Electronic and Optical Properties of Finite Gallium Sulfide Nano Ribbons: A First-Principles Study
Abstract
:1. Introduction
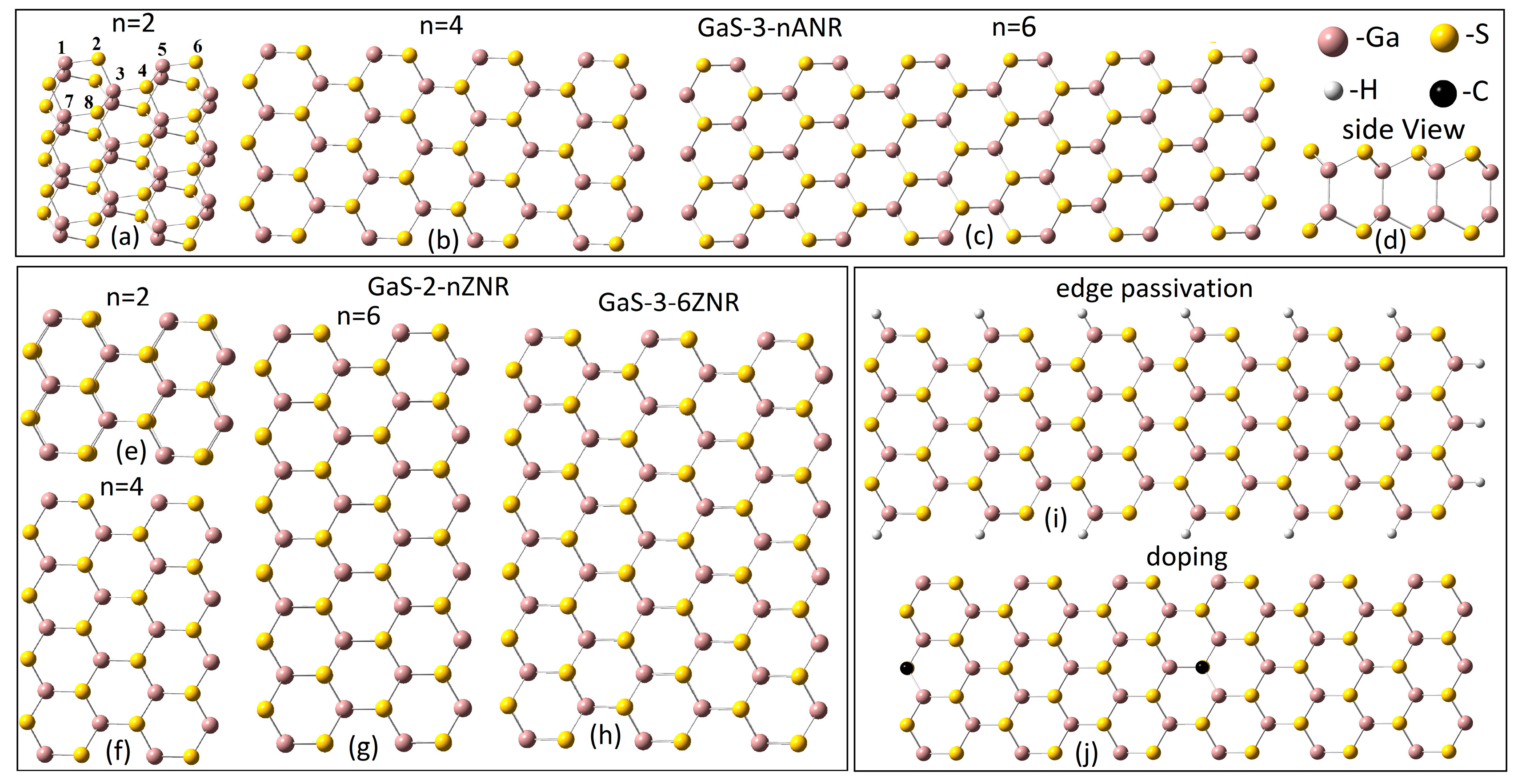
2. Computational Model
3. Results and Discussion
3.1. Structure Stability
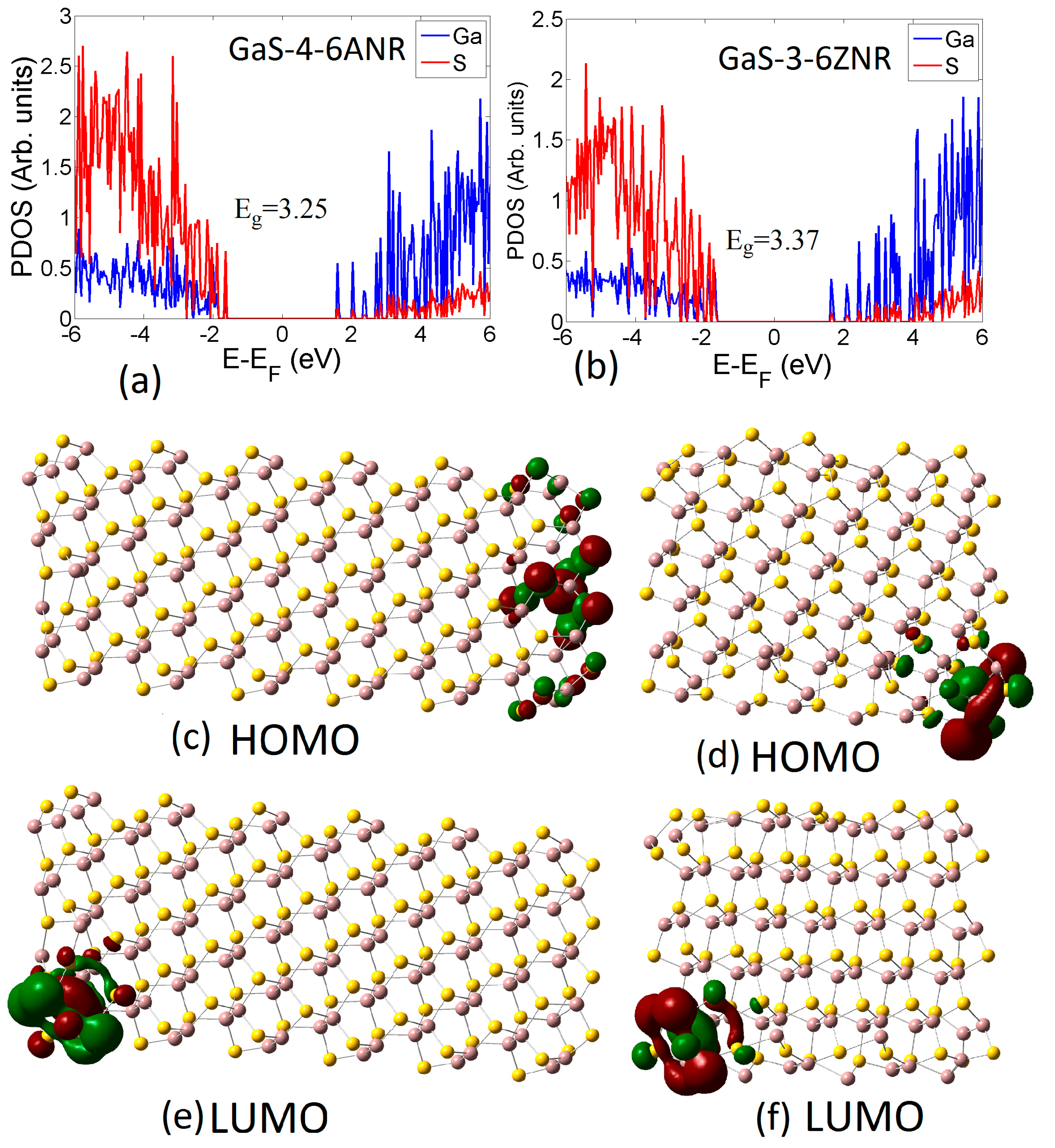
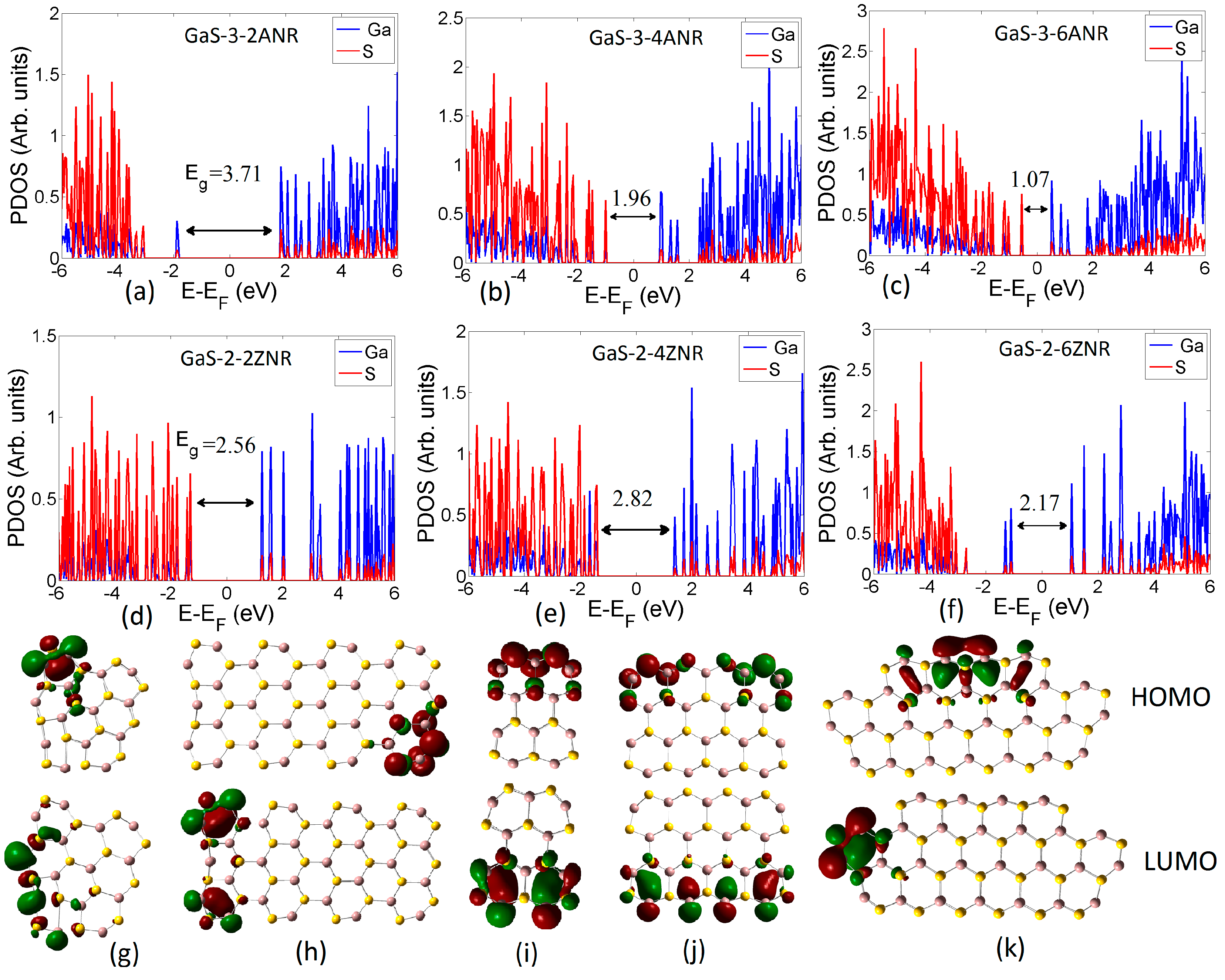
3.2. Electronic Properties
3.2.1. Pristine Nanoribbons
3.2.2. Chemical Modification
3.2.3. Quantum Stability
3.3. Characterization of Excited States
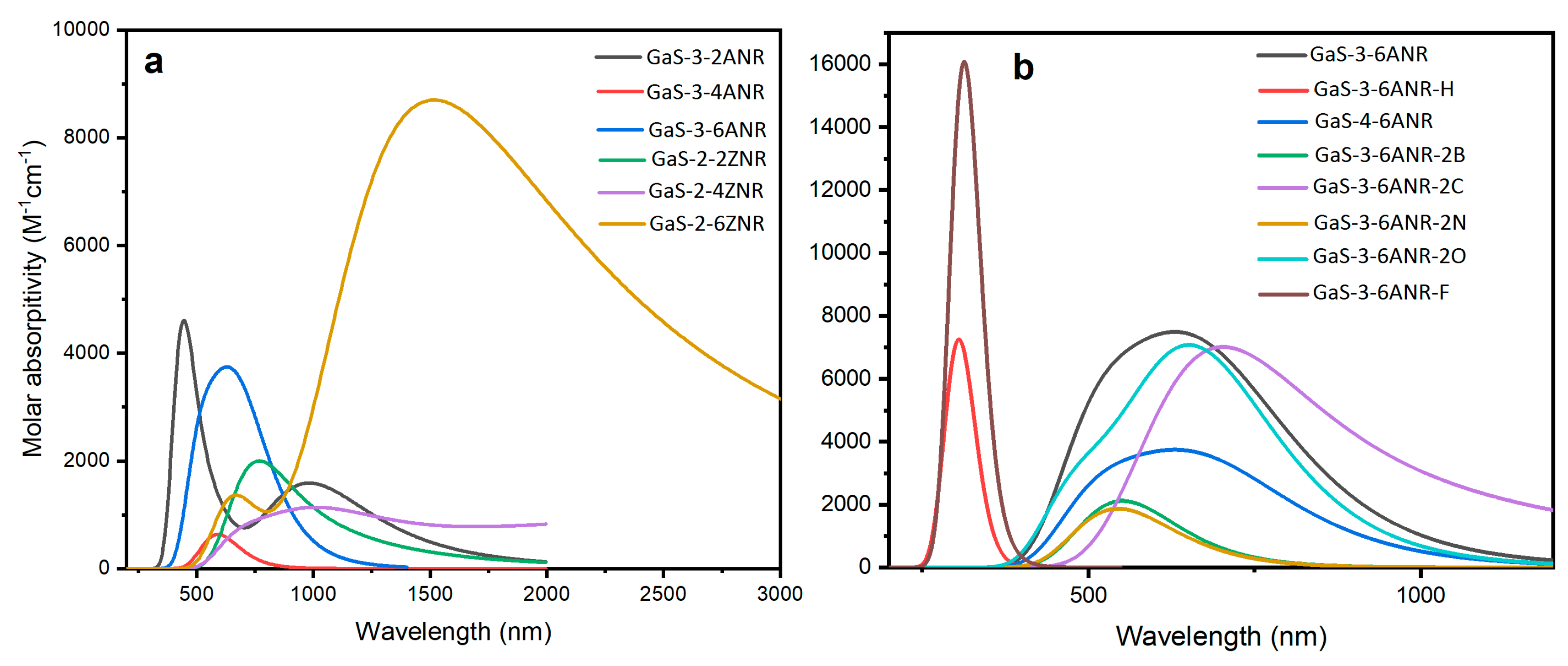
3.4. Optical Properties
4. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
Appendix C
| Compounds | EH (eV) | EL (eV) | IP (eV) | EF (eV) | Eg (eV) | Eg0 (eV) |
|---|---|---|---|---|---|---|
| GaS-3-6ANR | −6.31 | −5.23 | 5.93 | 5.78 | 1.07 | 0.15 |
| GaS-3-6ANR-2C | −6.62 | −5.19 | 6.14 | 5.45 | 1.43 | 0.69 |
| GaS-3-6ANR-2B | −7.67 | −4.89 | 7.18 | 5.18 | 2.78 | 2.04 |
| GaS-3-6ANR-H | −7.70 | −2.72 | 7.49 | 3.16 | 4.98 | 4.34 |
Appendix D
| Nanoribbon | Molecular Formula | Nanoribbon | Molecular Formula |
|---|---|---|---|
| GaS-3-2ANR | Ga28S28 | GaS-3-6ANR-F | Ga84S84F20 |
| GaS-3-4ANR | Ga56S56 | GaS-3-6ANR-2C | Ga84S82C2 |
| GaS-3-6ANR | Ga84S84 | GaS-3-6ANR-2N | Ga84S82N2 |
| GaS-2-2ZNR | Ga20S20 | GaS-3-6ANR-2O | Ga84S82O2 |
| GaS-2-4ZNR | Ga36S36 | GaS-3-6ANR-2B | Ga84S82B2 |
| GaS-2-6ZNR | Ga52S52 | GaS-4-6ANR | Ga108S108 |
| GaS-3-6ANR-H | Ga84S84H20 | GaS-3-6ZNR | Ga78S78 |
References
- Fiori, G.; Bonaccorso, F.; Iannaccone, G.; Palacios, T.; Neumaier, D.; Seabaugh, A.; Banerjee, S.K.; Colombo, L. Electronics based on two-dimensional materials. Nat. Nanotechnol. 2014, 9, 768–779. [Google Scholar] [CrossRef]
- Butler, S.Z.; Hollen, S.M.; Cao, L.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.; Ismach, A.F. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef]
- Akinwande, D.; Brennan, C.J.; Bunch, J.S.; Egberts, P.; Felts, J.R.; Gao, H.; Huang, R.; Kim, J.-S.; Li, T.; Li, Y. A review on mechanics and mechanical properties of 2D materials—Graphene and beyond. Extrem. Mech. Lett. 2017, 13, 42–77. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Chen, H.; Wang, S.; Liu, Q.; Jiang, Y.-G.; Zhang, D.W.; Liu, M.; Zhou, P. Two-dimensional materials for next-generation computing technologies. Nat. Nanotechnol. 2020, 15, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Cui, X.; Guan, B.; Wang, S.; Li, R.; Liu, Y.; Zhu, D.; Zheng, J. Synthesis of a monolayer fullerene network. Nature 2022, 606, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, D.; Kim, J.; Capasso, A.; Kang, H.S.; Park, J.-W.; Lee, C.-H.; Lee, G.-H. 2D semiconducting materials for electronic and optoelectronic applications: Potential and challenge. 2D Mater. 2020, 7, 022003. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Q.; Xing, J.; Liu, N.; Guo, Y.; Liu, Z.; Zhao, J. Recent progress on 2D magnets: Fundamental mechanism, structural design and modification. Appl. Phys. Rev. 2021, 8, 031305. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar]
- Vogt, P.; De Padova, P.; Quaresima, C.; Avila, J.; Frantzeskakis, E.; Asensio, M.C.; Resta, A.; Ealet, B.; Le Lay, G. Silicene: Compelling experimental evidence for graphenelike two-dimensional silicon. Phys. Rev. Lett. 2012, 108, 155501. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Naguib, M.; Barsoum, M.W.; Gogotsi, Y. Ten years of progress in the synthesis and development of MXenes. Adv. Mater. 2021, 33, 2103393. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Yoon, H.W.; Yoon, S.-M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S. Selective gas transport through few-layered graphene and graphene oxide membranes. Science 2013, 342, 91–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Yang, J.; Xu, R.; Wang, F.; Li, W.; Ghufran, M.; Zhang, Y.-W.; Yu, Z.; Zhang, G.; Qin, Q. Extraordinary photoluminescence and strong temperature/angle-dependent Raman responses in few-layer phosphorene. ACS Nano 2014, 8, 9590–9596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibertini, M.; Koperski, M.; Morpurgo, A.F.; Novoselov, K.S. Magnetic 2D materials and heterostructures. Nat. Nanotechnol. 2019, 14, 408–419. [Google Scholar] [CrossRef] [Green Version]
- Pham, P.V.; Bodepudi, S.C.; Shehzad, K.; Liu, Y.; Xu, Y.; Yu, B.; Duan, X. 2D heterostructures for ubiquitous electronics and optoelectronics: Principles, opportunities, and challenges. Chem. Rev. 2022, 122, 6514–6613. [Google Scholar] [CrossRef]
- Britnell, L.; Gorbachev, R.; Jalil, R.; Belle, B.; Schedin, F.; Mishchenko, A.; Georgiou, T.; Katsnelson, M.; Eaves, L.; Morozov, S. Field-effect tunneling transistor based on vertical graphene heterostructures. Science 2012, 335, 947–950. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Tang, Z.; Liu, C.; Zhou, P. Electronics based on two-dimensional materials: Status and outlook. Nano Res. 2021, 14, 1752–1767. [Google Scholar] [CrossRef]
- Choi, M.S.; Qu, D.; Lee, D.; Liu, X.; Watanabe, K.; Taniguchi, T.; Yoo, W.J. Lateral MoS2 p–n junction formed by chemical doping for use in high-performance optoelectronics. ACS Nano 2014, 8, 9332–9340. [Google Scholar] [CrossRef]
- Liu, Z.; Alshareef, H.N. MXenes for optoelectronic devices. Adv. Electron. Mater. 2021, 7, 2100295. [Google Scholar] [CrossRef]
- Ahn, E.C. 2D materials for spintronic devices. Npj 2D Mater. Appl. 2020, 4, 17. [Google Scholar] [CrossRef]
- Han, W.; Kawakami, R.K.; Gmitra, M.; Fabian, J. Graphene spintronics. Nat. Nanotechnol. 2014, 9, 794–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannaccone, G.; Bonaccorso, F.; Colombo, L.; Fiori, G. Quantum engineering of transistors based on 2D materials heterostructures. Nat. Nanotechnol. 2018, 13, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; He, J.; Yuan, M.; Lin, L.; Zhang, Z.; Kang, Z.; Liao, Q.; Li, H.; Sun, G.; Yang, X. Li+-clipping for edge S-vacancy MoS2 quantum dots as an efficient bifunctional electrocatalyst enabling discharge growth of amorphous Li2O2 film. Nano Energy 2019, 65, 103996. [Google Scholar] [CrossRef]
- Zhan, B.; Li, C.; Yang, J.; Jenkins, G.; Huang, W.; Dong, X. Graphene field-effect transistor and its application for electronic sensing. Small 2014, 10, 4042–4065. [Google Scholar] [CrossRef] [PubMed]
- Anichini, C.; Czepa, W.; Pakulski, D.; Aliprandi, A.; Ciesielski, A.; Samorì, P. Chemical sensing with 2D materials. Chem. Soc. Rev. 2018, 47, 4860–4908. [Google Scholar] [CrossRef] [Green Version]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef]
- Gao, G.; O’Mullane, A.P.; Du, A. 2D MXenes: A new family of promising catalysts for the hydrogen evolution reaction. Acs Catal. 2017, 7, 494–500. [Google Scholar] [CrossRef] [Green Version]
- Pomerantseva, E.; Gogotsi, Y. Two-dimensional heterostructures for energy storage. Nat. Energy 2017, 2, 17089. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Qiao, Q.; Yu, Y.; Peterson, D.; Zafar, A.; Kumar, R.; Curtarolo, S.; Hunte, F.; Shannon, S. All the catalytic active sites of MoS2 for hydrogen evolution. J. Am. Chem. Soc. 2016, 138, 16632–16638. [Google Scholar] [CrossRef]
- Suragtkhuu, S.; Sunderiya, S.; Myagmarsereejid, P.; Purevdorj, S.; Bati, A.S.; Bold, B.; Zhong, Y.L.; Davaasambuu, S.; Batmunkh, M. Graphene-Like Monoelemental 2D Materials for Perovskite Solar Cells. Adv. Energy Mater. 2023, 13, 2204074. [Google Scholar] [CrossRef]
- Shao, M.; Bie, T.; Yang, L.; Gao, Y.; Jin, X.; He, F.; Zheng, N.; Yu, Y.; Zhang, X. Over 21% efficiency stable 2D perovskite solar cells. Adv. Mater. 2022, 34, 2107211. [Google Scholar] [CrossRef]
- Harvey, A.; Backes, C.; Gholamvand, Z.; Hanlon, D.; McAteer, D.; Nerl, H.C.; McGuire, E.; Seral-Ascaso, A.; Ramasse, Q.M.; McEvoy, N. Preparation of gallium sulfide nanosheets by liquid exfoliation and their application as hydrogen evolution catalysts. Chem. Mater. 2015, 27, 3483–3493. [Google Scholar] [CrossRef]
- Zhang, C.; Park, S.H.; Ronan, O.; Harvey, A.; Seral-Ascaso, A.; Lin, Z.; McEvoy, N.; Boland, C.S.; Berner, N.C.; Duesberg, G.S. Enabling flexible heterostructures for Li-ion battery anodes based on nanotube and liquid-phase exfoliated 2D gallium chalcogenide nanosheet colloidal solutions. Small 2017, 13, 1701677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.; Cheng, P.K.; Qiao, J.; Gao, W.; Saleque, A.M.; Al Subri Ivan, M.N.; Wang, T.; Alam, T.I.; Hani, S.U.; Guo, Z.L. Nonlinear Optical Activities in Two-Dimensional Gallium Sulfide: A Comprehensive Study. ACS Nano 2022, 16, 12390–12402. [Google Scholar] [CrossRef] [PubMed]
- Zappia, M.I.; Bianca, G.; Bellani, S.; Curreli, N.; Sofer, Z.k.; Serri, M.; Najafi, L.; Piccinni, M.; Oropesa-Nuñez, R.; Marvan, P. Two-dimensional gallium sulfide nanoflakes for UV-selective photoelectrochemical-type photodetectors. J. Phys. Chem. C 2021, 125, 11857–11866. [Google Scholar] [CrossRef]
- Opoku, F.; Akoto, O.; Asare-Donkor, N.K.; Adimado, A.A. Defect-engineered two-dimensional layered gallium sulphide molecular gas sensors with ultrahigh selectivity and sensitivity. Appl. Surf. Sci. 2021, 562, 150188. [Google Scholar] [CrossRef]
- Ho, C.; Lin, S. Optical properties of the interband transitions of layered gallium sulfide. J. Appl. Phys. 2006, 100, 083508. [Google Scholar] [CrossRef]
- Lu, Y.; Warner, J.H. Synthesis and applications of wide bandgap 2D layered semiconductors reaching the green and blue wavelengths. ACS Appl. Electron. Mater. 2020, 2, 1777–1814. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Huang, L.; Li, J. Influential electronic and magnetic properties of the gallium sulfide monolayer by substitutional doping. J. Phys. Chem. C 2015, 119, 29148–29156. [Google Scholar] [CrossRef]
- Yagmurcukardes, M.; Senger, R.T.; Peeters, F.M.; Sahin, H. Mechanical properties of monolayer GaS and GaSe crystals. Phys. Rev. B 2016, 94, 245407. [Google Scholar] [CrossRef] [Green Version]
- Rai, J.; Gautam, S. Computational quantum chemical analysis of structural and electronic properties of functionalized gallium sulfide (GaS) nanoflakes. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Stolbov, S. Local defects in two-dimensional gallium sulfide as potential single-photon emitters: First-principles evaluation. Phys. Rev. B 2022, 106, 245205. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Talaat, M.H.; Lukyanchuk, I.; Portnoi, M.; Saroka, V. Electro-absorption of silicene and bilayer graphene quantum dots. J. Appl. Phys. 2016, 120, 014304. [Google Scholar] [CrossRef] [Green Version]
- Saroka, V.; Lukyanchuk, I.; Portnoi, M.; Abdelsalam, H. Electro-optical properties of phosphorene quantum dots. Phys. Rev. B 2017, 96, 085436. [Google Scholar] [CrossRef] [Green Version]
- Jing, F.M.; Zhang, Z.Z.; Qin, G.Q.; Luo, G.; Cao, G.; Li, H.O.; Song, X.X.; Guo, G.P. Gate-Controlled Quantum Dots Based on 2D Materials. Adv. Quantum Technol. 2022, 5, 2100162. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Zhang, Q.F. Properties and applications of quantum dots derived from two-dimensional materials. Adv. Phys. X 2022, 7, 2048966. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Li, X.; Teng, K.S.; Lau, S.P. Size-dependent structural and optical characteristics of glucose-derived graphene quantum dots. Part. Part. Syst. Charact. 2013, 30, 523–531. [Google Scholar] [CrossRef]
- Zhang, Z.; Chang, K.; Peeters, F. Tuning of energy levels and optical properties of graphene quantum dots. Phys. Rev. B 2008, 77, 235411. [Google Scholar] [CrossRef] [Green Version]
- Saroka, V.; Abdelsalam, H.; Demin, V.; Grassano, D.; Kuten, S.; Pushkarchuk, A.; Pulci, O. Absorption in finite-length chevron-type graphene nanoribbons. Semiconductors 2018, 52, 1890–1893. [Google Scholar] [CrossRef]
- Kundu, S.; Pillai, V.K. Synthesis and characterization of graphene quantum dots. Phys. Sci. Rev. 2019, 5, 20190013. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Saroka, V.A.; Atta, M.M.; Osman, W.; Zhang, Q. Tunable electro-optical properties of doped chiral graphene nanoribbons. Chem. Phys. 2021, 544, 111116. [Google Scholar] [CrossRef]
- Kundu, S.; Yadav, R.M.; Narayanan, T.; Shelke, M.V.; Vajtai, R.; Ajayan, P.M.; Pillai, V.K. Synthesis of N, F and S co-doped graphene quantum dots. Nanoscale 2015, 7, 11515–11519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, W.; Wang, L.; Zheng, Z.; Liu, F.; Yu, P.; Yang, G. Colossal Vacancy Effect of 2D CuInP2S6 Quantum Dots for Enhanced Broadband Photodetection. Cryst. Growth Des. 2023, 23, 1259–1268. [Google Scholar] [CrossRef]
- Ali, S.R.; De, M. Defect-Engineered Functionalized MoS2 Quantum Dots with Enhanced Antibacterial Activity. ACS Appl. Nano Mater. 2023, 6, 2193–2202. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Elhaes, H.; Ibrahim, M.A. Tuning electronic properties in graphene quantum dots by chemical functionalization: Density functional theory calculations. Chem. Phys. Lett. 2018, 695, 138–148. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Saroka, V.A.; Ali, M.; Teleb, N.H.; Elhaes, H.; Ibrahim, M.A. Stability and electronic properties of edge functionalized silicene quantum dots: A first principles study. Phys. E Low-Dimens. Syst. Nanostructures 2019, 108, 339–346. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H.; Wang, Z.; Li, J. Black phosphorus quantum dots: Synthesis, properties, functionalized modification and applications. Chem. Soc. Rev. 2018, 47, 6795–6823. [Google Scholar] [CrossRef]
- Musselman, K.P.; Ibrahim, K.H.; Yavuz, M. Research Update: Beyond graphene—Synthesis of functionalized quantum dots of 2D materials and their applications. APL Mater. 2018, 6, 120701. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Younis, W.; Saroka, V.; Teleb, N.; Yunoki, S.; Zhang, Q. Interaction of hydrated metals with chemically modified hexagonal boron nitride quantum dots: Wastewater treatment and water splitting. Phys. Chem. Chem. Phys. 2020, 22, 2566–2579. [Google Scholar] [CrossRef]
- Liu, B.; Yan, S.; Song, Z.; Liu, M.; Ji, X.; Yang, W.; Liu, J. One-step synthesis of boron nitride quantum dots: Simple chemistry meets delicate nanotechnology. Chem.–Eur. J. 2016, 22, 18899–18907. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Jiang, W.; Via, B.K. Facile synthesis of soluble graphene quantum dots and its improved property in detecting heavy metal ions. Colloids Surf. B Biointerfaces 2014, 118, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, H.; Teleb, N.; Yahia, I.; Zahran, H.; Elhaes, H.; Ibrahim, M. First principles study of the adsorption of hydrated heavy metals on graphene quantum dots. J. Phys. Chem. Solids 2019, 130, 32–40. [Google Scholar] [CrossRef]
- Prasongkit, J.; Shukla, V.; Grigoriev, A.; Ahuja, R.; Amornkitbamrung, V. Ultrahigh-sensitive gas sensors based on doped phosphorene: A first-principles investigation. Appl. Surf. Sci. 2019, 497, 143660. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Saroka, V.A.; Younis, W.O. Phosphorene quantum dot electronic properties and gas sensing. Phys. E Low-Dimens. Syst. Nanostructures 2019, 107, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Li, J. MoS2 quantum dots: Synthesis, properties and biological applications. Mater. Sci. Eng. C 2020, 109, 110511. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Tareen, A.K.; Iqbal, M.; Ye, Z.; Xie, Z.; Mahmood, A.; Mahmood, N.; Zhang, H. Recent Progress in Emerging Novel MXenes Based Materials and their Fascinating Sensing Applications. Small 2023, 19, 2206147. [Google Scholar] [CrossRef]
- Su, H.; Wang, W.; Shi, R.; Tang, H.; Sun, L.; Wang, L.; Liu, Q.; Zhang, T. Recent advances in quantum dot catalysts for hydrogen evolution: Synthesis, characterization, and photocatalytic application. Carbon Energy 2023, e280. [Google Scholar] [CrossRef]
- Mohanty, B.; Ghorbani-Asl, M.; Kretschmer, S.; Ghosh, A.; Guha, P.; Panda, S.K.; Jena, B.; Krasheninnikov, A.V.; Jena, B.K. MoS2 quantum dots as efficient catalyst materials for the oxygen evolution reaction. Acs Catal. 2018, 8, 1683–1689. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Teleb, N.; Wang, B.; Yunoki, S.; Zhang, Q. The electronic, adsorption, and catalytic properties of Bi-, Sb-, and As-nanoclusters. Catal. Today 2021, 376, 126–133. [Google Scholar] [CrossRef]
- Hu, W.; Lin, L.; Yang, C.; Dai, J.; Yang, J. Edge-modified phosphorene nanoflake heterojunctions as highly efficient solar cells. Nano Lett. 2016, 16, 1675–1682. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Atta, M.M.; Osman, W.; Zhang, Q. Two-dimensional quantum dots for highly efficient heterojunction solar cells. J. Colloid Interface Sci. 2021, 603, 48–57. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16 Revision C. 01. 2016; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Li, Y.; He, Z.; Xu, Y.; Yu, W. Theoretical investigations on charge transport properties of tetrabenzo [a, d, j, m] coronene derivatives using different density functional theory functionals (B3LYP, M06-2X, and wB97XD). J. Chem. Res. 2019, 43, 293–303. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Abdelsalam, H.; Abd-Elkader, O.H.; Zaghloul, N.S.; Zhang, Q. Magnetic and Electronic Properties of Edge-Modified Triangular WS2 and MoS2 Quantum Dots. Crystals 2023, 13, 251. [Google Scholar] [CrossRef]
- Wang, S.; Han, C.; Ye, L.; Zhang, G.; Hu, Y.; Li, W.; Jiang, Y. Electronic properties of triangle molybdenum disulfide (Mos2) clusters with different sizes and edges. Molecules 2021, 26, 1157. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.0. 16; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.D.; Adamo, C.; Jacquemin, D. Dye chemistry with time-dependent density functional theory. Phys. Chem. Chem. Physics 2014, 16, 14334–14356. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Osman, W.; Saad, M.; Ibrahim, M.; Yahia, I.; Abdelsalam, H.; Zhang, Q. Electronic, optical, and catalytic properties of finite antimonene nanoribbons: First principles study. Phys. Scr. 2022, 97, 035802. [Google Scholar] [CrossRef]
- O’boyle, N.M.; Tenderholt, A.L.; Langner, K.M. Cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Hamel, S.; Duffy, P.; Casida, M.E.; Salahub, D.R. Kohn–Sham orbitals and orbital energies: Fictitious constructs but good approximations all the same. J. Electron Spectrosc. Relat. Phenom. 2002, 123, 345–363. [Google Scholar] [CrossRef]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Bourass, M.; Benjelloun, A.T.; Benzakour, M.; Mcharfi, M.; Hamidi, M.; Bouzzine, S.M.; Bouachrine, M. DFT and TD-DFT calculation of new thienopyrazine-based small molecules for organic solar cells. Chem. Cent. J. 2016, 10, 67. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elkader, O.H.; Abdelsalam, H.; Sakr, M.A.; Shaltout, A.A.; Zhang, Q. First-Principles Study of MoS2, WS2, and NbS2 Quantum Dots: Electronic Properties and Hydrogen Evolution Reaction. Crystals 2023, 13, 994. [Google Scholar] [CrossRef]
- Sakr, M.A.; Sherbiny, F.F.; El-Etrawy, A.-A.S. Hydrazone-based materials; DFT, TD-DFT, NBO analysis, fukui function, MESP analysis, and solar cell applications. J. Fluoresc. 2022, 32, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Guido, C.A.; Cortona, P.; Mennucci, B.; Adamo, C. On the metric of charge transfer molecular excitations: A simple chemical descriptor. J. Chem. Theory Comput. 2013, 9, 3118–3126. [Google Scholar] [CrossRef]

| Design | Ga1-S2 (Å) | Ga3-S4 (Å) | S4-Ga5 (Å) | Ga7-S8 (Å) | Ga1-S2-Ga3 (°) | S4-Ga5-S6 (°) | Ga1-S2-Ga3-S4 (°) | Ga1-S2-Ga3-S8 (°) | Ga3-S4-Ga5-S6 (°) | |
|---|---|---|---|---|---|---|---|---|---|---|
| NRs | ||||||||||
| GaS-3-2ANR | 2.275 | 2.427 | 2.416 | 2.325 | 90.454 | 109.2 | 170.1 | 60.1 | 167.9 | |
| GaS-3-4ANR | 2.280 | 2.441 | 2.327 | 2.338 | 89.550 | 113.4 | 172.4 | 64.7 | 170.8 | |
| GaS-3-6ANR | 2.278 | 2.434 | 2.329 | 2.334 | 89.455 | 113.5 | 172.2 | 64.5 | 170.4 | |
| GaS-2-2ZNR | 2.331 | 2.591 | 2.294 | 2.718 | 87.868 | 109.5 | 172.7 | 66.2 | 174.9 | |
| GaS-2-4ZNR | 2.329 | 2.531 | 2.297 | 2.606 | 88.799 | 109.6 | 173.9 | 66.8 | 173.9 | |
| GaS-2-6ZNR | 2.541 | 2.374 | 2.381 | 4.944 | 93.307 | 113.9 | 174.3 | 62.3 | 158.1 | |
| GaS-3-6ANR-H | 2.335 | 2.378 | 2.321 | 2.383 | 115.37 | 120.3 | 86.0 | 21.2 | 135.6 | |
| GaS-3-6ANR-2C | 2.279 | 2.443 | 2.332 | 2.336 | 89.151 | 114.5 | 158.1 | 47.3 | 171.6 | |
| Compounds | EH (eV) | EL (eV) | ρ (eV) | χ (eV) | η (eV) | μ (D) |
|---|---|---|---|---|---|---|
| GaS-3-2ANR | −7.29 | −3.58 | −5.44 | 5.44 | 1.85 | 18.0 |
| GaS-3-4ANR | −6.71 | −4.75 | −5.73 | 5.73 | 0.98 | 91.1 |
| GaS-3-6ANR | −6.31 | −5.23 | −5.77 | 5.77 | 0.54 | 164.5 |
| GaS-2-2ZNR | −7.30 | −4.74 | −6.02 | 6.02 | 1.28 | 34.6 |
| GaS-2-4ZNR | −7.63 | −4.81 | −6.22 | 6.22 | 1.41 | 48.3 |
| GaS-2-6ZNR | −6.42 | −4.26 | −5.34 | 5.34 | 1.08 | 20.3 |
| GaS-3-6ANR-H | −7.70 | −2.72 | −5.21 | 5.21 | 2.49 | 65.1 |
| GaS-3-6ANR-2C | −6.62 | −5.19 | −5.91 | 5.91 | 0.72 | 141.9 |
| Compounds | Δr (Å) | D (Å) | Sr (au) | t (Å) | Ec (eV) |
|---|---|---|---|---|---|
| GaS-3-2ANR | 12.68 | 4.66 | 0.30 | 2.48 | 2.63 |
| GaS-3-4ANR | 39.93 | 20.63 | 0.002 | 18.76 | 0.68 |
| GaS-3-6ANR | 20.78 | 8.73 | 0.24 | 4.79 | 1.89 |
| GaS-2-2ZNR | 14.20 | 6.50 | 0.16 | 4.61 | 1.74 |
| GaS-2-4ZNR | 1.93 | 0.14 | 0.89 | −1.67 | 4.10 |
| GaS-2-6ZNR | 20.78 | 8.73 | 0.24 | 4.79 | 1.89 |
| GaS-3-6ANR-H | 19.56 | 3.75 | 0.34 | −2.22 | 0.67 |
| GaS-3-6ANR-F | 5.66 | 3.58 | 0.17 | −1.63 | 0.27 |
| GaS-3-6ANR-2C | 63.94 | 33.84 | 0.000 | 32.03 | 0.41 |
| GaS-3-6ANR-2N | 12.31 | 6.18 | 0.34 | 0.58 | 0.97 |
| GaS-3-6ANR-2O | 6.10 | 0.85 | 0.43 | −1.33 | 2.92 |
| GaS-3-6ANR-2B | 15.43 | 1.19 | 0.44 | −2.24 | 1.54 |
| GaS-3-6ANR-W | 8.84 | 4.31 | 0.29 | 2.49 | 2.79 |
| Nanoribbon | ES | λmax | TE (eV) | ET | f | TC |
|---|---|---|---|---|---|---|
| GaS-3-2ANR | 14 | 454.34 | 2.72 | H-4→L | 0.038 | 0.35 |
| GaS-3-4ANR | 16 | 590.58 | 2.09 | H-29→L | 0.01 | 0.25 |
| GaS-3-6ANR | 3 | 616.13 | 2.01 | H-49→L | 0.06 | 0.21 |
| GaS-2-2ZNR | 13 | 773.36 | 1.60 | H-5→L | 0.03 | 0.47 |
| GaS-2-4ZNR | 8 | 1088.1 | 1.14 | H-2→L+1 | 0.01 | 0.23 |
| GaS-2-6ZNR | 3 | 1504.15 | 0.82 | H→L | 0.19 | 0.18 |
| GaS-3-6ANR-H | 9 | 304.04 | 3.95 | H-27→L | 0.01 | 0.10 |
| GaS-3-6ANR-F | 13 | 313.38 | 3.96 | H-28→L | 0.04 | 0.19 |
| GaS-3-6ANR-2C | 14 | 692.02 | 1.79 | H-2→L+2 | 0.40 | 0.71 |
| GaS-3-6ANR-2N | 8 | 541.14 | 2.29 | H-1→L+2 | 0.01 | 0.68 |
| GaS-3-6ANR-2O | 4 | 644.09 | 1.93 | H-1→L+11 | 0.09 | 0.37 |
| GaS-3-6ANR-2B | 7 | 558.25 | 2.22 | H-1→L+2 | 0.02 | 0.66 |
| GaS-4-6ANR | 3 | 616.16 | 2.01 | H-49→L | 0.001 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd-Elkader, O.H.; Abdelsalam, H.; Sakr, M.A.S.; Teleb, N.H.; Zhang, Q. Electronic and Optical Properties of Finite Gallium Sulfide Nano Ribbons: A First-Principles Study. Crystals 2023, 13, 1215. https://doi.org/10.3390/cryst13081215
Abd-Elkader OH, Abdelsalam H, Sakr MAS, Teleb NH, Zhang Q. Electronic and Optical Properties of Finite Gallium Sulfide Nano Ribbons: A First-Principles Study. Crystals. 2023; 13(8):1215. https://doi.org/10.3390/cryst13081215
Chicago/Turabian StyleAbd-Elkader, Omar H., Hazem Abdelsalam, Mahmoud A. S. Sakr, Nahed H. Teleb, and Qinfang Zhang. 2023. "Electronic and Optical Properties of Finite Gallium Sulfide Nano Ribbons: A First-Principles Study" Crystals 13, no. 8: 1215. https://doi.org/10.3390/cryst13081215
APA StyleAbd-Elkader, O. H., Abdelsalam, H., Sakr, M. A. S., Teleb, N. H., & Zhang, Q. (2023). Electronic and Optical Properties of Finite Gallium Sulfide Nano Ribbons: A First-Principles Study. Crystals, 13(8), 1215. https://doi.org/10.3390/cryst13081215









