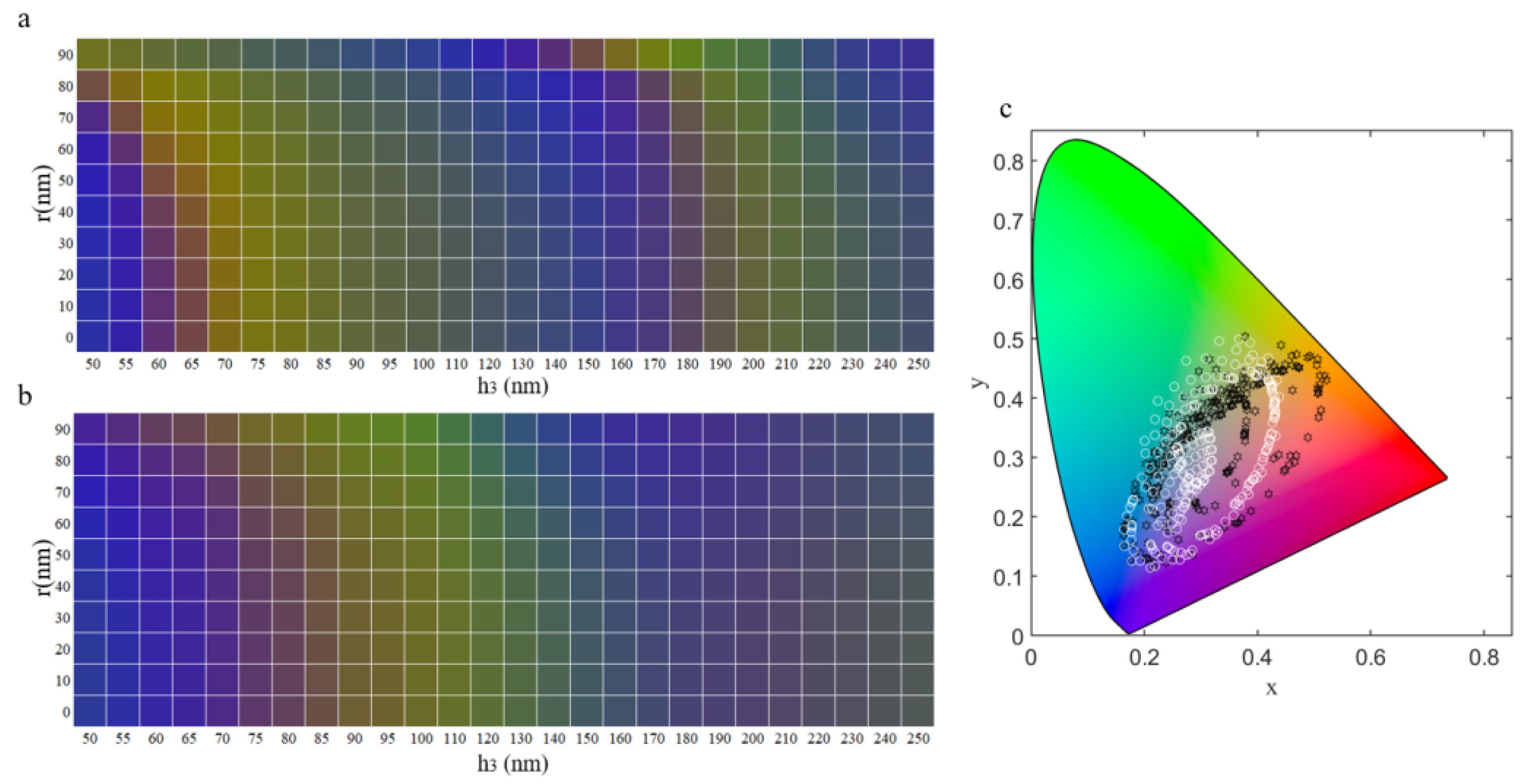
3.3. The Influence of Parameters on Structural Color
3.4. Practical Applications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vukusic, P.; Sambles, J.R. Photonic structures in biology. Nature 2003, 424, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.K.; Chen, Y.M.; Song, Q.H.; Xiao, S.M. Dynamic Structural Colors Based on All-Dielectric Mie Resonators. Adv. Opt. Mater. 2021, 9, 2002126. [Google Scholar] [CrossRef]
- Srinivasarao, M. Nano-optics in the biological world: Beetles, butterflies, birds, and moths. Chem. Rev. 1999, 99, 1935–1961. [Google Scholar] [CrossRef] [PubMed]
- Kulyk, O.; Rocard, L.; Maggini, L.; Bonifazi, D. Synthetic strategies tailoring colours in multichromophoric organic nanostructures. Chem. Soc. Rev. 2020, 49, 8400–8424. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.D.; Dong, Z.G.; Chan, J.Y.E.; Trisno, J.; Ng, R.J.H.; Ruan, Q.F.; Qiu, C.W.; Mortensen, N.A.; Yang, J.K.W. Nanophotonic Structural Colors. ACS Photonics 2021, 8, 18–33. [Google Scholar] [CrossRef]
- Lundberg, I.; Milatou-Smith, R. Mortality and cancer incidence among Swedish paint industry workers with long-term exposure to organic solvents. Scand. J. Work. Environ. Health 1998, 24, 270–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, F.F.; Shang, L.R.; Chen, Z.Y.; Yu, Y.R.; Zhao, Y.J. Bioinspired living structural color hydrogels. Sci. Robot. 2018, 3, eaar8580. [Google Scholar] [CrossRef] [Green Version]
- Glybovski, S.B.; Tretyakov, S.A.; Belov, P.A.; Kivshar, Y.S.; Simovski, C.R. Metasurfaces: From microwaves to visible. Phys. Rep. 2016, 634, 1–72. [Google Scholar] [CrossRef]
- Cheng, F.; Gao, J.; Luk, T.S.; Yang, X.D. Structural color printing based on plasmonic metasurfaces of perfect light absorption. Sci. Rep. 2015, 5, 11045. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.G.; Wang, W.; Rosenmann, D.; Czaplewski, D.A.; Yang, X.D.; Gao, J. All-metal structural color printing based on aluminum plasmonic metasurfaces. Opt. Express 2016, 24, 20472–20480. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liang, Y.; Qu, J.; Chen, M.K.; Cui, M.; Cheng, Z.; Zhang, J.; Yao, J.; Chen, S.; Tsai, D.P.; et al. Plasmonic bound states in the continuum for unpolarized weak spatially coherent light. Photonics Res. 2023, 11, 260. [Google Scholar] [CrossRef]
- Zheng, D.; Wen, Y.; Xu, X.; Lin, Y.-S. Metamaterial grating for colorimetric chemical sensing applications. Mater. Today Phys. 2023, 33, 101056. [Google Scholar] [CrossRef]
- Cerjan, B.; Gerislioglu, B.; Link, S.; Nordlander, P.; Halas, N.J.; Griep, M.H. Towards scalable plasmonic Fano-resonant metasurfaces for colorimetric sensing. Nanotechnology 2022, 33, 405201. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, Z.X.; Zhang, C.; Gao, Y.S.; Duan, Z.H.; Xiao, S.M.; Song, Q.H. All-Dielectric Full-Color Printing with TiO2 Metasurfaces. ACS Nano 2017, 11, 4445–4452. [Google Scholar] [CrossRef]
- Yang, W.H.; Xiao, S.M.; Song, Q.H.; Liu, Y.L.; Wu, Y.K.; Wang, S.; Yu, J.; Han, J.C.; Tsai, D.P. All-dielectric metasurface for high-performance structural color. Nat. Commun. 2020, 11, 1864. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, T.; Yan, R.Q.; Yue, X.Z.; Wang, H.M.; Wang, Y.D.; Zhang, J.Y. Tunable structural colors generated by hybrid Si3N4 and Al metasurfaces. Opt. Express 2022, 30, 7299–7307. [Google Scholar] [CrossRef] [PubMed]
- Ellenbogen, T.; Seo, K.; Crozier, K.B. Chromatic Plasmonic Polarizers for Active Visible Color Filtering and Polarimetry. Nano Lett. 2012, 12, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; An, B.W.; Jiang, S.M.; Gao, J.; Chen, Y.L.; Pan, S.D. Plasmonic induced triple-band absorber for sensor application. Opt. Express 2015, 23, 17607–17612. [Google Scholar] [CrossRef]
- Bai, Y.; Jiang, Y.Y.; Liu, L.H. Role of surface plasmon polaritons on the enhancement of the near-field thermal radiation from fishnet metamaterial. J. Phys. D Appl. Phys. 2014, 47, 445304. [Google Scholar] [CrossRef]
- Kuznetsov, A.I.; Miroshnichenko, A.E.; Brongersma, M.L.; Kivshar, Y.S.; Luk’yanchuk, B. Optically resonant dielectric nanostructures. Science 2016, 354, aag2472. [Google Scholar] [CrossRef] [Green Version]
- Moitra, P.; Slovick, B.A.; Li, W.; Kraychencko, I.I.; Briggs, D.P.; Krishnamurthy, S.; Valentine, J. Large-Scale All-Dielectric Metamaterial Perfect Reflectors. ACS Photonics 2015, 2, 692–698. [Google Scholar] [CrossRef]
- Jahani, S.; Jacob, Z. All-dielectric metamaterials. Nat. Nanotechnol. 2016, 11, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.Y.; Kamin, S.; Liu, N. Dynamic plasmonic colour display. Nat. Commun. 2017, 8, 14606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.Q.; Duan, X.Y.; Matuschek, M.; Zhou, Y.M.; Neubrech, F.; Duan, H.G.; Liu, N. Dynamic Color Displays Using Stepwise Cavity Resonators. Nano Lett. 2017, 17, 5555–5560. [Google Scholar] [CrossRef] [Green Version]
- Seeboth, A.; Loetzsch, D.; Ruhmann, R. Piezochromic Polymer Materials Displaying Pressure Changes in Bar-Ranges. Am. J. Mater. Sci. 2012, 1, 139–142. [Google Scholar] [CrossRef] [Green Version]
- Song, S.C.; Ma, X.L.; Pu, M.B.; Li, X.; Liu, K.P.; Gao, P.; Zhao, Z.Y.; Wang, Y.Q.; Wang, C.T.; Luo, X.G. Actively Tunable Structural Color Rendering with Tensile Substrate. Adv. Opt. Mater. 2017, 5, 1600829. [Google Scholar] [CrossRef]
- Wang, G.P.; Chen, X.C.; Liu, S.; Wong, C.P.; Chu, S. Mechanical Chameleon through Dynamic Real Time-Plasmonic Tuning. ACS Nano 2016, 10, 1788–1794. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Walter, E.C.; Agrawal, A.; Bohn, C.; Velmurugan, J.; Zhu, W.Q.; Lezec, H.J.; Talin, A.A. High-contrast and fast electrochromic switching enabled by plasmonics. Nat. Commun. 2016, 7, 10479. [Google Scholar] [CrossRef] [Green Version]
- Xiong, K.L.; Emilsson, G.; Maziz, A.; Yang, X.X.; Shao, L.; Jager, E.W.H.; Dahlin, A.B. Plasmonic Metasurfaces with Conjugated Polymers for Flexible Electronic Paper in Color. Adv. Mater. 2016, 28, 9956–9960. [Google Scholar] [CrossRef] [Green Version]
- Shu, F.Z.; Yu, F.F.; Peng, R.W.; Zhu, Y.Y.; Xiong, B.; Fan, R.H.; Wang, Z.H.; Liu, Y.M.; Wang, M. Dynamic Plasmonic Color Generation Based on Phase Transition of Vanadium Dioxide. Adv. Opt. Mater. 2018, 6, 1700939. [Google Scholar] [CrossRef]
- Zhao, J.C.; Zhou, Y.; Huo, Y.H.; Gao, B.; Ma, Y.G.; Yu, Y.T. Flexible dynamic structural color based on an ultrathin asymmetric Fabry–Perot cavity with phase-change material for temperature perception. Opt. Express 2021, 29, 23273–23281. [Google Scholar] [CrossRef] [PubMed]
- He, J.Q.; Zhang, M.; Shu, S.W.; Yan, Y.; Wang, M.X. VO2 based dynamic tunable absorber and its application in switchable control and real-time color display in the visible region. Opt. Express 2020, 28, 37590–37599. [Google Scholar] [CrossRef]
- Rui, G.; Ding, C.; Gu, B.; Gan, Q.; Zhan, Q.; Cui, Y. Symmetric Ge2Sb2Te5 based metamaterial absorber induced dynamic wide-gamut structural color. J. Opt. 2020, 22, 085003. [Google Scholar] [CrossRef]
- He, Q.; Youngblood, N.; Cheng, Z.G.; Miao, X.S.; Bhaskaran, H. Dynamically tunable transmissive color filters using ultra-thin phase-change materials. Opt. Express 2020, 28, 39841–39849. [Google Scholar] [CrossRef]
- Loke, D.; Lee, T.H.; Wang, W.J.; Shi, L.P.; Zhao, R.; Yeo, Y.C.; Chong, T.C.; Elliott, S.R. Breaking the Speed Limits of Phase-Change Memory. Science 2012, 336, 1566–1569. [Google Scholar] [CrossRef] [Green Version]
- Rios, C.; Hosseini, P.; Taylor, R.A.; Bhaskaran, H. Color Depth Modulation and Resolution in Phase-Change Material Nanodisplays. Adv. Mater. 2016, 28, 4720–4726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, F.; Yang, Y.Q.; Bozhevolnyi, S.I. Dynamic Metasurfaces Using Phase-Change Chalcogenides. Adv. Opt. Mater. 2019, 7, 1801709. [Google Scholar] [CrossRef] [Green Version]
- Morin, F.J. Oxides Which Show a Metal-to-Insulator Transition at the Neel Temperature. Phys. Rev. Lett. 1959, 3, 34–36. [Google Scholar] [CrossRef]
- Dicken, M.J.; Aydin, K.; Pryce, I.M.; Sweatlock, L.A.; Boyd, E.M.; Walavalkar, S.; Ma, J.; Atwater, H.A. Frequency tunable near-infrared metamaterials based on VO2 phase transition. Opt. Express 2009, 17, 18330–18339. [Google Scholar] [CrossRef] [Green Version]
- Savaliya, P.B.; Thomas, A.; Dua, R.; Dhawan, A. Tunable optical switching in the near-infrared spectral regime by employing plasmonic nanoantennas containing phase-change materials. Opt. Express 2017, 25, 23755–23772. [Google Scholar] [CrossRef]
- Kats, M.A.; Blanchard, R.; Genevet, P.; Yang, Z.; Qazilbash, M.M.; Basov, D.N.; Ramanathan, S.; Capasso, F. Thermal tuning of mid-infrared plasmonic antenna arrays using a phase-change material. Opt. Lett. 2013, 38, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Barreda, A.; Zou, C.J.; Sinelnik, A.; Menshikov, E.; Sinev, I.; Pertsch, T.; Staude, I. Tuning and switching effects of quasi-BIC states combining phase-change materials with all-dielectric metasurfaces. Opt. Mater. Express 2022, 12, 3132–3142. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, D.; Chae, J.Y.; Ko, B.; Lee, H.; Paik, T.; Hong, S.H. Reconfigurable, vivid reflective colors based on solution-processed Fabry–Perot absorber using thermochromic vanadium dioxide. Appl. Surf. Sci. 2021, 565, 150610. [Google Scholar] [CrossRef]
- Du, Z.Y.; Li, M.; Xu, S.C.; Li, K.B.; Zou, F.X.; Zhang, R.R.; Li, G.H. VO2-based intelligent thermal control coating for spacecraft by regulating infrared emittance. J. Alloys Compd. 2022, 895, 162679. [Google Scholar] [CrossRef]
- Sun, K.; Riedel, C.A.; Urbani, A.; Simeoni, M.; Mengali, S.; Zalkovskij, M.; Bilenberg, B.; de Groot, C.H.; Muskens, O.L. VO2 Thermochromic Metamaterial-Based Smart Optical Solar Reflector. ACS Photonics 2018, 5, 2280–2286. [Google Scholar] [CrossRef]
- He, D.; Liu, Z.J.; Fernandes, G.E.; Shen, T.Y.; Oller, D.; Pacifici, D.; Kim, J.H.; Xu, J. High-purity red coloration via mode-selective absorption in a layered thin-film cavity. Aip Adv. 2018, 8, 065226. [Google Scholar] [CrossRef] [Green Version]
- Palik, E.D.; Ghosh, G. Handbook of Optical Constants of Solids; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Ke, Y.; Wen, X.; Zhao, D.; Che, R.; Xiong, Q.; Long, Y. Controllable Fabrication of Two-Dimensional Patterned VO(2) Nanoparticle, Nanodome, and Nanonet Arrays with Tunable Temperature-Dependent Localized Surface Plasmon Resonance. ACS Nano 2017, 11, 7542–7551. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Ho, H.-C.; Lai, Y.-C.; Nagao, T.; Hsueh, C.-H. Thermochromic vanadium dioxide film on textured silica substrate for smart window with enhanced visible transmittance and tunable infrared radiation. Infrared Phys. Technol. 2019, 102, 103019. [Google Scholar] [CrossRef]
- Li, S.Y.; Niklasson, G.A.; Granqvist, C.G. Nanothermochromics: Calculations for VO2 nanoparticles in dielectric hosts show much improved luminous transmittance and solar energy transmittance modulation. J. Appl. Phys. 2010, 108, 063525. [Google Scholar] [CrossRef] [Green Version]
- Mlyuka, N.R.; Niklasson, G.A.; Granqvist, C.G. Thermochromic VO2-based multilayer films with enhanced luminous transmittance and solar modulation. Phys. Status Solidi (A) 2009, 206, 2155–2160. [Google Scholar] [CrossRef]
- Huang, X.; Huang, J.; Qian, Y.; Yang, L.; Zhang, Z.; Yang, J. Nonvolatile phase-change materials color display designed by evolutionary search. Results Phys. 2021, 29, 104701. [Google Scholar] [CrossRef]
- Santos, G.; Losurdo, M.; Moreno, F.; Gutierrez, Y. Directional Scattering Switching from an All-Dielectric Phase Change Metasurface. Nanomaterials 2023, 13, 496. [Google Scholar] [CrossRef]
- Westland, S.; Ripamonti, C.; Cheung, V. Computational Colour Science Using MATLAB, 2nd ed.; Wiley: Hoboken, NJ, USA, 2012; p. xii. [Google Scholar]
- Huang, X.; Huang, J.; Yang, L.N.; Zhang, Z.F.; Ma, H.S.; Qian, Y.; Li, C.X.; Zhang, Z.R.; Yang, J.B. All-Dielectric Metasurfaces Color Filter Arrays Designed by Evolutionary Search. IEEE Photonics J. 2021, 13, 7100409. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Tian, X.; Fang, J.K.; Shi, Y.P.; Shi, S.N.; Zhang, S.; Song, J.M.; Li, M.P.; Liu, X.Y.; Wang, X.D.; et al. Independently tunable all-dielectric synthetic multi-spectral metamaterials based on Mie resonance. RSC Adv. 2022, 12, 20765–20770. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Shi, S.; Sun, K.; Di, C.; Lin, Y.; Zhu, Y.; Zhang, S.; Shi, Y. Thermally Tunable Structural Color Based on Patterned Ultra-Thin Asymmetric Fabry–Perot Cavity with Phase-Change Material. Crystals 2023, 13, 996. https://doi.org/10.3390/cryst13070996
Fang J, Shi S, Sun K, Di C, Lin Y, Zhu Y, Zhang S, Shi Y. Thermally Tunable Structural Color Based on Patterned Ultra-Thin Asymmetric Fabry–Perot Cavity with Phase-Change Material. Crystals. 2023; 13(7):996. https://doi.org/10.3390/cryst13070996
Chicago/Turabian StyleFang, Jiukai, Shengnan Shi, Kaixiang Sun, Chengzhe Di, Yuwen Lin, Yeqing Zhu, Shan Zhang, and Yanpeng Shi. 2023. "Thermally Tunable Structural Color Based on Patterned Ultra-Thin Asymmetric Fabry–Perot Cavity with Phase-Change Material" Crystals 13, no. 7: 996. https://doi.org/10.3390/cryst13070996




