Water Adsorption on MgO Surfaces: A Vibrational Analysis
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
- The reactivity of MgO nanoparticles with water present in the ambient air is very high. Brucite (Mg(OH)2) is rapidly formed.
- The first step for the transformation of MgO into Mg(OH)2 is the dissociation of water.
- Intermediate species resulting from this dissociation can be identified through Raman spectroscopy.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, H.; Guan, D.; Ma, L. The bio-inspired heterogeneous single-cluster catalyst Ni100–Fe4S4 for enhanced electrochemical CO2 reduction to CH4. Nanoscale 2023, 15, 2756–2766. [Google Scholar] [CrossRef]
- Lv, Z.; Xu, H.; Xu, W.; Peng, B.; Zhao, C.; Xie, M.; Lv, X.; Gao, Y.; Hu, K.; Fang, Y.; et al. Quasi-Topological Intercalation Mechanism of Bi0.67NbS2 Enabling 100 C Fast-Charging for Sodium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2300790. [Google Scholar] [CrossRef]
- Obregón, Á.; Rodríguez-galicia, J.L.; López-cuevas, J.; Pena, P.; Baudín, C. MgO—CaZrO3-based refractories for cement kilns. J. Eur. Ceram. Soc. 2011, 31, 61–74. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Shi, R.; Ma, X.; Tan, L.; Ji, Q.; Xia, Y. Enhanced flame-retardant properties of cellulose fibers by incorporation of acid-resistant magnesium-oxide microcapsules. Carbohydr. Polym. 2017, 176, 246–256. [Google Scholar] [CrossRef]
- Carpenter, T.O.; Delucia, M.C.; Zhang, J.H.; Bejnerowicz, G.; Tartamella, L.; Dziura, J.; Petersen, K.F.; Befroy, D.; Cohen, D. A Randomized Controlled Study of Effects of Dietary Magnesium Oxide Supplementation on Bone Mineral Content in Healthy Girls. J. Clin. Endocrinol. Metab. 2006, 91, 4866–4872. [Google Scholar] [CrossRef] [PubMed]
- Kastyuchik, A.; Karam, A.; Aïder, M. Environmental Technology & Innovation Effectiveness of alkaline amendments in acid mine drainage remediation. Environ. Technol. Innov. 2016, 6, 49–59. [Google Scholar] [CrossRef]
- Kathrein, H.; Freund, F.J. Electrical conductivity of magnesium oxide single crystal below 1200 K. Phys. Chem. Solids 1983, 44, 177–186. [Google Scholar] [CrossRef]
- Itatani, K.; Tsujimoto, T.; Kishimoto, A. Thermal and optical properties of transparent magnesium oxide ceramics fabricated by post hot-isostatic pressing. J. Eur. Ceram. Soc. 2006, 26, 639–645. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.; Abdel-rahman, M.A.; Farag, M.M.S.; Shehal-deen, A.; Mohamed, A.A.; Alsharif, S.M.; Saied, E.; Moghanim, S.A.; Salah, M. Current Research in Biotechnology Catalytic degradation of wastewater from the textile and tannery industries by green synthesized hematite (α-Fe2O3) and magnesium oxide (MgO) nanoparticles. Curr. Res. Biotechnol. 2021, 3, 29–41. [Google Scholar] [CrossRef]
- Song, G.; Zhu, X.; Chen, R.; Liao, Q.; Ding, Y.; Chen, L. An investigation of CO2 adsorption kinetics on porous magnesium oxide. Chem. Eng. J. 2016, 283, 175–183. [Google Scholar] [CrossRef]
- Hornak, J. Synthesis, Properties, and Selected Technical Applications of Magnesium Oxide Nanoparticles: A Review. Int. J. Mol. Sci. 2021, 22, 12752. [Google Scholar] [CrossRef]
- Naguib, G.H.; Nassar, H.M.; Hamed, M.T. Bioactive Materials Antimicrobial properties of dental cements modified with zein-coated magnesium oxide nanoparticles. Bioact. Mater. 2022, 8, 49–56. [Google Scholar] [CrossRef]
- Fernandes, M.; RB Singh, K.; Sarkar, T.; Singh, P.; Pratap Singh, R. Recent Applications of Magnesium Oxide (MgO) Nanoparticles in various domains. Adv. Mater. Lett. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Sastry, S.V.; Nyshadham, J.R.; Fix, J.A. Recent technological advances in oral drug delivery—A review. PSTT 2000, 3, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guan, D. Exceptional Anisotropic Noncovalent Interactions in Ultrathin Nanorods: The Terminal σ-Hole. ACS Appl. Mater. Interfaces 2022, 14, 51190–51199. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liu, P.; Zhang, W.; Wang, Q.; Yang, Y. Structure, stability, electronic and magnetic properties of monometallic Pd, Pt, and bimetallic Pd-Pt core—Shell nanoparticles. Chem. Phys. 2020, 539, 110953. [Google Scholar] [CrossRef]
- Khairallah, F.; Glisenti, A. Synthesis, characterization and reactivity study of nanoscale magnesium oxide. J. Mol. Catal. A Chem. 2007, 274, 137–147. [Google Scholar] [CrossRef]
- Singh, J.P.; Singh, V.; Sharma, A.; Pandey, G.; Chae, K.H.; Lee, S. Approaches to synthesize MgO nanostructures for diverse applications. Heliyon 2020, 6, e04882. [Google Scholar] [CrossRef]
- Syrlybekov, A.; Arca, E.; Verre, R.; O Coileain, C.; Toktarbaiuly, O.; Khalid, A.; Zhang, H.; Shvets, I.V. Induced morphological changes on vicinal MgO (100) subjected to high-temperature annealing: Step formation and surface stability. Surf. Interface Anal. 2015, 47, 969–977. [Google Scholar] [CrossRef]
- Geler-Kremer, J.; Posadas, A.B.; Demkov, A.A. Preparation of clean MgO surface by oxygen plasma: Comparison with standard substrate cleaning procedures. J. Vac. Sci. Technol. B 2020, 38, 062201. [Google Scholar] [CrossRef]
- Thomele, D.; Gheisi, A.R.; Niedermaier, M.; Elsässer, M.S.; Bernardi, J.; Grönbeck, H.; Diwald, O. Thin water films and particle morphology evolution in nanocrystalline MgO. J. Am. Ceram. Soc. 2018, 101, 4994–5003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Z.; Selloni, A. Hydration structure of flat and stepped MgO surfaces. J. Chem. Phys. 2021, 154, 114708. [Google Scholar] [CrossRef] [PubMed]
- Kebede, G.G.; Spångberg, D.; Mitev, P.D.; Broqvist, P.; Hermansson, K. Comparing van der Waals DFT methods for water on NaCl (001) and MgO (001). J. Chem. Phys. 2017, 146, 064703. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Huang, L.; Dong, L.; Zhang, H.; Huang, Z.; Li, F.; Zhang, S. Hydration behavior of MgO surface: A first-principles study. Appl. Surf. Sci. 2023, 611, 155441. [Google Scholar] [CrossRef]
- Ončák, M.; Włodarczyk, R.; Sauer, J. Water on the MgO (001) surface: Surface reconstruction and ion solvation. J. Phys. Chem. Lett. 2015, 6, 2310–2314. [Google Scholar] [CrossRef]
- Jug, K.; Heidberg, B.; Bredow, T. Molecular Dynamics Study of Water Adsorption Structures on the MgO (100) Surface. J. Phys. Chem. C 2007, 111, 6846–6851. [Google Scholar] [CrossRef]
- Hadj Youssef, A.; Zhang, J.; Ehteshami, A.; Kolhatkar, G.; Dab, C.; Berthomieu, D.; Merlen, A.; Légaré, F.; Ruediger, A. Symmetry-Forbidden-Mode Detection in SrTiO3 Nanoislands with Tip-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2021, 125, 6200–6208. [Google Scholar] [CrossRef]
- Manson, N.B.; Von der Ohe, W.; Chodos, S.L. Second-Order Raman Spectrum of MgO. Phys. Rev. B 1971, 3, 1968. [Google Scholar] [CrossRef]
- Ishikawa, K.; Fujima, N.; Komura, H. First-order Raman scattering in MgO microcrystals. J. Appl. Phys. 1985, 57, 973–975. [Google Scholar] [CrossRef]
- Dovesi, R.; Erba, A.; Orlando, R.; Zicovich-Wilson, C.M.; Civalleri, B.; Maschio, L.; Rérat, M.; Casassa, S.; Baima, J.; Salustro, S.; et al. Quantum-mechanical condensed matter simulations with CRYSTAL. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1360. [Google Scholar] [CrossRef]
- Dovesi, R.; Pascale, F.; Civalleri, B.; Doll, K.; Harrison, N.M.; Bush, I.; D’Arco, P.; Noel, Y.; Rera, M.; Carbonniere, P.; et al. The CRYSTAL code, 1976–2020 and beyond, a long story. J. Chem. Phys. 2020, 152, 204111. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the collesalvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Vilela Oliveira, D.; Laun, J.; Peintinger, M.F.; Bredow, T. BSSE-correction scheme for consistent gaussian basis sets of double- and triple-zeta valence with polarization quality for solid-state calculations. J. Comput. Chem. 2019, 40, 2364–2376. [Google Scholar] [CrossRef]
- Available online: https://www.crystal.unito.it/basis_sets.html (accessed on 24 July 2023).
- McCarthy, M.I.; Harrison, N.M. Ab Initio determination of the bulk properties of MgO. Phys. Rev. B 1994, 49, 8574–8582. [Google Scholar] [CrossRef] [Green Version]
- Scaranto, J.; Giorgianni, S. A quantum-mechanical study of CO adsorbed on TiO2: A comparison of the Lewis acidity of the rutile (1 1 0) and the anatase (1 0 1) surfaces. J. Mol. Struct. 2008, 858, 72–76. [Google Scholar] [CrossRef]
- Towler, M.D.; Allan, N.L.; Harrison, N.M.; Saunders, V.R.; Mackrodt, W.C.; Apra, E. An ab initio Hartree-Fock study of MnO and NiO. Phys. Rev. B 1994, 50, 5041–5054. [Google Scholar] [CrossRef] [PubMed]
- Dovesi, R.; Ermondi, E.; Ferrero, E.; And, C.P.; Roetti, C. Hartree-Fock study of lithium hydride with the use of a polarizable basis set. Phys. Rev. B 1983, 29, 3591–3600. [Google Scholar] [CrossRef]
- Maschio, L.; Kirtman, B.; Rérat, M.; Orlando, R.; Dovesi, R. Ab initio analytical Raman intensities for periodic systems through a coupled perturbed Hartree-Fock/Kohn-Sham method in an atomic orbital basis. I. Theory. J. Chem. Phys. 2013, 139, 164101. [Google Scholar] [CrossRef] [Green Version]
- Maschio, L.; Kirtman, B.; Rérat, M.; Orlando, R.; Dovesi, R. Ab initio analytical Raman intensities for periodic systems through a coupled perturbed Hartree-Fock/Kohn-Sham method in an atomic orbital basis. II. Validation and comparison with experiments. J. Chem. Phys. 2013, 139, 164102. [Google Scholar] [CrossRef] [Green Version]
- Pascale, F.; Zicovich-Wilson, C.M.; López Gejo, F.; Civalleri, B.; Orlando, R.; Dovesi, R. The calculation of the vibrational frequencies of crystalline compounds and its implementation in the CRYSTAL code. J. Comput. Chem. 2004, 25, 888–897. [Google Scholar] [CrossRef]
- Zicovich-Wilson, C.M.; Pascale, F.; Roetti, C.; Saunders, V.R.; Orlando, R.; Dovesi, R. Calculation of the vibration frequencies of α-quartz: The effect of hamiltonian and basis set. J. Comput. Chem. 2004, 25, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Erba, A.; Maul, J.; Ferrabone, M.; Carbonniere, P.; Rerat, M.; Dovesi, R. Anharmonic Vibrational States of Solids from DFT Calculations. Part I: Description of the Potential Energy Surface. J. Chem. Theory Comput. 2019, 15, 3755–3765. [Google Scholar] [CrossRef] [PubMed]
- Erba, A.; Maul, J.; Ferrabone, M.; Dovesi, R.; Rerat, M.; Carbonniere, P. Anharmonic Vibrational States of Solids from DFT Calculations. Part II: Implementation of the VSCF and VCI Methods. J. Chem. Theory Comput. 2019, 15, 3766–3777. [Google Scholar] [CrossRef] [PubMed]
- Carbonniere, P.; Erba, A.; Richter, F.; Dovesi, R.; Rérat, M. Calculation of Anharmonic IR and Raman Intensities for Periodic Systems from DFT Calculations: Implementation and Validation. J. Chem. Theory Comput. 2020, 16, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Heidberg, J.; Redlich, B.; Wetter, D. Adsorption of Water Vapor on the MgO (100) Single Crystal Surface. Phys. Chem. 1995, 11, 1333–1337. [Google Scholar] [CrossRef]
- Odelius, M. Mixed Molecular and Dissociative Water Adsorption on MgO [100]. Phys. Rev. Lett. 1999, 82, 3919–3922. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Goodman, D.W. Structure and geometry of water adsorbed on the MgO (100) surface. Chem. Phys. Lett. 1997, 265, 341–346. [Google Scholar] [CrossRef]
- Gardeh, M.G.; Kistanov, A.A.; Nguyen, H.; Manzano, H.; Cao, W.; Kinnunen, P. Exploring Mechanisms of Hydration and Carbonation of MgO and Mg(OH)2 in Reactive Magnesium Oxide-Based Cements. J. Phys. Chem. C 2022, 126, 6196–6206. [Google Scholar] [CrossRef] [PubMed]
- Alessio, M.; Usvyat, D.; Sauer, J. Chemically Accurate Adsorption Energies: CO and H2O on the MgO (001) Surface. J. Chem. Theory Comput. 2019, 15, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W. Effect of C and H contamination on the MgO surface properties studied using the first-principles method. J. Inf. Disp. 2013, 14, 97–102. [Google Scholar] [CrossRef]
- Broda, M.A.; Buczek, A.; Kupka, T.; Kaminsky, J. Anharmonic vibrational frequency calculations for solvated molecules in the B3LYP Kohn—Sham basis set limit. Vib. Spectrosc. 2012, 63, 432–439. [Google Scholar] [CrossRef]
- Pascale, F.; Tosoni, S.; Zicovich-wilson, C.; Ugliengo, P.; Orlando, R.; Dovesi, R. Vibrational spectrum of brucite, Mg(OH)2: A periodic ab initio quantum mechanical calculation including OH anharmonicity. Chem. Phys. Lett. 2004, 396, 308–315. [Google Scholar] [CrossRef]
- Available online: https://rruff.info/Brucite/R050455 (accessed on 24 July 2023).
- Leung, Y.H.; Ng, A.M.C.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.N.; Guo, M.Y.; Ng, Y.H.; Djurisic, A.B.; et al. Mechanisms of Antibacterial Activity of MgO Non-ROS Mediated Toxicity of MgO Nanoparticles Towards Escherichia coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef]

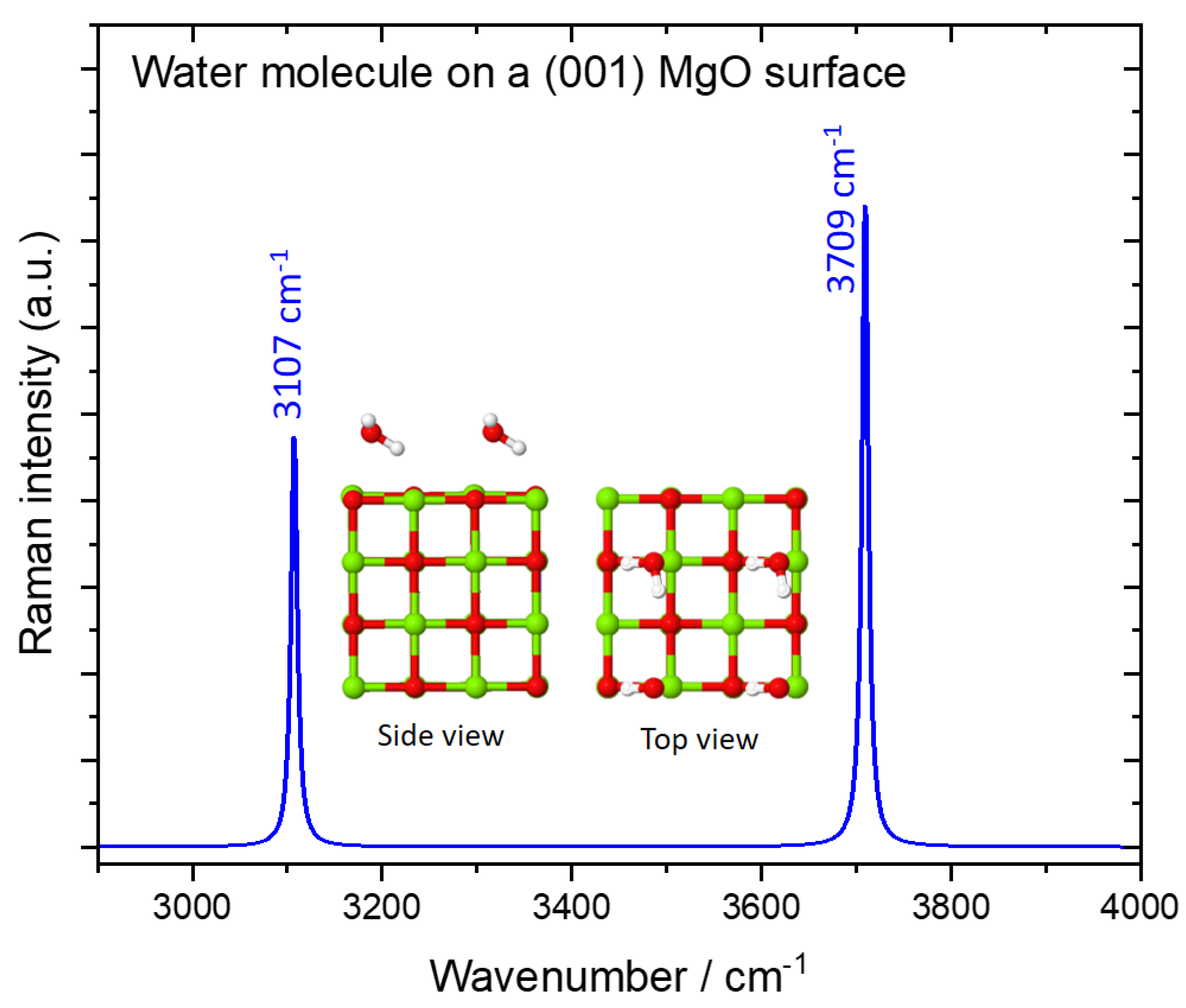
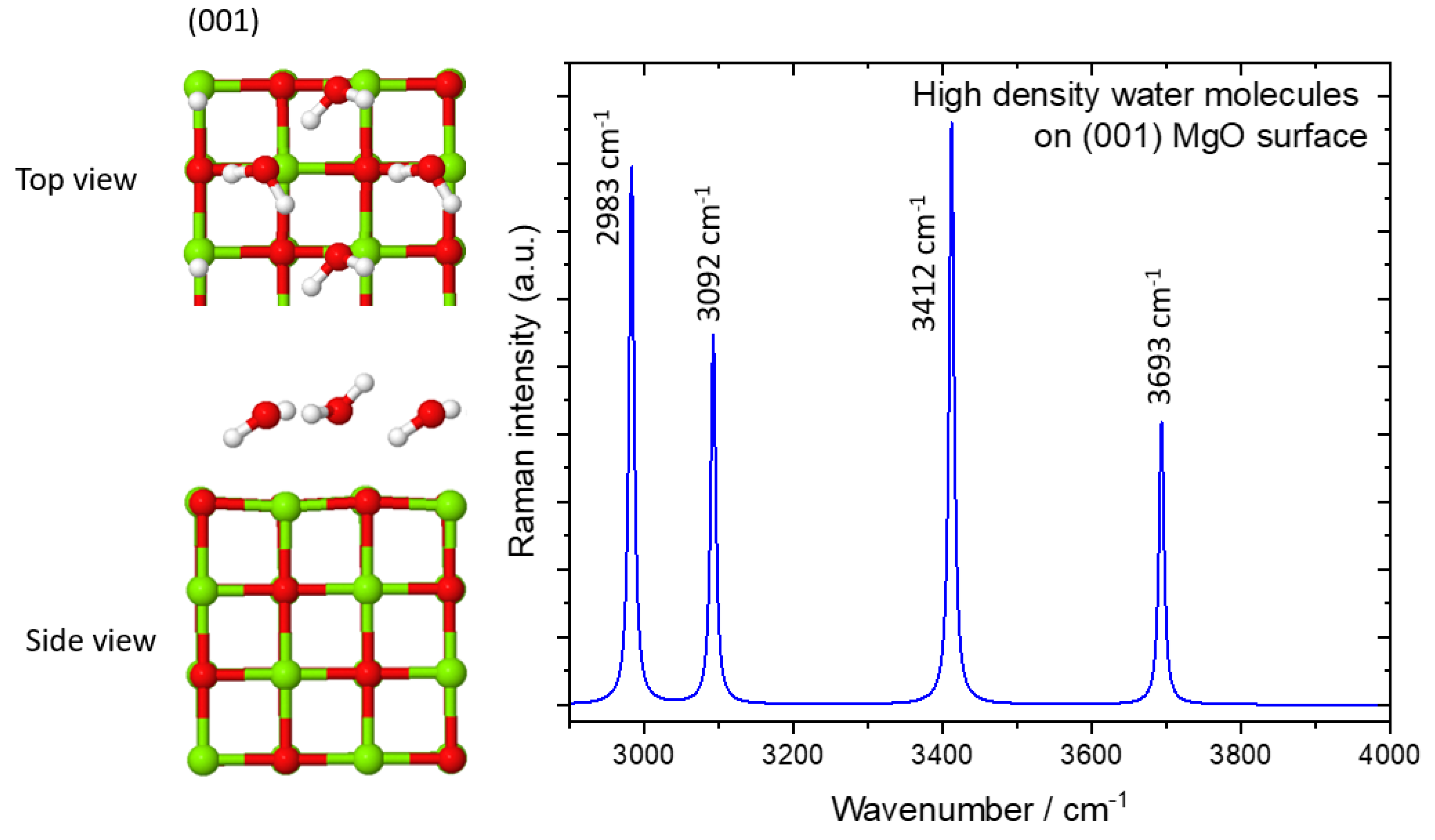
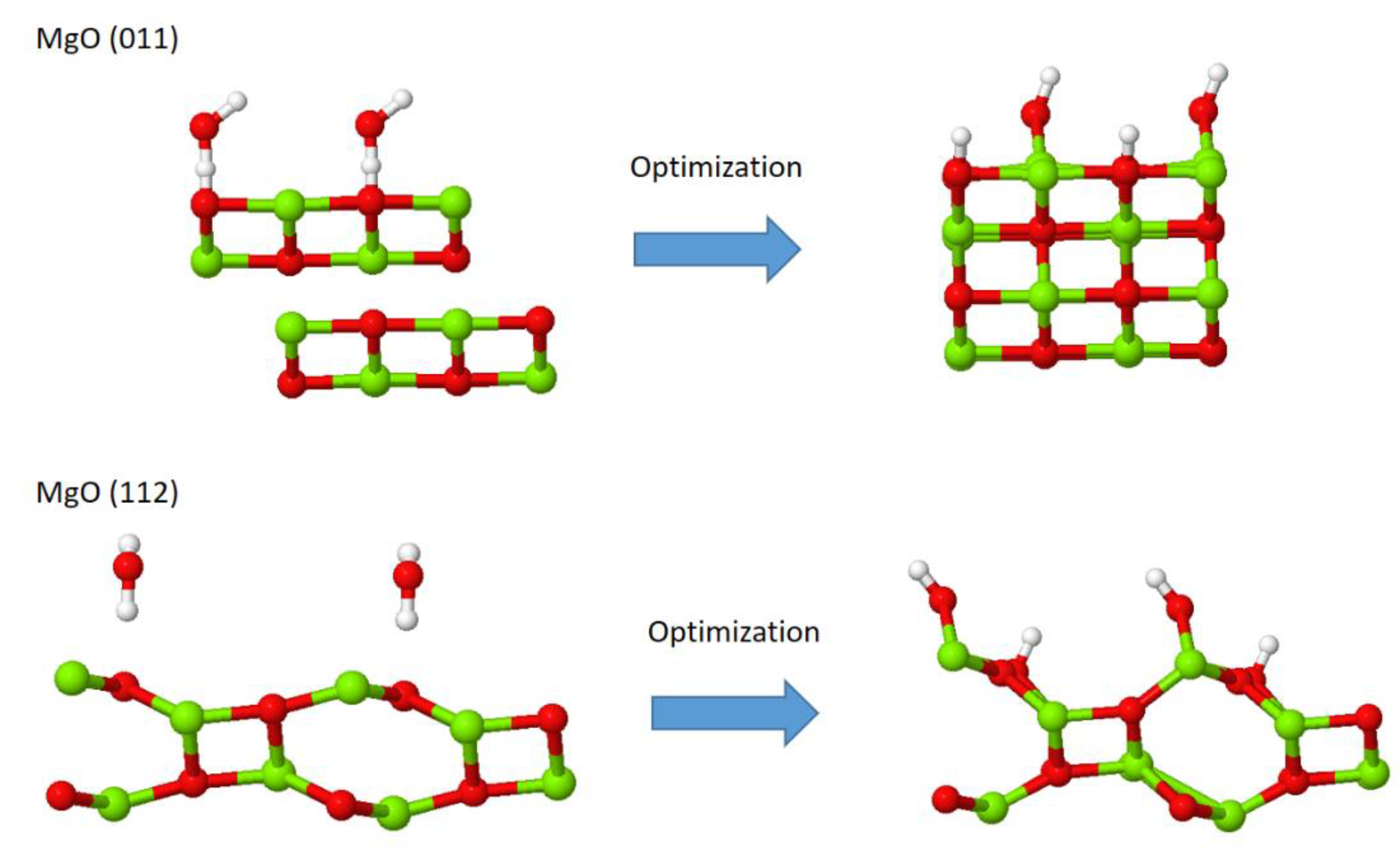

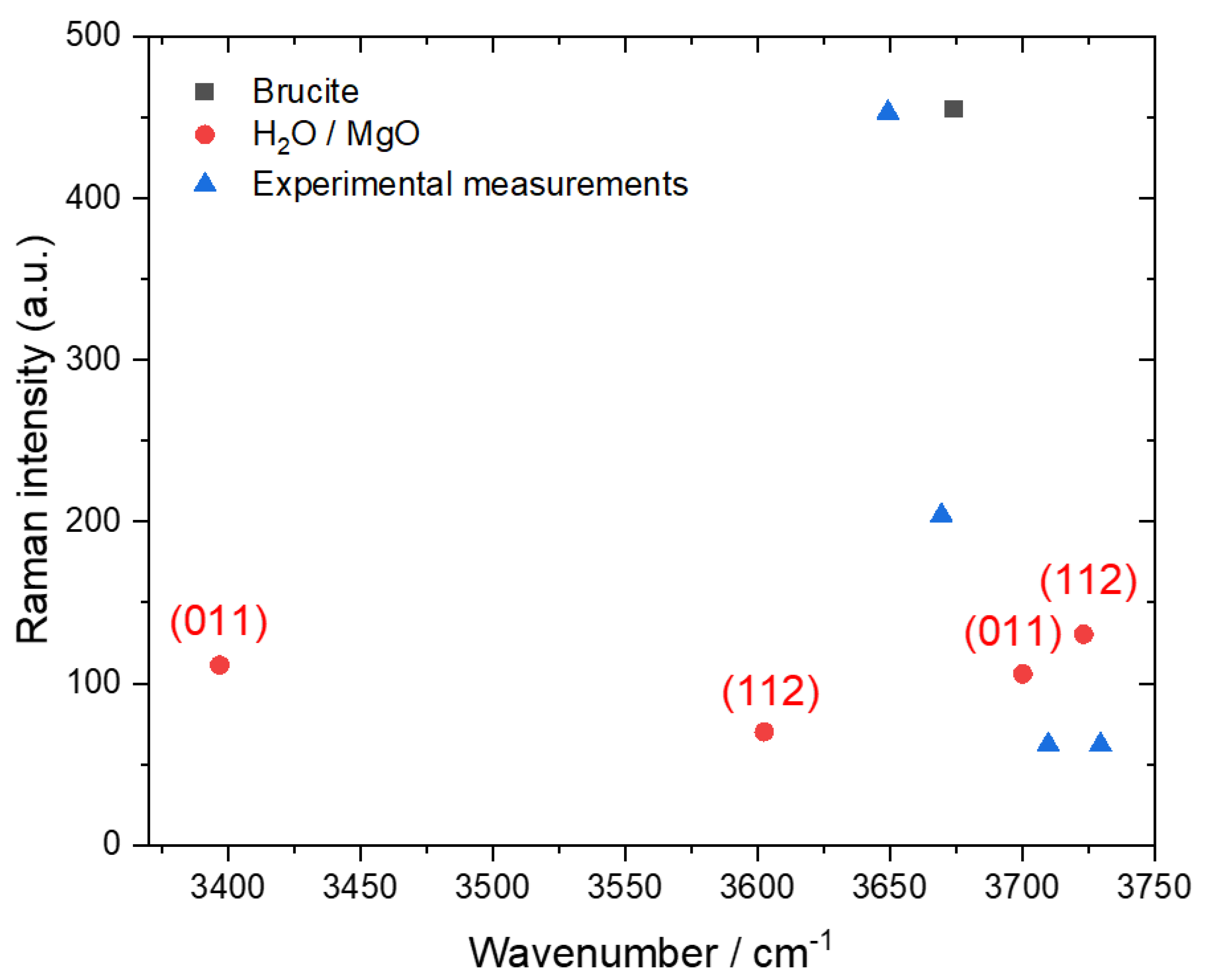
| Configuration | Frequencies/Intensities | |
|---|---|---|
| Harmonic | Anharmonic | |
| H2O | 3846/30, 3731/86 (3778/28, 3649/87) | 3684/31, 3603/86 (3619/29, 3527/87) |
| 1 H2O/MgO(001) | 3768/103, 3321/67 | 3672/108, 3182/67 |
| 2 H2O/MgO(001) | 3753/104, 3618/245 3378/242, 3126/122 | 3654/106, 3481/270 3182/228, 2947/138 |
| H2O-MgO(011) | 3805/102, 3584/114 | 3701/106, 3397/112 |
| H2O-MgO(112) | 3816/125, 3696/68 | 3724/130, 3603/70 |
| Mg(OH)2 (brucite) | 3805/459 (3746/405) | 3676/455 (3611/391) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dekermenjian, M.; Merlen, A.; Ruediger, A.; Rérat, M. Water Adsorption on MgO Surfaces: A Vibrational Analysis. Crystals 2023, 13, 1153. https://doi.org/10.3390/cryst13081153
Dekermenjian M, Merlen A, Ruediger A, Rérat M. Water Adsorption on MgO Surfaces: A Vibrational Analysis. Crystals. 2023; 13(8):1153. https://doi.org/10.3390/cryst13081153
Chicago/Turabian StyleDekermenjian, Maria, Alexandre Merlen, Andreas Ruediger, and Michel Rérat. 2023. "Water Adsorption on MgO Surfaces: A Vibrational Analysis" Crystals 13, no. 8: 1153. https://doi.org/10.3390/cryst13081153
APA StyleDekermenjian, M., Merlen, A., Ruediger, A., & Rérat, M. (2023). Water Adsorption on MgO Surfaces: A Vibrational Analysis. Crystals, 13(8), 1153. https://doi.org/10.3390/cryst13081153







