3.2. Inert Gas Atmosphere Annealing Treatment
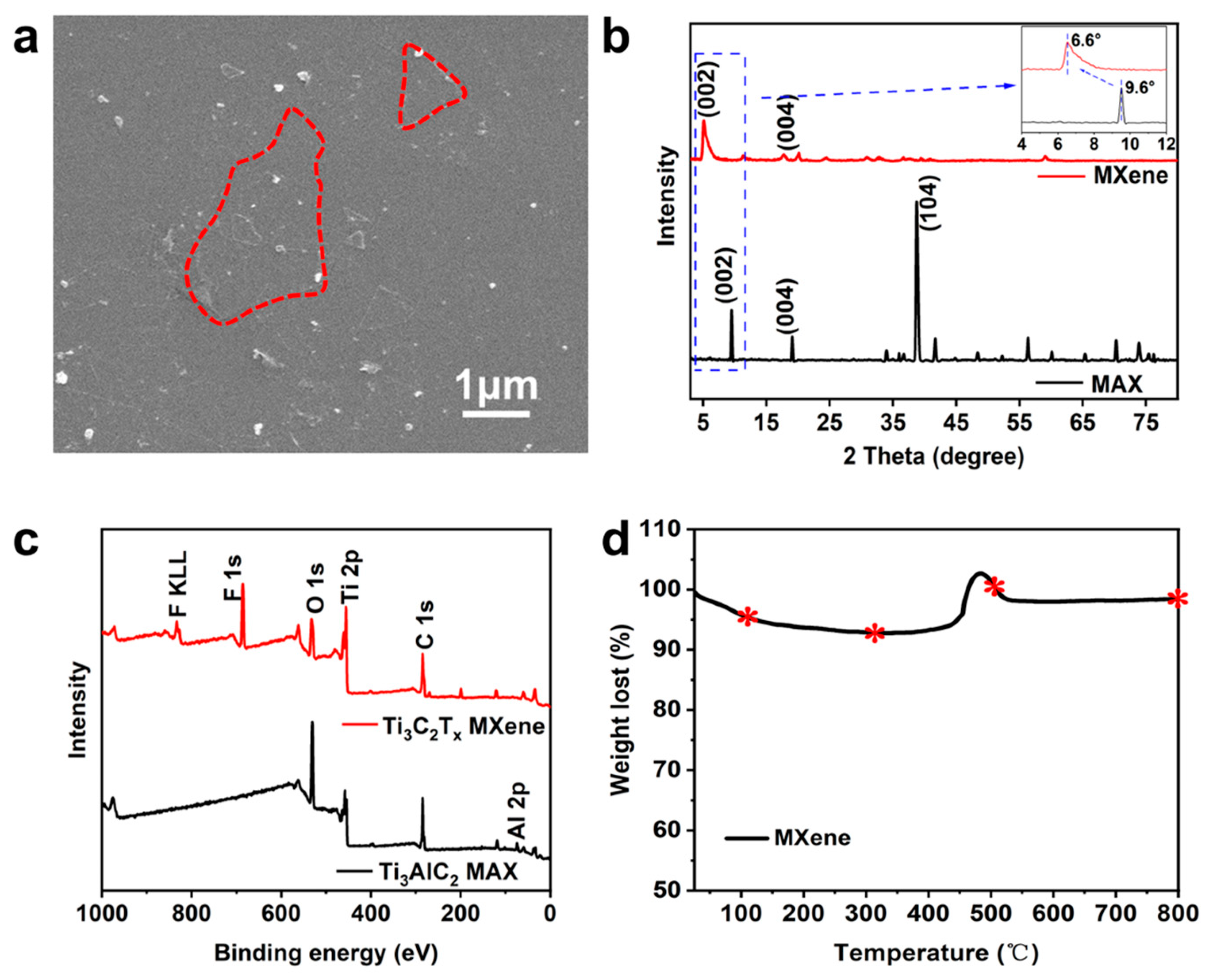
Ti
3AlC
2 MAX was etched into Ti
3C
2T
x MXene with LiF-HCl, and then the product was sonicated to form multilayer MXene nanosheets, which were used to anneal in N
2, Ar and CO
2 atmospheres at different temperatures. X-ray diffraction analysis (XRD) was used to study the crystal structure of the nanosheets after annealing with an inert atmosphere, as shown in
Figure 2. A diffraction peak near 6.6° can be seen in each sample, which corresponds to the characteristic peak of the (002) crystal plane of Ti
3C
2T
x MXene. It is worth mentioning that the position of the (002) peak shifted to the left with the increase in treatment temperature under the three inert gases. The position of the characteristic peak is determined by its crystal plane spacing of the (002) plane. The retention of the peak position indicates that the two-dimensional structure of MXene nanosheets after annealing was not destroyed by the annealing treatment. However, the left shift of the peak position indicates an increase in the layer spacing. In addition to the change in peak position, the area of the (002) peak became wider, indicating that the grain size of the nanosheets became smaller. Except for M
Ar-100 (as shown in
Figure 2b), the height of the (002) peak was reduced, indicating that the crystal structure was slightly damaged. The higher (002) peak intensity of the M
Ar-100 sample may be due to the reduction in defects caused by adsorbed H
2O molecules on the surface of the nanosheet after annealing at 100 °C in the Ar atmosphere, resulting in a more complete crystal structure, while the lower temperature does not destroy the crystal structure.
In the XRD patterns of the MXene nanosheets treated in a N
2 atmosphere at different temperatures (
Figure 2a), no characteristic peaks of anatase phase and rutile phase TiO
2 were observed, indicating that high temperatures did not oxidize the Ti atomic layer in the N
2 environment. In contrast, the characteristic TiO
2 peaks of anatase (24.8°) and rutile (26.9°) phases appear at 800 °C for MXene nanosheets treated in an Ar atmosphere, as shown in
Figure 2b. Similarly, MXene nanosheets treated in a CO
2 atmosphere showed a characteristic peak of anatase phase TiO
2 at 500 °C. At high temperatures, the anatase phase TiO
2 changes to the more stable rutile phase TiO
2. As the temperature increases to 800 °C, the characteristic peak of TiO
2 in the rutile phase appears, and both phases show extremely high peak intensity, as shown in
Figure 2c. There was no obvious characteristic TiO
2 peak at 100 °C and 300 °C.
Furthermore, we compared the XRD patterns of the treated M
N2-800, M
Ar-800, M
CO2-800 and the original MXene nanosheets at 800 °C, as shown in
Figure 3a. Except for the characteristic (002) peak, almost no diffraction peaks representing crystals appeared in the M
N2-800 sample, but the (002) peak became wider and the intensity became lower, indicating that the crystal structure of the nanosheet treated with the N
2 atmosphere at 800 °C was still damaged, but no oxidation of Ti atoms occurred and no new crystals were formed. The (002) peak of the M
Ar-800 sample was relatively well retained, and the characteristic peak positions of the anatase phase and rutile phase TiO
2 appeared at the same time, indicating that Ti atoms reacted with O-containing functional groups on the surface. In contrast, the (002) peak of the M
CO2-800 sample almost completely disappeared and was replaced by the strong characteristic peak of anatase and rutile phase TiO
2. In addition to the O-containing functional groups on the surface, CO
2 provides a source of O during the oxidation of Ti atoms. Meanwhile, the original crystal structure of MXene was almost completely destroyed.
To further confirm the above speculation, Raman spectra (the laser wavelength was 633 nm) have been collected and provided in
Figure 3b. The A
1g (Ti, O, Tx) mode near 201 cm
−1 (blue dashed line position in
Figure 3b), which is the out-of-plane vibration of the outer Ti atoms as well as the carbon and surface groups, could be observed for all samples, confirming that the majority of the sample remains as Ti
3C
2T
x MXene. In particular, the M
Ar-800 and M
CO2-800 samples showed vibrational peaks near 151 cm
−1, a characteristic peak position for anatase phase oxides of Ti. This is consistent with the results of the previous analysis. For the M
CO2-800 sample, obvious vibrational peaks appeared around 253 cm
−1, 421 cm
−1 and 615 cm
−1, which were characteristic peak positions for the rutile phase oxide of Ti. A weak vibration signal could also be observed in the M
Ar-800 sample. We further observed the surface of the sample after observation, and no ablation occurred, which proved that the oxide production was not caused by laser irradiation. This is consistent with the conclusion of XRD. At the same time, the enhancement of the D and G peaks representing the C-layer structure between 1300 and 1600 cm
−1 of the M
CO2-800 sample indicated a large area of bare leakage in the C-layer. Combined with the attenuation of the signal at 201 cm
−1 and the enhancement of the oxide signal of Ti, the structure of the M
CO2-800 sample is presumed to evolve into a C-TiO
2 structure. In contrast, the Raman signals of M
N2-800 and MXene were almost identical, which is also consistent with the analytical conclusions of XRD.
X-ray photoelectron spectroscopy (XPS) has been widely used to characterize the surface element content of nanomaterials. The total XPS spectra of samples annealed with a N
2 atmosphere and CO
2 atmosphere at different temperatures are provided in
Figure 4a,b. It can be observed in the spectrum that four characteristic peaks are present, corresponding to F 1s, O 1s, Ti 2p and C 1s. The terminal functional group of Ti
3C
2T
x MXene is grafted on the Ti atoms in the surface layer, and its type and content are determined by the etching and post-processing processes. The -OH, =O and -F groups are common termination groups in the products prepared via the LiF-HCl etching process. We restricted the surface functional group composition of the nanosheets to a combination of these three groups in different proportions. It can be seen from
Figure 4a,b that the content of F atoms is decreasing. It has been shown that the F-containing functional group does not exist stably at high temperatures, which is consistent with the trend in the figure.
For the M
N2 series samples, a clear characteristic peak of N 1s can be observed, which indicates a small amount of N element doping in the sample annealed under a N
2 atmosphere. In order to confirm this point of view, the content of N atoms and Ti atoms are compared and plotted in
Figure 4c. It can be seen that with the increase in temperature, the doping amount of N gradually increases from 0.03 to 0.29. In combination with the XRD pattern of
Figure 2a, no new crystal characteristic peaks appear, and it is speculated that N elements mainly exist in the M
N2 series samples in the way of adsorption. Similarly, in order to confirm that CO
2 participates in the reaction during the processing of M
CO2 series samples, the content of O atoms and Ti atoms are compared and plotted in
Figure 4d. It can be seen that with the increase in temperature, the content of O increases gradually, from 1.27 to 4.29. The O content did not increase significantly during the low-temperature treatment, indicating that CO
2 was mainly involved in the reaction above 500 °C in this study.
We compared the total XPS spectra of the treated M
N2-800, M
Ar-800, M
CO2-800 and the original MXene nanosheet, as shown in
Figure 5a. The decrease in F after treatment under 800 °C can be clearly observed, indicating that the F-containing functional group on the MXene surface cannot withstand the high temperatures. High-resolution XPS analysis of C 1s peaks was performed for the four samples, and the results are shown in
Figure 5b. In M
N2-800, M
Ar-800 and raw MXene nanosheets, the C 1s peak can be divided into four secondary peaks located at 281.6 eV, 284.8 eV, 286.9 eV and 288.7 eV, corresponding to C-Ti, C-C, C-O and C=O/C-F bonds, respectively. In the M
N2-800 sample, the C-C bond ratio increased substantially and the C-Ti bond ratio decreased, which we believe is due to the destruction of the layered structure of the nanosheets during the treatment, which corresponds to the loss of the (002) peak in XRD spectra. However, for the M
Ar-800 sample, the decrease in the ratio of C-Ti bonds is mainly due to the oxidation of Ti atoms. The process of bonding Ti atoms to O is bound to be accompanied by the breaking of the C-Ti bond. More obviously, C-Ti bonds are hardly observed in the C 1s fine spectrum of the M
CO2-800 sample, supporting the formation of a large amount of TiO
2. Correspondingly, the Ti 2p peaks of the four samples were analyzed using high-resolution XPS, and the results are shown in
Figure 5c. In M
N2-800, M
Ar-800 and raw MXene nanosheets, Ti 2p peaks can be divided into six secondary peaks located at 454.9 eV, 456.4 eV, 459.2 eV, 461.4 eV, 463.0 eV and 464.9 eV, corresponding to Ti-C 2p
3, Ti (II) 2p
3, Ti-O 2p
3, Ti-C 2p
1, Ti (II) 2p
1 and Ti-O 2p
1 bonds, respectively. Similarly, the large increase in the Ti-O bond ratio in the M
N2-800 sample is due to the destruction of the layered structure of the nanosheets, and the increase in the Ti-O bond ratio in the M
Ar-800 sample is mainly due to the oxidation of Ti atoms. This is also supported by the relative content of Ti (II) bonds in the two samples. Similarly, the Ti-C bond almost disappears in the Ti 2p fine spectrum of the M
CO2-800 sample and is replaced by Ti-O. This is consistent with the conclusions of the above analysis.
High-resolution XPS analysis was used to perform the split-peak fitting of the O 1s peak to further study the existence form and changing trend of O-containing functional groups on the surface, and the results are shown in
Figure 5c. The O 1s peak can be divided into four secondary peaks, about Ti-O, C-Ti-O
x, C-Ti- (OH)
x and O
2−. In addition, the positions and area ratios of O 1s differentiation peaks are shown in
Table 2. It can be seen that compared with pure MXene, the free oxygen contents of M
N2-800, M
Ar-800 and M
CO2-800 are significantly decreased, which is inseparable from the effect of temperature. Free oxygen binds to surface Ti atoms to form -OH under the influence of temperature. Meanwhile, the increase in the proportion of Ti-O peaks for M
Ar-800 and M
CO2-800 samples was accompanied by a decrease in the proportion of C-Ti-O
x peaks, indicating the formation of TiO
2 after the annealing treatment. In particular, the presence of specific O-valence bonds on the surface of M
N2-800 is closely related to the destruction of the layered structure. The above conclusions are consistent with the aforementioned XRD, Raman and XPS total spectroscopic results.
The SEM was performed on M
N2-800, M
Ar-800 and M
CO2-800, and the results are shown in
Figure 6a–c.
Figure 6a showed that the M
N2-800 sample size became smaller, and a large number of dark holes existed in the nanosheets, and the two-dimensional structure of the nanosheets was destroyed. Nanoparticles were formed on the surface of the M
Ar-800 and M
CO2-800 samples, but not on M
N2-800. The nanoparticles of M
CO2-800 were scanned by HRTEM, and the results are shown in
Figure 6d,e. HRTEM image analysis of the two nanoparticles found that
Figure 6d showed two lattice stripes with crystal plane spacing of 0.352 nm and 0.184 nm, corresponding to the (101) and (200) crystal planes of the TiO
2 anatase phase. Similarly,
Figure 6e shows three lattice stripes with lattice spacing of 0.203 nm, 0.324 nm and 0.230 nm, corresponding to the (210), (110) and (200) crystal facets of the TiO
2 rutile phase, indicating that TiO
2 was generated during the synthesis.
The coaxial method was used to characterize the dielectric properties of the samples before and after the inert gas annealing treatment in the form of nanosheets/paraffin composites. The test results for M
N2-800, M
Ar-800, M
CO2-800 and the original MXene nanosheets are shown in
Figure 7. In general, the dielectric properties of nanosheets are affected by the surface composition and morphology, and the surface functional groups and their positional relationships determine the number and efficiency of polarized channels, which significantly increase the dielectric constant of the composite. The density of samples MXene/paraffin, M
N2-800/paraffin, M
Ar-800/paraffin and M
CO2-800/paraffin were 0.25, 0.24, 0.23 and 0.25 g·cm
−1, respectively. The density values of the four samples were basically the same and had no significant effect on the measurement of dielectric parameters.
When the external electromagnetic wave reaches, the different types of polarization behaviors caused by the periodic changes in the electromagnetic wave determine the dielectric constant performance of the material, and the size of the dielectric constant determines the energy storage property. In the low-frequency region, due to the slow speed of electromagnetic wave change, the polarization of relatively large grains, ions and other particles can undergo polarization relaxation in response to the change in electromagnetic waves, which then cause the change in dielectric constant. However, in the high-frequency region, particles with large particle sizes cannot respond to the changes in electromagnetic waves in time, and the polarization of electrons becomes the main polarization form. It can be seen from
Figure 7a that the lowest permittivity of M
CO2-800 is because the presence of a large number of TiO
2 particles makes it difficult to respond to the changes in electromagnetic waves. At the same time, the large loss of surface functional groups makes the sample unable to respond to the rapidly changing external alternating electromagnetic field in time. Both together lead to the maintenance of the dielectric constant of M
CO2-800 at a low level. The permittivity of pure MXene in the low-frequency region (<4GHz) is extremely high, mainly due to the presence of free oxygen in response to the change in electromagnetic waves. The permittivity of M
N2-800 and M
Ar-800 varies similarly with frequency, mainly due to the existence of similar O-containing functional groups on the surface. In the high-frequency region, the dielectric constant of M
N2-800 becomes rapidly higher. This is due to the effect of the N element doped on its surface on the response of the external electromagnetic field. Similarly, the increase in O-containing functional groups (as shown in
Table 2) in the M
Ar-800 sample led to a rapid increase in the dielectric constant in the high-frequency region. Among them, the small amount of TiO
2 particles on the surface of M
Ar-800 causes it to fail to reach the same corresponding level of M
N2-800. The imaginary part of the dielectric constant represents the loss ability of electromagnetic waves in the polarization process, and its variation law is similar to that of the real part of the dielectric constant, as shown in
Figure 7b. Using this result, the inert gas atmosphere heat treatment process can be applied to the improvement of the electronic product raw material design.
Based on the above analysis, we can summarize the structural changes of Ti
3C
2T
x MXene after the annealing treatment in an inert gas atmosphere, as shown in
Figure 8.
Figure 8a shows the two-dimensional nanosheet structure of the original Ti
3C
2T
x MXene. When annealed in a N
2 atmosphere, due to the protective effect, the large area of the Ti atom oxidation behavior will not occur on the MXene surface, but the doping of N element will occur, accompanied by the destruction of the 2D layered structure, as shown in
Figure 8b. When annealed in an Ar atmosphere, most of the 2D structure can be effectively preserved. When the temperature is increased to 800 °C, trace oxidation of Ti atoms occurs on the MXene surface, resulting in anatase and rutile phases, as shown in
Figure 8c. In the process of annealing in CO
2 atmosphere, anatase phase and rutile phase are formed at 500 °C. When the temperature is increased to 800 °C, the Ti-C bond almost disappears and is replaced by the oxidation of a large number of Ti atoms. In this process, CO
2 participates in the reaction as the source of O, and finally generates the complete C-TiO
2 structure, as shown in
Figure 8d. Either treatment is accompanied by the loss of a large amount of F-containing functional groups and the conversion of free oxygen.