X-ray Structures and Hirshfeld Studies of Two Dinuclear Cd(II) Complexes with a s-Triazine/Pyrazolo Ligand and Pesudohalides as a Linker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Syntheses
Synthesis of the Cd(II) Complexes[Cd(BPMST)(SCN)]2 (1) and [Cd(BPMST)(N3)Cl]2 (2)
2.2. Crystal Structure Determination
2.3. Hirshfeld Surface Analysis
3. Results and Discussion
3.1. Synthesis and Characterizations
3.2. Crystal Structure Description
3.3. Analysis of Molecular Packing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shibutani, M.; Mitsumori, K.; Niho, N.; Satoh, S.; Hiratsuka, H.; Satoh, M.; Sumiyoshi, M.; Nishijima, M.; Katsuki, Y.; Suzuki, J.; et al. Assessment of renal toxicity by analysis of regeneration of tubular epithelium in rats given low-dose cadmium chloride or cadmium-polluted rice for 22 months. Arch. Toxicol. 2000, 74, 571–577. [Google Scholar] [CrossRef]
- Chen, L.; Lei, L.; Jin, T.; Nordberg, M.; Nordberg, G.F. Plasma Metallothionein Antibody, Urinary Cadmium, and Renal Dysfunction in a Chinese Type 2 Diabetic Population. Diabetes Care 2006, 29, 2682–2687. [Google Scholar] [CrossRef] [Green Version]
- Antonio, M.T.; Corredor, L.; Leret, M.L. Study of the activity of several brain enzymes like markers of the neurotoxicity induced by perinatal exposure to lead and/or cadmium. Toxicol. Lett. 2003, 143, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Rikans, L.E.; Yamano, T. Mechanisms of cadmium-mediated acute hepatotoxicity. J. Biochem. Mol. Toxicol. 2000, 14, 110–117. [Google Scholar] [CrossRef]
- Lane, T.W.; Morel, F.M.M. A biological function for cadmium in marine diatoms. Proc. Natl. Acad. Sci. USA 2000, 97, 4627–4631. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Bi, C.F.; Fan, Y.H.; Yan, X.C.; Zhang, X.; Zhang, P.F.; Huang, G.M. Synthesis, crystal structures, luminescent properties, theoretical calculation, and DNA interaction of the cadmium (II) and lead (II) complexes with o-aminobenzoic acid and 1, 10-phenanthroline. Russ. J. Coord. Chem. 2015, 41, 274–284. [Google Scholar] [CrossRef]
- Jana, S.K.; Mandal, A.K.; Seth, S.K.; Puschmann, H.; Hossain, M.; Dalai, S. Synthesis, Characterization and Crystal Structure of a New 3D Cadmium(II) Coordination Polymer: Binding Interaction with DNA and Double Stranded RNA. J. Inorg. Organomet. Polym. 2016, 26, 806–818. [Google Scholar] [CrossRef]
- Zianna, A.; Ristović, M.Š.; Psomas, G.; Hatzidimitriou, A.; Coutouli-Argyropoulou, E.; Lalia-Kantouri, M. Cadmium(II) complexes of 5-bromo-salicylaldehyde and α-diimines: Synthesis, structure and interaction with calf-thymus DNA and albumins. Polyhedron 2016, 107, 136–147. [Google Scholar] [CrossRef]
- Wazeer, M.I.M.; Isab, A.A.; Fettouhi, M. New cadmium chloride complexes with imidazolidine-2-thione and its derivatives: X-ray structures, solid state and solution NMR and antimicrobial activity studies. Polyhedron 2007, 26, 1725–1730. [Google Scholar] [CrossRef]
- Ruíz, M.; Perelló, L.; Server-Carrió, J.; Ortiz, R.; García-Granda, S.; Díaz, M.R.; Cantón, E. Cinoxacin complexes with divalent metal ions. Spectroscopic characterization. Crystal structure of a new dinuclear Cd(II) complex having two chelate-bridging carboxylate groups. Antibacterial studies. J. Inorg. Biochem. 1998, 69, 231–239. [Google Scholar] [CrossRef]
- Dubler, E.; Gyr, E. New metal complexes of the antitumor drug 6-mercaptopurine. Syntheses and X-ray structural characterizations of dichloro(6-mercaptopurinium)copper(I), dichlorotetrakis(6-mercaptopurine)cadmium(II), and bis(6-mercaptopurinato)cadmium(II) dehydrate. Inorg. Chem. 1988, 27, 1466–1473. [Google Scholar] [CrossRef]
- Perez, J.M.; Cerrillo, V.; Matesanz, A.I.; Millan, J.M.; Navaro, P.; Alonso, C.; Souza, P. DNA Interstrand Cross-Linking Efficiency and Cytotoxic Activity of Novel Cadmium(II)–Thiocarbodiazone Complexes. ChemBioChem 2001, 2, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Ignatius, I.C.; Rajathi, S.; Kirubavathi, K.; Selvaraju, K. Synthesis, crystal growth and characterization of novel semiorganic nonlinear optical crystal: Dichloro(beta-alanine)cadmium(II). Optik-Int. J. Light Electron. Opt. 2014, 125, 5144–5147. [Google Scholar] [CrossRef]
- Saghatforoush, L.; Khoshtarkib, Z.; Keypour, H.; Hakimi, M. Mononuclear, tetranuclear and polymeric cadmium(II) complexes with the 3,6-bis(2-pyridyl)-1,2,4,5-tetrazine ligand: Synthesis, crystal structure, spectroscopic and DFT studies. Polyhedron 2016, 119, 160–174. [Google Scholar] [CrossRef]
- Wang, W.; Qiao, J.; Wang, L.; Duan, L.; Zhang, D.; Yang, W.; Qiu, Y. Synthesis, Structures, and Optical Properties of Cadmium Iodide/Phenethylamine Hybrid Materials with Controlled Structures and Emissions. Inorg. Chem. 2007, 46, 10252–10260. [Google Scholar] [CrossRef]
- Biswas, F.B.; Roy, T.G.; Rahman, A.; Emran, T.B. An in vitro antibacterial and antifungal effects of cadmium (II) complexes of hexamethyltetraazacyclotetradecadiene and isomers of its saturated analogue. Asian. Pac. J. Trop. Med. 2014, 7, S534–S539. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.C.; Huang, M.X.; Qi, Y.; Gao, E.Q. Synthesis, structure, luminescence and catalytic properties of cadmium(II) coordination polymers with 9H-carbazole-2,7-dicarboxylic acid. Dalton Trans. 2014, 43, 3691–3697. [Google Scholar] [CrossRef]
- Jia, W.G.; Li, D.D.; Gu, C.; Dai, Y.C.; Zhou, Y.H.; Yuan, G.; Sheng, E.H. Two cadmium(II) complexes with oxazoline-based ligands as effective catalysts for C–N cross-coupling reactions. Inorg. Chim. Acta 2015, 427, 226–231. [Google Scholar] [CrossRef]
- Gong, Y.-Q.; Fan, J.; Wang, R.-H.; Zhou, Y.-F.; Yuan, D.-Q.; Hong, M.-C. Syntheses, crystal structures and photoluminescence of two Cd(II) coordination polymers derived from a flexible bipyridyl ligand. J. Mol. Struct. 2004, 705, 29–34. [Google Scholar] [CrossRef]
- Li, W.; Evans, O.-R.; Xiong, R.-G.; Wang, Z. Supramolecular Engineering of Chiral and Acentric 2D Networks. Synthesis, Structures, and Second-Order Nonlinear Optical Properties of Bis(nicotinato)zinc and Bis{3-[2-(4-pyridyl)ethenyl]benzoato}cadmium. J. Am. Chem. Soc. 1998, 120, 13272–13273. [Google Scholar]
- Lin, W.; Wang, Z.; Ma, L. A Novel Octupolar Metal−Organic NLO Material Based on a Chiral 2D Coordination Network. J. Am. Chem. Soc. 1999, 121, 11249–11250. [Google Scholar] [CrossRef]
- Chen, Y.-B.; Zhang, J.; Cheng, J.-K.; Kang, Y.; Li, Z.-J.; Yao, Y.-G. 1D chain structure, NLO and luminescence properties of [Zn2 (bpp) (pht)2]. Inorg. Chem. Commun. 2004, 7, 1139–1141. [Google Scholar] [CrossRef]
- Cooke, N.H.C.; Viavattene, R.L.; Eksteen, R.; Wong, W.S.; Davies, G.; Karger, B.L. Use of metal ions for selective separations in high-performance liquid chromatography. J. Chromatogr. 1978, 149, 391–415. [Google Scholar] [CrossRef]
- Le Page, J.N.; Lindner, W.; Davies, G.; Seitz, D.E.; Karger, B.L. Resolution of the optical isomers of dansyl amino acids by reversed phase liquid chromatography with optically active metal chelate additives. Anal. Chem. 1979, 51, 433–435. [Google Scholar] [CrossRef]
- Lindner, W. HPLC-Enantiomerentrennung an gebundenen chiralen Phasen. Naturwissenschaften 1980, 67, 354–356. [Google Scholar] [CrossRef]
- Hashemi, L.; Hosseinifard, M.; Amani, V.; Morsali, A.J. Sonochemical Synthesis of Two New Nano-structured Cadmium (II) Supramolecular Complexes. Inorg. Organomet. Polym. 2013, 23, 519–524. [Google Scholar] [CrossRef]
- Mlowe, S.; Lewis, D.J.; Malik, M.A.; Raftery, J.; Mubofu, E.B.; O’Brien, P.; Revaprasadu, N. Bis(piperidinedithiocarbamato)pyridinecadmium(II) as a single-source precursor for the synthesis of CdS nanoparticles and aerosol-assisted chemical vapour deposition (AACVD) of CdS thin films. New J. Chem. 2014, 38, 6073–6080. [Google Scholar] [CrossRef] [Green Version]
- Stang, P.J.; Olenyuk, B. Self-Assembly, Symmetry, and Molecular Architecture: Coordination as the Motif in the Rational Design of Supramolecular Metallacyclic Polygons and Polyhedra. Acc. Chem. Res. 1997, 30, 502–518. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Desiraju, G.R. Crystal Engineering and Organometallic Architecture. Chem. Rev. 1998, 98, 1375–1406. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H.; Davis, C.; Richardson, D.; Groy, T.L. Synthetic Strategies, Structure Patterns, and Emerging Properties in the Chemistry of Modular Porous Solids. Acc. Chem. Res. 1998, 31, 474–484. [Google Scholar] [CrossRef]
- Purdy, A.P.; Gilardi, R.; Luther, J.; Butcher, R.J. Synthesis, crystal structure, and reactivity of alkali and silver salts of sulfonated imidazoles. Polyhedron 2007, 26, 3930–3938. [Google Scholar] [CrossRef]
- Zhang, Z.-T.; Shi, J.; He, Y.; Guo, Y.-N. Self-assembly and crystal structure of a barium sulfonate chrysin coordination polymer. Inorg. Chem. Commun. 2006, 9, 579–581. [Google Scholar] [CrossRef]
- Yang, X.-L.; Ren, S.-B.; Zhang, J.; Li, Y.-Z.; Du, H.-B.; You, X.-Z. Syntheses and structures of three coordination polymers based on 4-methylbenzenethiolates of Zn(II) and Cd(II) and bipyridine. J. Coord. Chem. 2009, 62, 3782–3794. [Google Scholar] [CrossRef]
- Ghoshal, D.; Maji, T.K.; Mostafa, G.; Lu, T.H.; Chaudhuri, N.R. A Three-dimensional honeycomb-Like network constructed with novel “Sinusoidal” One-Dimensional Chains via Hydrogen Bonding and π−π Interactions. Cryst. Growth Des. 2003, 3, 9–11. [Google Scholar] [CrossRef]
- Rashidi-Ranjbar, Z.; Hamidi, S.; Heshmatpour, F.; Morsali, A. Thermal, spectroscopic, X-ray powder diffraction, and structural studies on a new Cd(II) mixed-ligand coordination polymer. J. Coord. Chem. 2009, 62, 2022–2027. [Google Scholar] [CrossRef]
- Smith, G.; Wermuth, U.D.; Young, D.J.; White, J.M. Polymeric structures in the metal complexes of 5-sulfosalicylic acid: The rubidium(I), caesium(I) and lead(II) analogues. Polyhedron 2007, 26, 3645–3652. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-J.; Ren, P.-D.; Zhang, Z.-B.; Fang, Y.; Wang, Y.-Y. Synthesis and characterization of a nickel-organic framework encapsulating hetero-chiral helical water chains in the 1-D channels. J. Coord. Chem. 2009, 62, 2814–2823. [Google Scholar] [CrossRef]
- De Silva, C.-R.; Maeyer, J.-R.; Dawson, A.; Zheng, Z. Adducts of lanthanide β-diketonates with 2,4,6-tri(2-pyridyl)-1,3,5-triazine: Synthesis, structural characterization, and photoluminescence studies. Polyhedron 2007, 26, 1229–1238. [Google Scholar] [CrossRef]
- Ionova, G.; Raber, C.; Guillaumont, R.; Ionov, S.; Madic, C.; Krupa, D.; Guillaneux, J.-C. A donor–acceptor model of Ln(III) complexation with terdentate nitrogen planar ligands. New J. Chem. 2002, 26, 234–242. [Google Scholar] [CrossRef]
- Yan, C.; Chen, Q.; Chen, L.; Feng, R.; Shan, X.; Jiang, F.; Hong, M. crystal structures and luminescence behaviour of d10 Metal–Organic Complexes with multipyridineligands. Aust. J. Chem. 2011, 64, 104–118. [Google Scholar] [CrossRef]
- Wu, G.; Wang, X.-F.; Guo, L.; Li, H.-H. Zn(II) and Cd(II) Complexes extended structures sustained by hydrogen bonding, π–π and C–H···π interactions. J. Chem. Crystallogr. 2011, 41, 1071–1076. [Google Scholar] [CrossRef]
- Glaser, T.; Lügger, T.; Fröhlich, R. Synthesis, crystal structures, and magnetic properties of a mono- and a dinuclearcopper(II) complex of the 2,4,6-tris(2-pyridyl)-1,3,5-triazine ligand. Eur. J. Inorg. Chem. 2004, 394–400. [Google Scholar] [CrossRef]
- Schwalbe, M.; Karnahl, M.; Görls, H.; Chartrand, D.; Laverdiere, F.; Hanan, G.-S.; Tschierlei, S.; Dietzek, B.; Schmitt, M.; Popp, J.; et al. Ruthenium polypyridine complexes of tris-(2-pyridyl)-1,3,5-triazine—Unusual building blocks for the synthesis of photochemical molecular devices. Dalton Trans. 2009, 4012–4022. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.M.; El-Faham, A. Synthesis, characterization, and structural studies of two heteroleptic Mn(II) complexes with tridentate N,N,N-pincer type ligand. J. Coord. Chem. 2018, 71, 2373–2388. [Google Scholar] [CrossRef]
- Soliman, S.M.; El-Faham, A. One pot synthesis of two Mn(II) perchlorate complexes with s-triazine NNN-pincer ligand; molecular structure, Hirshfeld analysis and DFT studies. J. Mol. Struct. 2018, 1164, 344–353. [Google Scholar] [CrossRef]
- Soliman, S.M.; El-Faham, A.; Elsilk, S.E.; Farooq, M. Two heptacoordinatedmanganese(II) complexes of giant pentadentate s-triazine bis-Schiff base ligand: Synthesis, crystal structure, biological and DFT studies. Inorg. Chim. Acta 2018, 479, 275–285. [Google Scholar] [CrossRef]
- Moulton, C.J.; Shaw, B.L. Transition metal–carbon bonds. Part XLII. Complexes of nickel, palladium, platinum, rhodium and iridium with the tridentate ligand 2,6-bis[(di-t-butylphosphino)methyl]phenyl. J. Chem. Soc. Dalton Trans. 1976, 1020–1040. [Google Scholar] [CrossRef]
- Van Koten, G. Tuning the reactivity of metals held in a rigid ligand environment. Pure Appl. Chem. 1989, 61, 1681–1694. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, M.; Van Koten, G. Platinum Group Organometallics Based on “Pincer” Complexes: Sensors, Switches, and Catalysts. Angew. Chem. Int. Ed. Engl. 2001, 40, 3750–3781. [Google Scholar] [CrossRef]
- Asay, M.; Morales-Morales, D. Non-symmetric pincer ligands: Complexes and applications in catalysis. J. Chem. Soc. Dalton Trans. 2015, 44, 17432–17447. [Google Scholar] [CrossRef]
- Li, H.; Zheng, B.; Huang, K.-W. A new class of PN3-pincer ligands for metal–ligand cooperative catalysis. Coord. Chem. Rev. 2015, 293, 116–138. [Google Scholar] [CrossRef] [Green Version]
- Szabó, K.J. Mechanism of the oxidative addition of hypervalent iodonium salts to palladium(II) pincer-complexes. J. Mol. Catal. A Chem. 2010, 324, 56–63. [Google Scholar] [CrossRef]
- De Hoog, P.; Gamez, P.; Driessen, L.W.; Reedijk, J. New polydentate and polynucleating N-donor ligands from amines and 2,4,6-trichloro-1,3,5-triazine. Tetrahedron Lett. 2002, 43, 6783–6786. [Google Scholar] [CrossRef]
- Das, A.; Demeshko, S.; Dechert, S.; Meyer, F. A New Triazine-Based Tricompartmental Ligand for Stepwise Assembly of Mononuclear, Dinuclear, and 1D-Polymeric Heptacoordinate Manganese(II)/Azido Complexes. Eur. J. Inorg. Chem. 2011, 2011, 1240–1248. [Google Scholar] [CrossRef]
- Medlycott, E.A.; Udachin, K.A.; Hanan, G.S. Non-covalent polymerisation in the solid state: Halogen–halogen vs. methyl–methyl interactions in the complexes of 2,4-di(2-pyridyl)-1,3,5-triazine ligands. Dalton Trans. 2007, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Mooibroek, T.J.; Gamez, P. The s-triazine ring, a remarkable unit to generate supramolecular interactions. Inorg. Chim. Acta 2007, 360, 381–404. [Google Scholar] [CrossRef]
- Gamez, P.; Reedijk, J. 1,3,5-Triazine-Based Synthons in Supramolecular Chemistry. Eur. J. Inorg. Chem. 2006, 2006, 29–42. [Google Scholar] [CrossRef]
- Ranganathan, A.; Heisen, B.C.; Dix, I.; Meyer, F. A triazine-based three-directional rigid-rod tecton forms a novel 1D channel structure. Chem. Commun. 2007, 3637–3639. [Google Scholar] [CrossRef]
- Galan-Mascaros, J.R.; Clemente-Juan, J.M.; Dunbar, K.R. Synthesis, structure and magnetic properties of the one-dimensional chain compound {K[Fe(1,3,5-triazine-2,4,6-tricarboxylate)(H2O)2]·2H2O}∞. J. Chem. Soc. Dalton Trans. 2002, 2710–2713. [Google Scholar] [CrossRef]
- Wietzke, R.; Mazzanti, M.; Latour, J.M.; Percaut, J. Crystal Structure and Solution Fluxionality of Lanthanide Complexes of 2,4,6,-Tris-2-pyridyl-1,3,5-triazine. Inorg. Chem. 1999, 38, 3581–3585. [Google Scholar] [CrossRef]
- Ramirez, J.; Stadler, A.-M.; Kyritskas, N.; Lehn, J.-M. Solvent-modulated reversible conversion of a [2 × 2]-grid into a pincer-like complex. Chem. Commun. 2007, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Stadler, A.M.; Harrowfield, J.M.; Brelot, L.; Huuskonen, J.; Rissanen, K.; Allouche, L.; Lehn, J.M. Coordination Architectures of Large Heavy Metal Cations (Hg2+ and Pb2+) with Bis-tridentate Ligands: Solution and Solid-State Studies. Z. Anorg. Allg. Chem. 2007, 633, 2435–2444. [Google Scholar] [CrossRef]
- Ramirez, J.; Stadler, A.-M.; Brelot, L.; Lehn, J.-M. Coordinative, conformational and motional behaviour of triazine-based ligand strands on binding of Pb(II) cations. Tetrahedron 2008, 64, 8402–8410. [Google Scholar] [CrossRef]
- Hsu, G.-Y.; Misra, P.; Cheng, S.-C.; Wei, H.-H.; Mohanta, S. Syntheses, structures, and magnetic properties of dicyanamide bridged one-dimensional double chain and discrete dinuclear complexes of manganese(II) derived from 6,7-dimethyl-2,3-di(2-pyridyl)quinoxaline or 2,4,6-tri(2-pyridyl)-1,3,5-triazine. Polyhedron 2006, 25, 3393–3398. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, R.; Zhao, Q. Synthesis and Crystal Structure of [Mn(H2O)(tptz)(CH3COO)][N(CN)2] · 2H2O (tptz = 2,4,6-tris-(2-pyridyl)-1,3,5-triazine). J. Chem. Crystallogr. 2008, 38, 601–604. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, U.P. Chloro and azido bonded manganese complexes: Synthesis, structural, and magnetic studies. J. Coord. Chem. 2009, 62, 1613–1622. [Google Scholar] [CrossRef]
- Soliman, S.M.; Almarhoon, Z.; El-Faham, A. Synthesis, Molecular and Supramolecular Structures of New Cd(II) Pincer-Type Complexes with s-Triazine Core Ligand. Crystals 2019, 9, 226. [Google Scholar] [CrossRef] [Green Version]
- Soliman, S.M.; Elsilk, S.E.; El-Faham, A. Syntheses, structure, Hirshfeld analysis and antimicrobial activity of four new Co(II) complexes with s-triazine-based pincer ligand. Inorg. Chim. Acta 2020, 510, 119753. [Google Scholar] [CrossRef]
- Soliman, S.M.; El-Faham, A. Synthesis, molecular structure and DFT studies of two heteroleptic nickel(II) s-triazine pincer type complexes. J. Mol. Struct. 2019, 1185, 461–468. [Google Scholar] [CrossRef]
- Soliman, S.M.; Elsilk, S.E.; El-Faham, A. Synthesis, structure and biological activity of zinc(II) pincer complexes with 2,4-bis(3,5-dimethyl-1H-pyrazol-1-yl)-6-methoxy-1,3,5-triazine. Inorg. Chim. Acta 2020, 508, 119627. [Google Scholar] [CrossRef]
- Soliman, S.M.; El-Faham, A. Synthesis, X-ray structure, and DFT studies of five- and eight-coordinated Cd(II) complexes with s-triazine N-pincer chelate. J. Coord. Chem. 2019, 73, 1621–1636. [Google Scholar] [CrossRef]
- Barakat, A.; El-Faham, A.; Haukka, M.; Al-Majid, A.M.; Soliman, S.M. s-Triazine Pincer Ligands: Synthesis of their Metal Complexes, Coordination Behavior, and Applications. App. Organomet. Chem. 2021, 35, e6317. [Google Scholar] [CrossRef]
- Soliman, S.M.; Almarhoon, Z.; Sholkamy, E.N.; El-Faham, A. Bis-pyrazolyl-s-triazine Ni(II) pincer complexes as selective gram positive antibacterial agents; synthesis, structural and antimicrobial studies. J. Mol. Struct. 2019, 1195, 315–322. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Y.H.; Bai, F.Y.; Wang, X.Y.; Guan, Q.L.; Hou, Y.N.; Zhang, R.; Shi, Z. Synthesis, structure, and surface photovoltage properties of a series of novel d7–d10 metal complexes with pincer N-heterocycle ligands. RSC Adv. 2013, 3, 16021. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Jelsch, C.; Ejsmont, K.; Hudera, L. The enrichment ratio of atomic contacts in crystals, an indicator derived from the Hirshfeld surface analysis. IUCrJ 2014, 1, 119–128. [Google Scholar] [CrossRef]
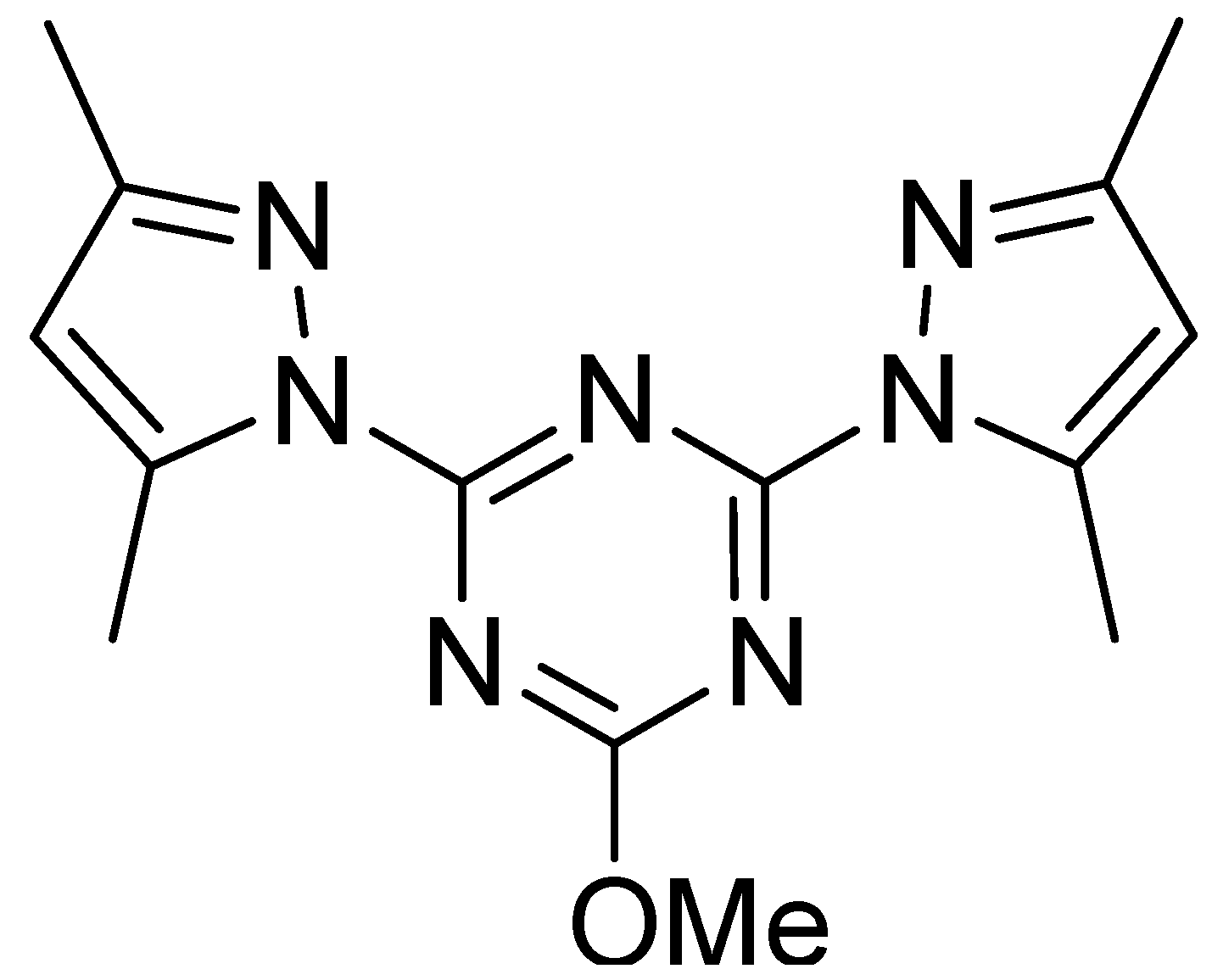








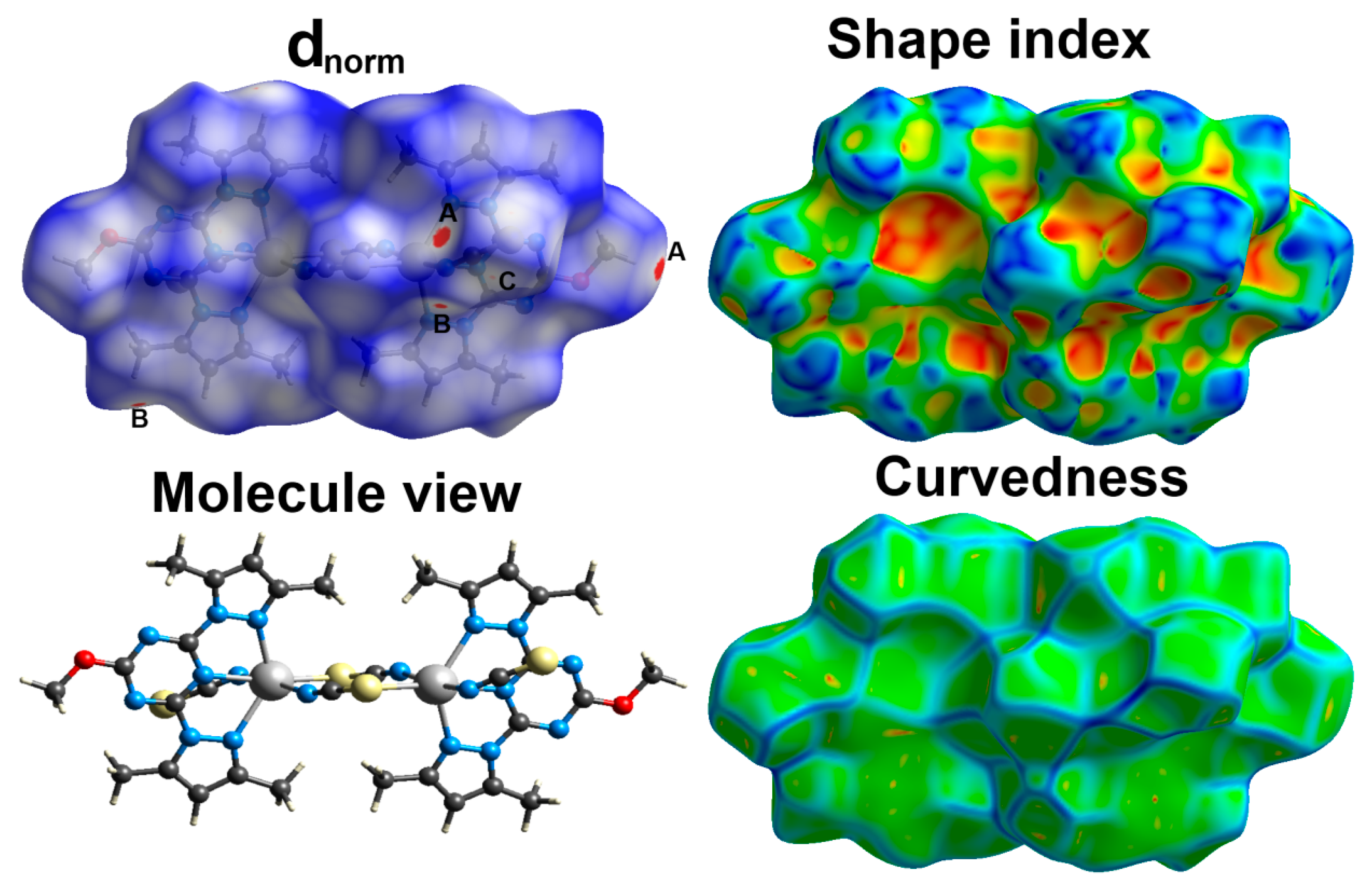





| Bond | Length/Å | Bond | Length/Å |
|---|---|---|---|
| Bond distances | |||
| Cd1-N9 # | 2.258(3) | Cd1-N4 | 2.402(3) |
| Cd1-N8 | 2.287(3) | Cd1-N6 | 2.454(3) |
| Cd1-N1 | 2.348(3) | Cd1-S1 | 2.6170(11) |
| Bond angles | |||
| N9 #-Cd1-N8 | 171.72(12) | N1-Cd1-N6 | 66.06(9) |
| N9 #-Cd1-N1 | 99.30(10) | N4-Cd1-N6 | 131.93(10) |
| N8-Cd1-N1 | 84.99(11) | N9 #-Cd1-S1 | 92.07(8) |
| N9 #-Cd1-N4 | 87.14(11) | N8-Cd1-S1 | 84.40(9) |
| N8-Cd1-N4 | 101.09(12) | N1-Cd1-S1 | 167.45(7) |
| N1-Cd1-N4 | 66.63(10) | N4-Cd1-S1 | 108.99(8) |
| N9 #-Cd1-N6 | 92.31(11) | N6-Cd1-S1 | 119.06(7) |
| N8-Cd1-N6 | 82.93(12) | ||
| Bond | Length/Å | Bond | Length/Å |
|---|---|---|---|
| Bond distances | |||
| Cd1-Cl1 | 2.4700(13) | Cd1-N8 # | 2.322(3) |
| Cd1-N4 | 2.409(2) | Cd1-N1 | 2.411(2) |
| Cd1-N8 | 2.415(3) | Cd1-N6 | 2.451(2) |
| Bond angles | |||
| N8 #-Cd1-N4 | 109.11(10) | N8 #-Cd1-N1 | 154.32(9) |
| N4-Cd1-N1 | 65.64(7) | N8-Cd1-N8 # | 73.49(12) |
| N4-Cd1-N8 | 90.55(10) | N1-Cd1-N8 | 81.31(8) |
| N8 #-Cd1-N6 | 116.69(10) | N4-Cd1-N6 | 130.14(7) |
| N1-Cd1-N6 | 64.57(7) | N8-Cd1-N6 | 84.58(10) |
| N8 #-Cd1-Cl1 | 95.60(8) | N4-Cd1-Cl1 | 95.94(6) |
| N1-Cd1-Cl1 | 109.80(6) | N8-Cd1-Cl1 | 168.70(7) |
| N6-Cd1-Cl1 | 98.15(7) | ||
| Enrichment Ratio (EXY) | |||
|---|---|---|---|
| [Cd(BPMST)(SCN)]2 (1) | [Cd(BPMST)(N3)Cl]2 (2) | ||
| O…H | 1.4 | O…H | 1.4 |
| H…H | 1.0 | H…H | 0.8 |
| N…H | 1.1 | N…H | 1.3 |
| C…H | 1.0 | C…H | 1.0 |
| S…S | 0.3 | N…O | 0.1 |
| N…S | 1.6 | N…N | 0.5 |
| C…N | 1.3 | C…N | 0.8 |
| C…S | 1.2 | Cl…H | 1.5 |
| C…C | 0.7 | ||
| S…H | 1.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, S.M.; Al-Rasheed, H.H.; AL-khamis, S.A.; Haukka, M.; El-Faham, A. X-ray Structures and Hirshfeld Studies of Two Dinuclear Cd(II) Complexes with a s-Triazine/Pyrazolo Ligand and Pesudohalides as a Linker. Crystals 2023, 13, 1198. https://doi.org/10.3390/cryst13081198
Soliman SM, Al-Rasheed HH, AL-khamis SA, Haukka M, El-Faham A. X-ray Structures and Hirshfeld Studies of Two Dinuclear Cd(II) Complexes with a s-Triazine/Pyrazolo Ligand and Pesudohalides as a Linker. Crystals. 2023; 13(8):1198. https://doi.org/10.3390/cryst13081198
Chicago/Turabian StyleSoliman, Saied M., Hessa H. Al-Rasheed, Sarah A. AL-khamis, Matti Haukka, and Ayman El-Faham. 2023. "X-ray Structures and Hirshfeld Studies of Two Dinuclear Cd(II) Complexes with a s-Triazine/Pyrazolo Ligand and Pesudohalides as a Linker" Crystals 13, no. 8: 1198. https://doi.org/10.3390/cryst13081198






