High-Temperature Oxidation Behaviors of AlCrTiSi0.2 High-Entropy Alloy Doped with Rare Earth La and Y
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Characterization
3. Results and Discussion
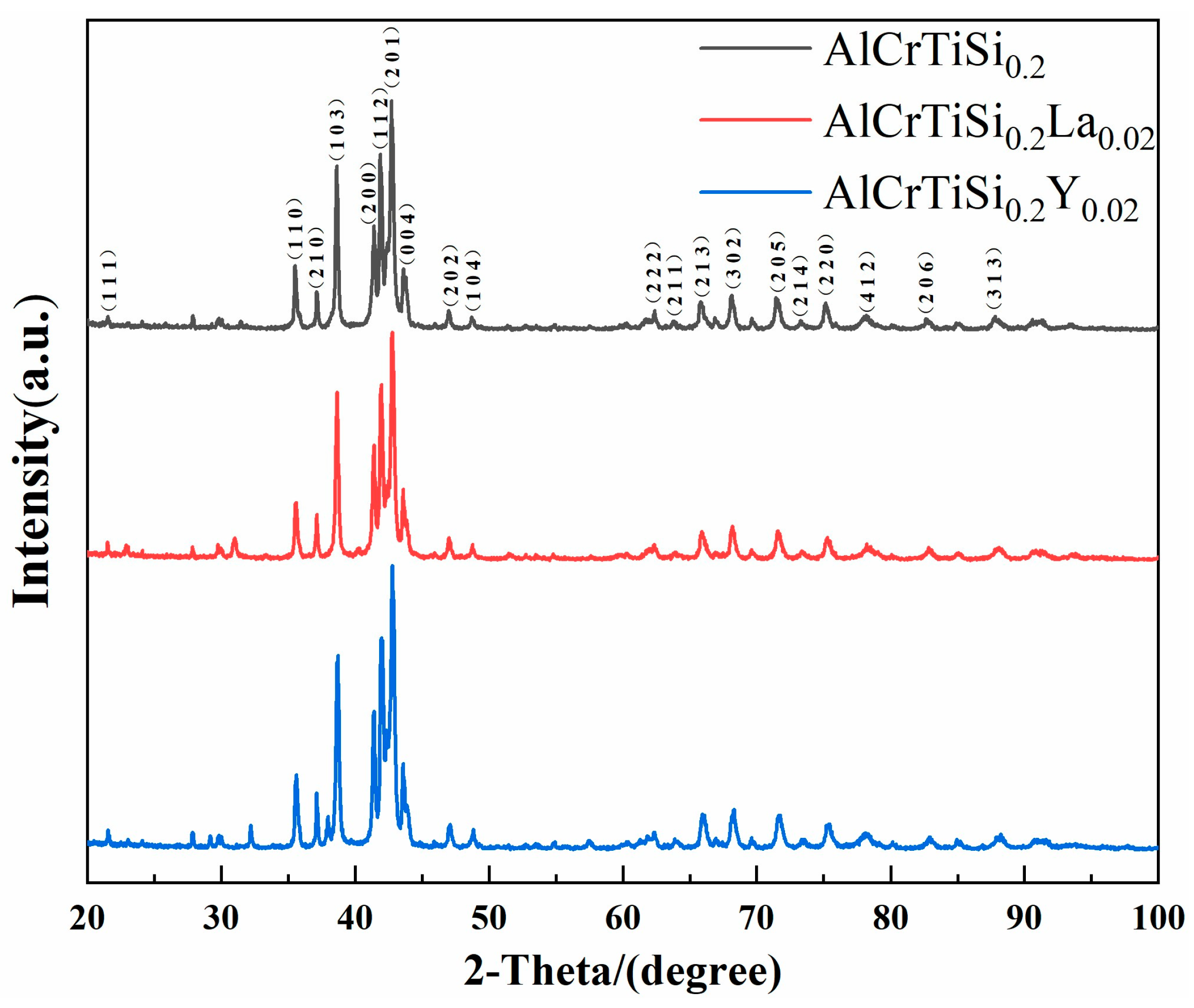
3.1. Microstructures of Cast Alloys
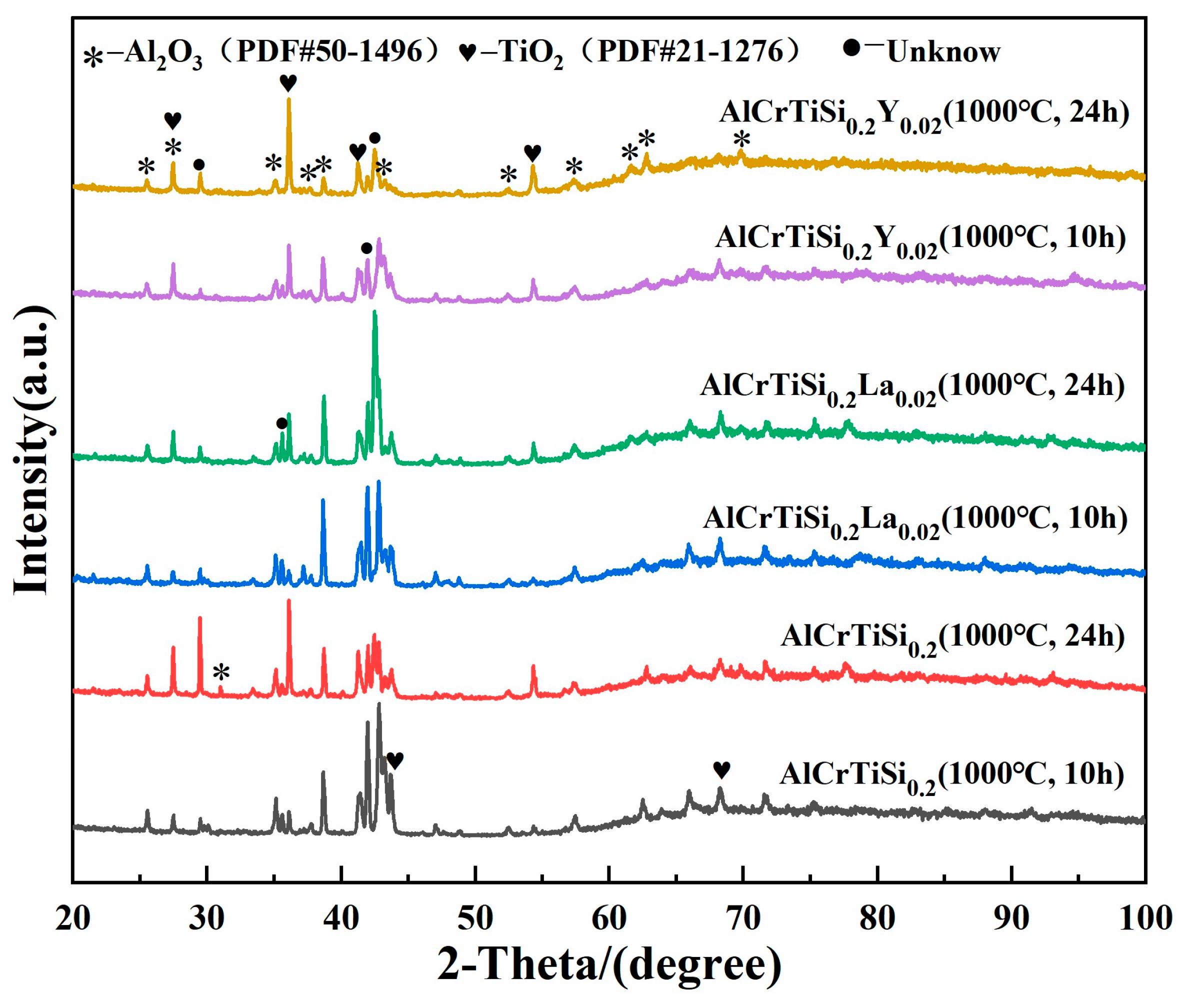
3.2. Oxidation Behaviors of AlCrTiSi0.2La0.02 and AlCrTiSi0.2Y0.02 HEAs
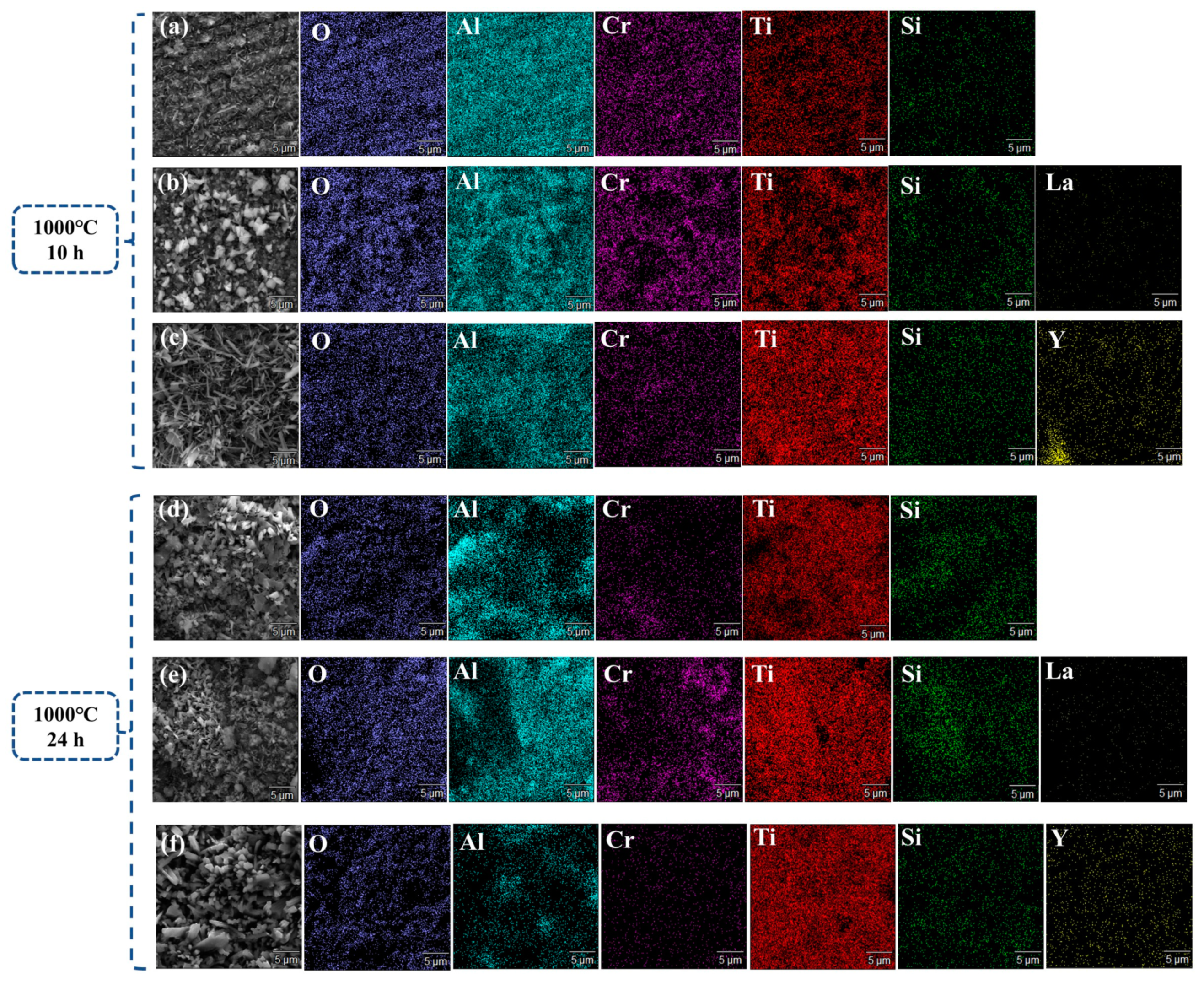
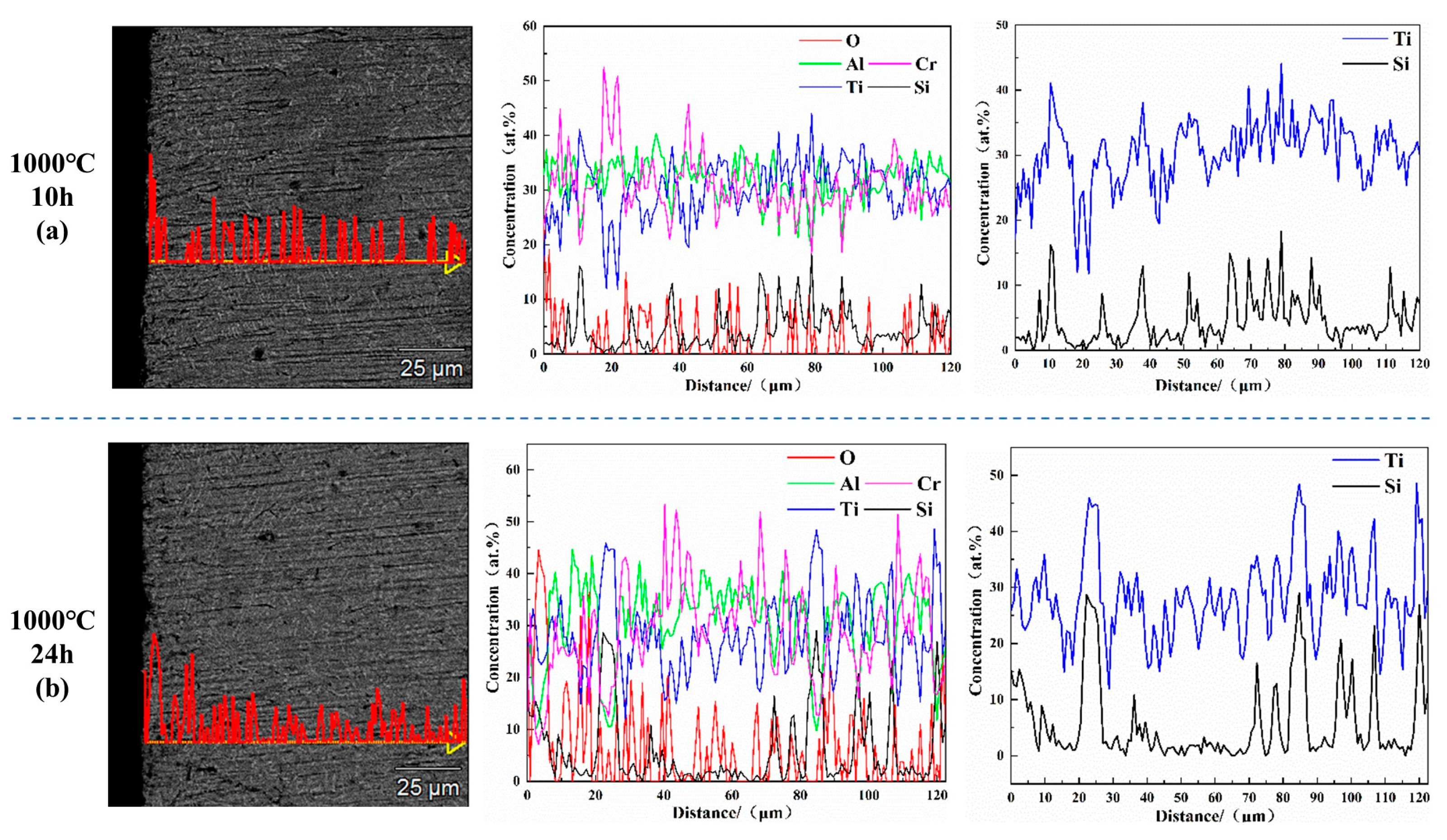
3.3. Oxidation Mechanism of AlCrTiSi0.2La0.02 and AlCrTiSi0.2Y0.02HEAs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.; Lin, S.; Gan, J.; Chin, T.; Shun, T.; Tsau, C.H.; Chang, S. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Pradeep, K.G.; Tasan, C.C.; Yao, M.; Deng, Y.; Raabe, D. Non-equiatomic high entropy alloys: Approach towards rapid alloy screening and property-oriented design. Mater. Sci. Eng. A 2015, 648, 183–192. [Google Scholar] [CrossRef]
- Wei, Y.; Fu, Y.; Pan, Z.; Ma, Y.; Cheng, H.; Zhao, Q.; Luo, H.; Li, X. Influencing factors and mechanism of high-temperature oxidation of high-entropy alloys: A review. Int. J. Miner. Metall. Mater. 2021, 28, 915–930. [Google Scholar] [CrossRef]
- Pickering, E.; Jones, N.G. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhang, L.; Fan, J.; Yu, P.; Li, G. Novel Co-free CrFeNiNb0.1Ti high-entropy alloys with ultrahigh hardness and strength. Mater. Sci. Eng. A 2019, 764, 138212. [Google Scholar] [CrossRef]
- Tan, M.; Meng, L.; Fang, S.; Lin, C.; Ke, L.; Yu, Z.; Qu, J.; Qi, T. Organizational Evolution during Performance Meritocracy of AlSi0.5CrxCo0.2Ni Lightweight High Entropy Alloys. Crystals 2022, 12, 1828. [Google Scholar] [CrossRef]
- Huang, C.; Tang, Y.; Zhang, Y.; Dong, A.; Tu, J.; Chai, L.; Zhou, Z. Microstructure and dry sliding wear behavior of laser clad AlCrNiSiTi multi-principal element alloy coatings. Rare Met. 2017, 36, 562–568. [Google Scholar] [CrossRef]
- Tan, M.; Meng, L.; Lin, C.; Ke, L.; Liu, Y.; Qu, J.; Qi, T. Variation of Microstructures and Properties of Co0.2CrAlNi High Entropy Alloy Doped Si. J. Alloys Compd. 2022, 927, 167081. [Google Scholar] [CrossRef]
- Butler, T.M.; Chaput, K.J. Native oxidation resistance of Al20Nb30Ta10Ti30Zr10 refractory complex concentrated alloy (RCCA). J. Alloys Compd. 2019, 787, 606–617. [Google Scholar] [CrossRef]
- Xiao, Y.; Kuang, W.; Xu, Y.; Wu, L.; Gong, W.; Qian, J.; Zhang, Q.; He, Y. Microstructure and oxidation behavior of the CrMoNbTaV high-entropy alloy. J. Mater. Res. 2019, 34, 301–308. [Google Scholar] [CrossRef]
- Butler, T.M.; Chaput, K.J.; Dietrich, J.R.; Senkov, O.N. High temperature oxidation behaviors of equimolar NbTiZrV and NbTiZrCr refractory complex concentrated alloys (RCCAs). J. Alloys Compd. 2017, 729, 1004–1019. [Google Scholar] [CrossRef]
- Lin, C.; Meng, L.; Tan, M.; Ke, L.; Qi, T. Microstructures and properties of Crx-FeNiCu0.5Ti0.5 high-entropy alloys for corrosion resistance. Intermetallics 2023, 153, 107781. [Google Scholar] [CrossRef]
- Praveen, S.; Kim, H.S. High-Entropy Alloys: Potential Candidates for High-Temperature Applications—An Overview. Adv. Eng. Mater. 2018, 20, 1700645. [Google Scholar] [CrossRef]
- Jiang, W.; Yuan, S.; Cao, Y. Mechanical properties and deformation mechanisms of a Ni2Co1Fe1V0.5Mo0.2 medium-entropy alloy at elevated temperatures. Acta Mater. 2021, 213, 116982. [Google Scholar] [CrossRef]
- Antonaglia, J.; Xie, X.; Tang, Z.; Tsai, C.W.; Qiao, J.; Zhang, Y.; Laktionova, M.O.; Tabachnikova, E.D.; Carroll, R.; Yeh, J.W.; et al. Erratum to: Temperature Effects on Deformation and Serration Behavior of High-Entropy Alloys (HEAs). JOM 2014, 66, 2593. [Google Scholar] [CrossRef] [Green Version]
- Ham, G.S.; Kim, Y.K.; Na, Y.S.; Lee, K.A. Effect of Ti Addition on the Microstructure and High-Temperature Oxidation Property of AlCoCrFeNi High-Entropy Alloy. Met. Mater. Int. 2021, 27, 156–165. [Google Scholar] [CrossRef]
- RAO, Z.; Wang, X.; Wang, Q.; Liu, T.; Chen, X.; Wang, L.; Hui, X. Microstructure, Mechanical Properties, and Oxidation Behavior of AlxCr0.4CuFe0.4MnNi High Entropy Alloys: AlxCr0.4CuFe0.4MnNi High Entropy Alloys. Adv. Eng. Mater. 2017, 19, 1600726. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Zhang, S.; Tang, H.; Zhang, A. Microstructure and oxidation behavior of new refractory high entropy alloys. J. Alloys Compd. 2014, 583, 162–169. [Google Scholar] [CrossRef]
- Gorr, B.; Mueller, F.; Christ, H.J.; Mueller, T.; Chen, H.; Kauffmann, A.; Heilmaier, M. High temperature oxidation behavior of an equimolar refractory metal-based alloy 20Nb20Mo20Cr20Ti20Al with and without Si addition. J. Alloys Compd. 2016, 688, 468–477. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, A.; Sampreeth, J.K.; Bembalge, O.; Hascoet, J.Y.; Panigrahi, S.K. High temperature oxidation study of direct laser deposited AlXCoCrFeNi (X = 0.3, 0.7) high entropy alloys. Surf. Coat. Technol. 2019, 380, 125028. [Google Scholar] [CrossRef]
- Erdogan, A.; Doleker, K.M.; Zeytin, S. Effect of Al and Ti on High-Temperature Oxidation Behavior of CoCrFeNi-Based High-Entropy Alloys. JOM 2019, 71, 3499–3510. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.; Zhang, P.; Zhang, K.; Chen, C.; Shen, B. Influence of Al content on high temperature oxidation behavior of AlxCoCrFeNiTi0.5 high entropy alloys. Vacuum 2019, 163, 263–268. [Google Scholar] [CrossRef]
- Butler, T.M.; Weaver, M.L. Oxidation behavior of arc melted AlCoCrFeNi multi-component high-entropy alloys. J. Alloys Compd. 2016, 674, 229–244. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Jin, J.; Gong, P.; Wang, X. Oxidation behavior of CoCrFeMnNi high entropy alloy after plastic deformation. Corros. Sci. 2020, 163, 108285. [Google Scholar] [CrossRef]
- Holcomb, G.R.; Tylczak, J.; Carney, C. Oxidation of CoCrFeMnNi High Entropy Alloys. JOM 2015, 67, 2326–2339. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Zhang, J.; Chen, Z.; Shen, B. Oxidation behavior of AlCoCrFeNiSi high-entropy alloys at 1100 °C. Corros. Sci. 2021, 190, 109633. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.; Tan, Z.; He, D.; Bobzin, K.; Zhao, L.; Öte, M.; Königstein, T. High temperature oxidation behavior of Al0.6CrFeCoNi and Al0.6CrFeCoNiSi0.3 high entropy alloys. J. Alloys Compd. 2018, 764, 845–852. [Google Scholar] [CrossRef]
- Senkov, O.N.; Senkova, S.V.; Dimiduk, D.M.; Woodward, C.; Miracle, D.B. Oxidation behavior of a refractory NbCrMo0.5Ta0.5TiZr alloy. J. Mater. Sci. 2012, 47, 6522–6534. [Google Scholar] [CrossRef]
- Mallikarjuna, H.T.; Caley, W.F.; Richards, N.L. Oxidation Kinetics and Oxide Scale Characterization of Nickel-Based Superalloy IN738LC at 900 °C. J. Mater. Eng. Perform. 2017, 26, 4838–4846. [Google Scholar] [CrossRef]
- Yuan, K.; Peng, R.; Li, X.; Johansson, S.; Wang, Y. Some aspects of elemental behaviour in HVOF MCrAlY coatings in high-temperature oxidation. Surf. Coat. Technol. 2015, 261, 86–101. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ji, H.; Chen, M.; Bao, Z.; Wang, F. High temperature oxidation and interdiffusion behavior of recoated NiCoCrAlY coating on a nickel-based superalloy. Corros. Sci. 2020, 175, 108894. [Google Scholar] [CrossRef]
- Yoshioka, T.; Arikawa, H.; Okada, M.; Hisamatsu, T.; Kojima, Y. Influence of Surface Treatment of Bondcoat and Yttrium in Bondcoat on Oxidation Behavior of Thermal Barrier Coatings. J. Jpn. Inst. Met. 2006, 70, 7–13. [Google Scholar] [CrossRef]
- Peng, H.; Guo, H.; He, J.; Gong, S. Cyclic oxidation and diffusion barrier behaviors of oxides dispersed NiCoCrAlY coatings. J. Alloys Compd. 2010, 502, 411–416. [Google Scholar] [CrossRef]
- Aryal, A.; Pandey, S.; Dubenko, I.; Mazumdar, D.; Ali, N. Effects of Rare-Earth (R = Pr, Gd, Ho, Er) Doping on Magnetostructural Phase Transitions and Magnetocaloric Properties in Ni43−xRxMn46Sn11 Shape Memory Alloys. IEEE Trans. Magn. 2018, 55, 2500505. [Google Scholar]
- Zhu, S.; Nie, J.; Gibson, M.A.; Easton, M.A. On the unexpected formation of rare earth hydrides in magnesium–rare earth casting alloys. Scr. Mater. 2014, 77, 21–24. [Google Scholar] [CrossRef]
- Hu, Z.; Zhan, Y.; Zhang, G.; She, J.; Li, C. Effect of rare earth Y addition on the microstructure and mechanical properties of high entropy AlCoCrCuNiTi alloys. Mater. Des. 2010, 31, 1599–1602. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Cieślak, G.; Stygar, M.; Mroczka, K.; Berent, K.; Kulik, T.; Danielewski, M. Influence of Cu content on high temperature oxidation behavior of AlCoCrCuxFeNi high entropy alloys (x = 0; 0.5; 1). Intermetallics 2017, 84, 52–61. [Google Scholar] [CrossRef]




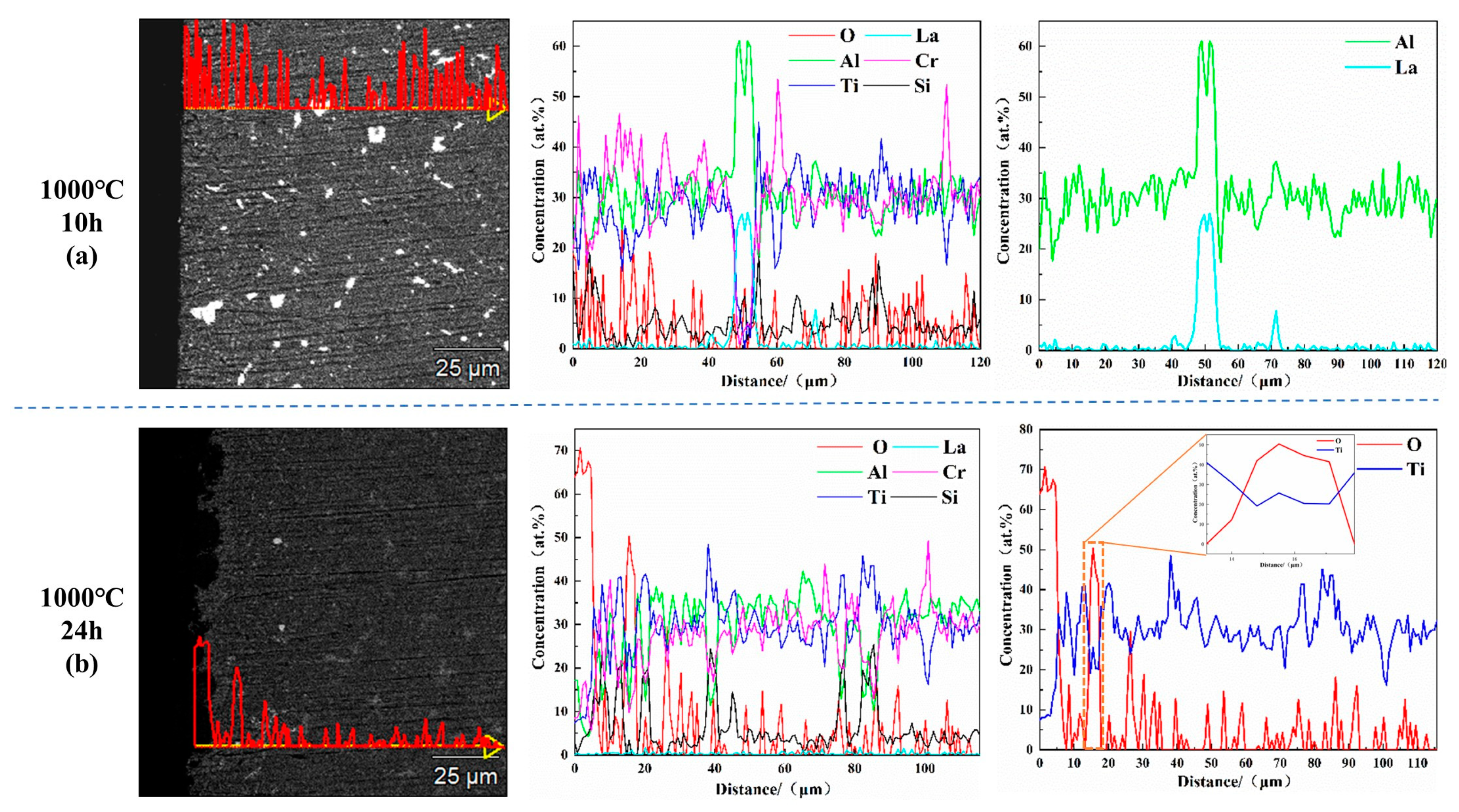
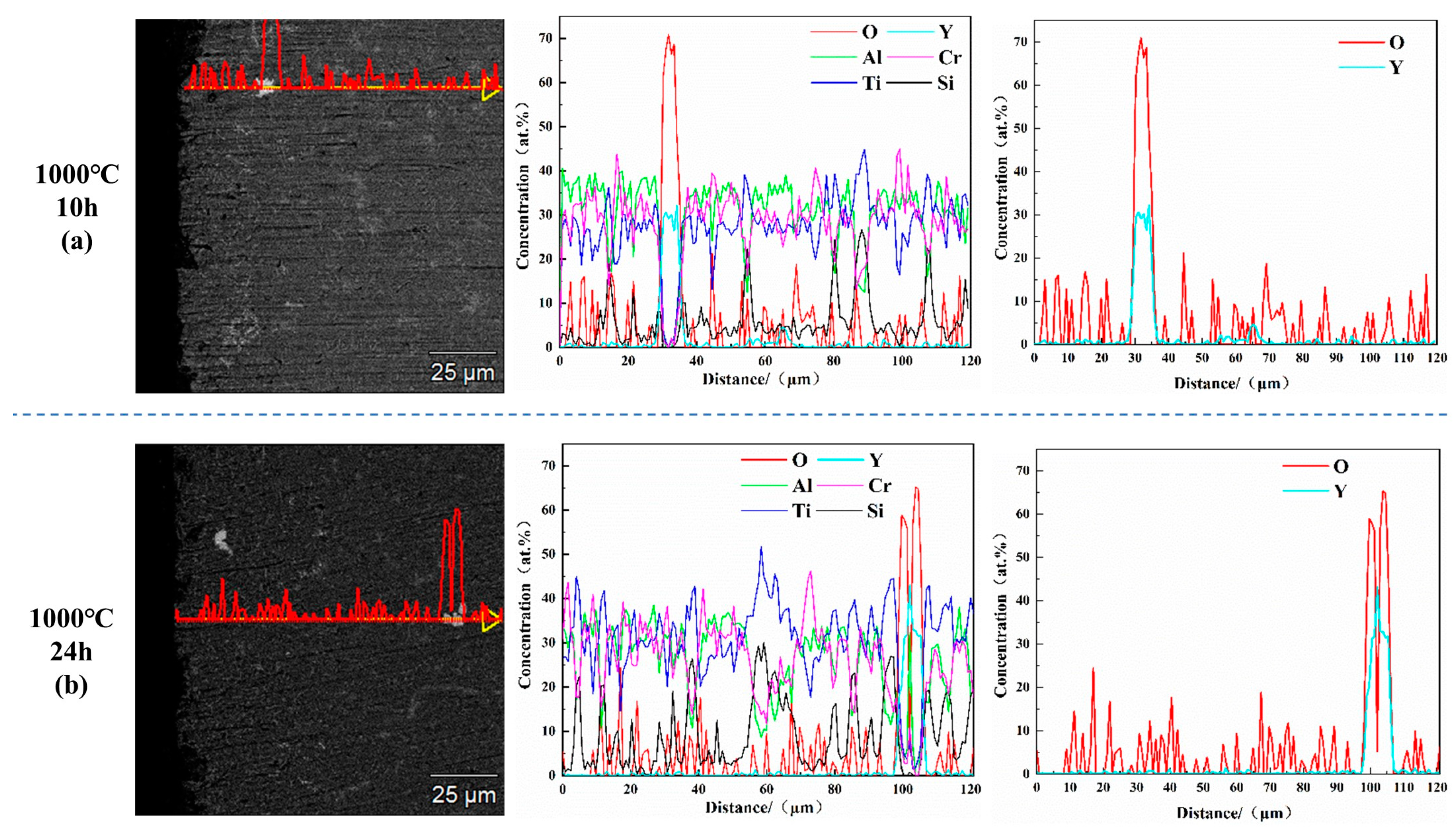
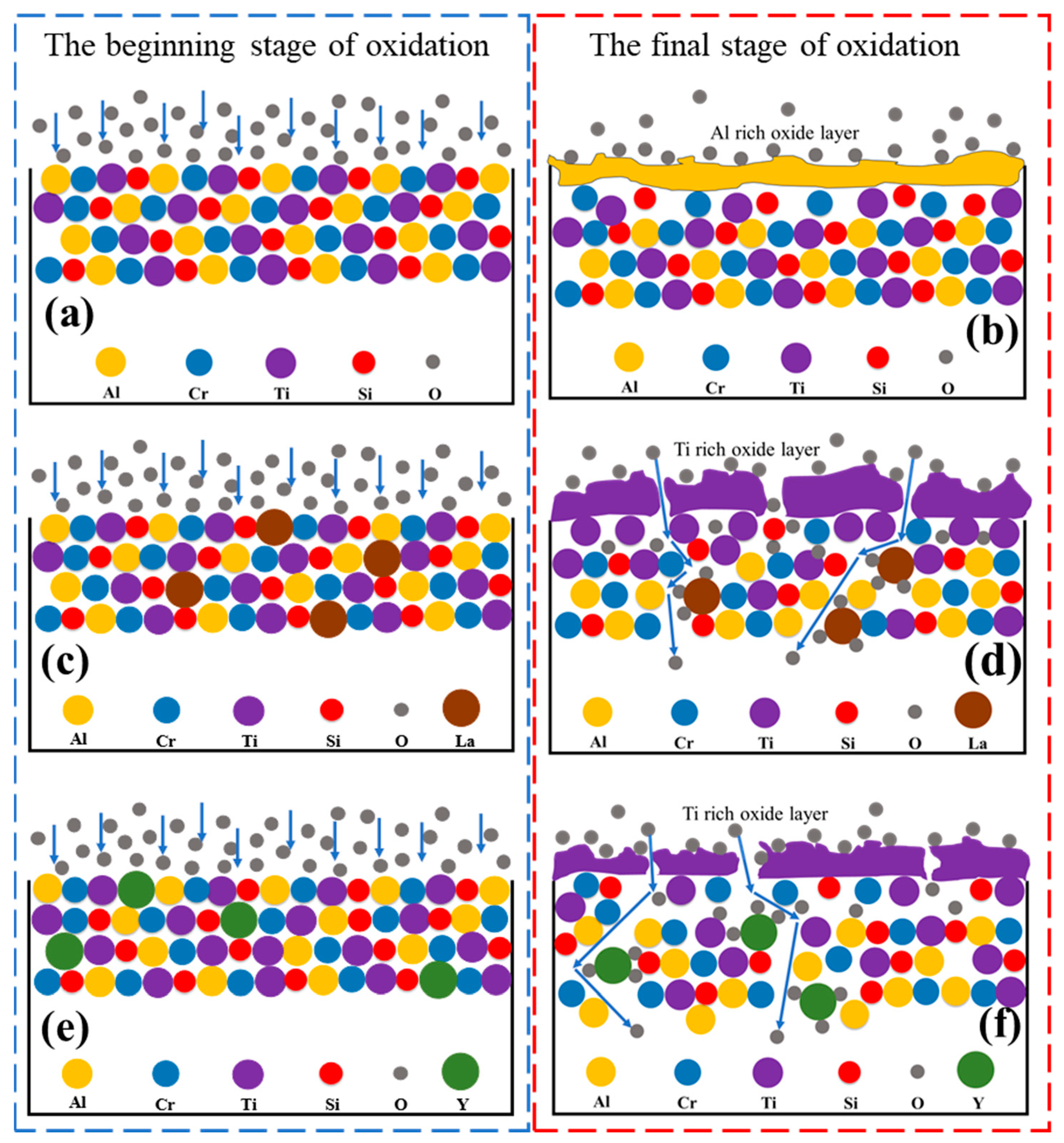
| Alloys | Al | Cr | Ti | Si | La | Y |
|---|---|---|---|---|---|---|
| AlCrTiSi0.2 (nominal) | 31.25 | 31.25 | 31.25 | 6.25 | - | - |
| AlCrTiSi0.2 (measured) | 31.11 | 31.10 | 30.81 | 6.98 | - | - |
| AlCrTiSi0.2La0.02 (nominal) | 31.056 | 31.056 | 31.056 | 6.211 | 0.621 | - |
| AlCrTiSi0.2La0.02 (measured) | 30.79 | 31.19 | 31.38 | 6.18 | 0.46 | - |
| AlCrTiSi0.2Y0.02 (nominal) | 31.056 | 31.056 | 31.056 | 6.211 | - | 0.621 |
| AlCrTiSi0.2Y0.02 (measured) | 30.91 | 31.42 | 30.95 | 5.87 | - | 0.85 |
| Alloys | Al | Cr | Ti | Si | La | Y |
|---|---|---|---|---|---|---|
| AlCrTiSi0.2 | 32.41 ± 0.24 | 29.87 ± 0.24 | 30.60 ± 0.21 | 7.12 ± 0.16 | - | - |
| AlCrTiSi0.2La0.02 | 30.12 ± 0.20 | 30.76 ± 0.22 | 31.89 ± 0.20 | 6.77 ± 0.13 | 0.46 ± 0.07 | - |
| AlCrTiSi0.2Y0.02 | 30.91 ± 0.23 | 31.00 ± 0.26 | 31.42 ± 0.18 | 5.77 ± 0.10 | - | 0.91 ± 0.06 |
| Alloys | Oxide Layer | O | Al | Cr | Ti | Si | La | Y |
|---|---|---|---|---|---|---|---|---|
| AlCrTiSi0.2 | (a) | 58.06 ± 1.17 | 32.26 ± 0.15 | 4.66 ± 0.08 | 4.73 ± 0.06 | 0.29 ± 0.05 | - | - |
| (d) | 60.30 ± 3.61 | 12.26 ± 0.11 | 2.07 ± 0.09 | 23.3 ± 0.13 | 2.06 ± 0.05 | - | - | |
| AlCrTiSi0.2La0.02 | (b) | 58.23 ± 1.41 | 28.73 ± 0.14 | 5.35 ± 0.08 | 6.78 ± 0.06 | 0.82 ± 0.03 | 0.09 ± 0.03 | - |
| (e) | 59.56 ± 2.19 | 20.89 ± 0.13 | 4.26 ± 0.06 | 13.32 ± 0.11 | 1.93 ± 0.04 | 0.04 ± 0.04 | - | |
| AlCrTiSi0.2Y0.02 | (c) | 58.37 ± 2.69 | 21.73 ± 0.14 | 3.48 ± 0.09 | 14.87 ± 0.10 | 1.14 ± 0.04 | - | 0.41 ± 0.03 |
| (f) | 65.98 ± 0.98 | 3.15 ± 0.05 | 0.74 ± 0.07 | 29.43 ± 0.19 | 0.67 ± 0.03 | - | 0.03 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, L.; Meng, L.; Fang, S.; Lin, C.; Tan, M.; Qi, T. High-Temperature Oxidation Behaviors of AlCrTiSi0.2 High-Entropy Alloy Doped with Rare Earth La and Y. Crystals 2023, 13, 1169. https://doi.org/10.3390/cryst13081169
Ke L, Meng L, Fang S, Lin C, Tan M, Qi T. High-Temperature Oxidation Behaviors of AlCrTiSi0.2 High-Entropy Alloy Doped with Rare Earth La and Y. Crystals. 2023; 13(8):1169. https://doi.org/10.3390/cryst13081169
Chicago/Turabian StyleKe, Lingsheng, Long Meng, Sheng Fang, Chun Lin, Mingtian Tan, and Tao Qi. 2023. "High-Temperature Oxidation Behaviors of AlCrTiSi0.2 High-Entropy Alloy Doped with Rare Earth La and Y" Crystals 13, no. 8: 1169. https://doi.org/10.3390/cryst13081169






