Robust Conveniently Sealable Container for High-Temperature Single-Crystal Growth Out of Reactive Melts with High Vapor Pressure
Abstract
:1. Introduction
2. Materials and Methods
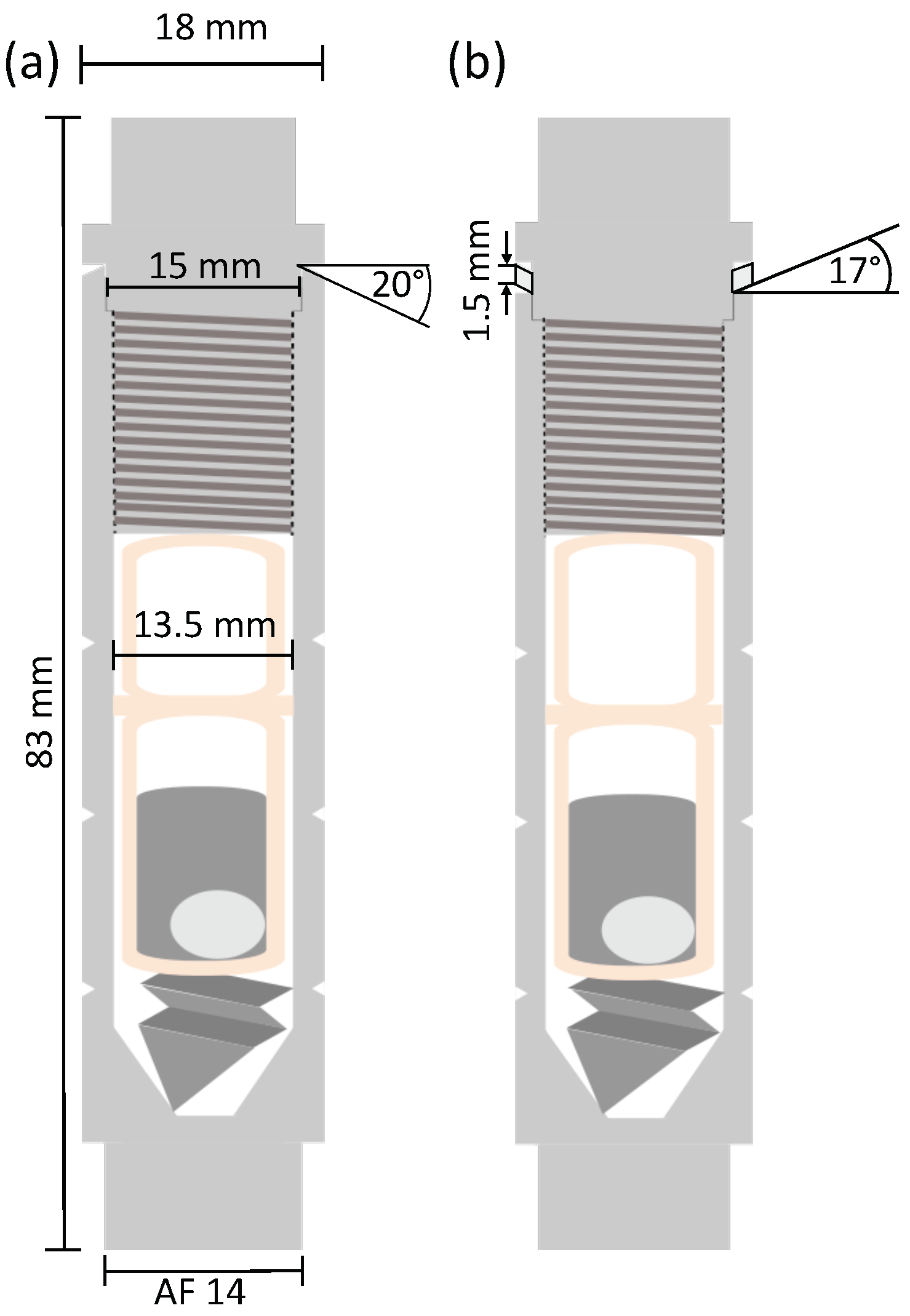
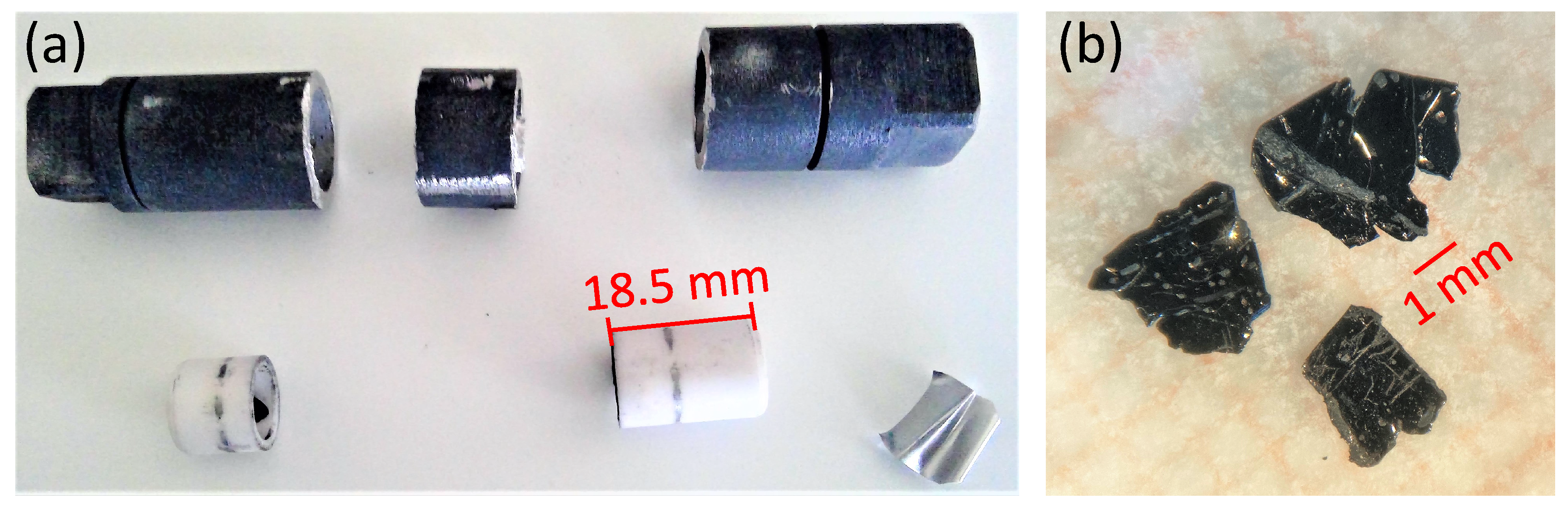
2.1. Novel Steel Container
2.2. Growth of KFeAg Single Crystals
2.3. Characterization Methods
3. Results
3.1. Optimization of Crystal Growth
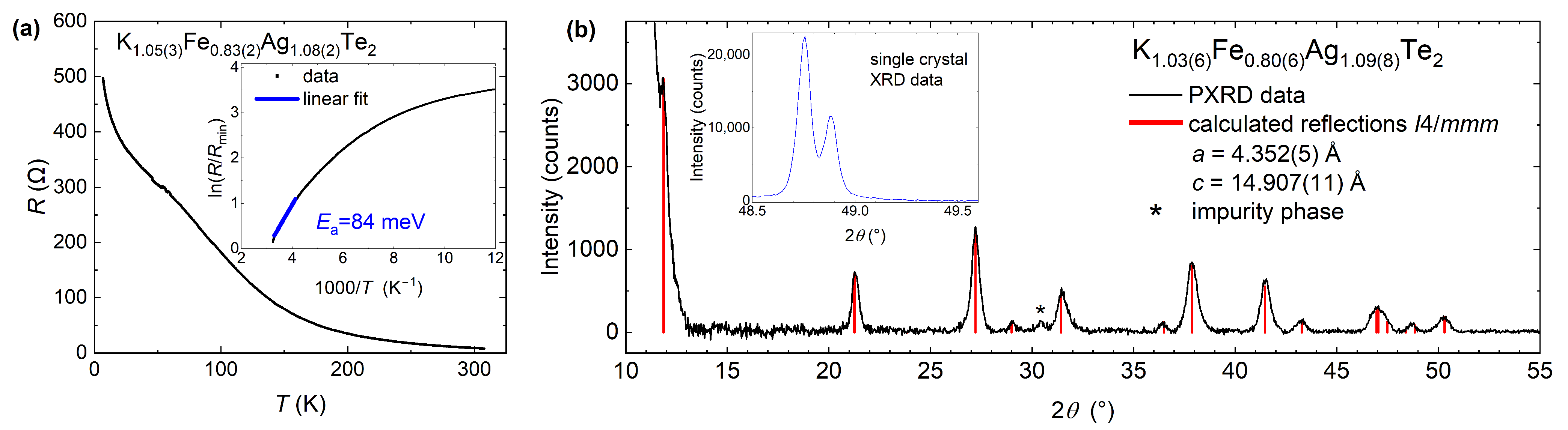
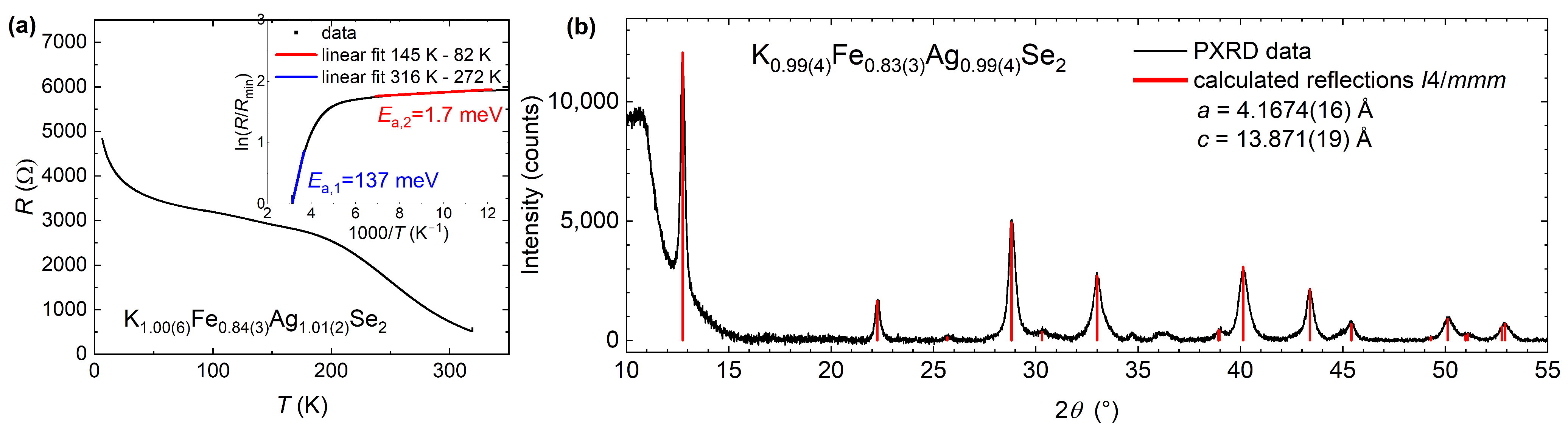
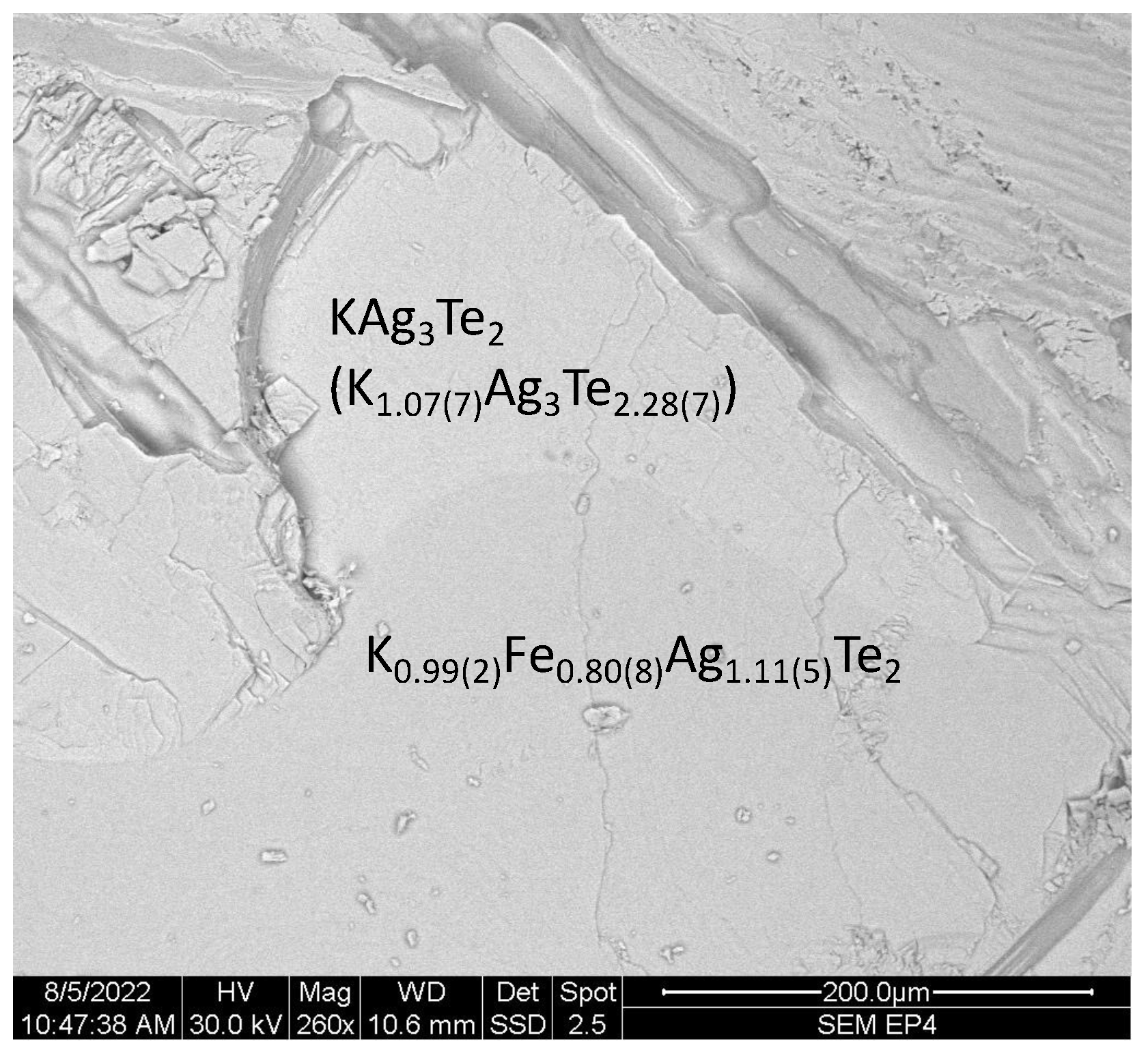
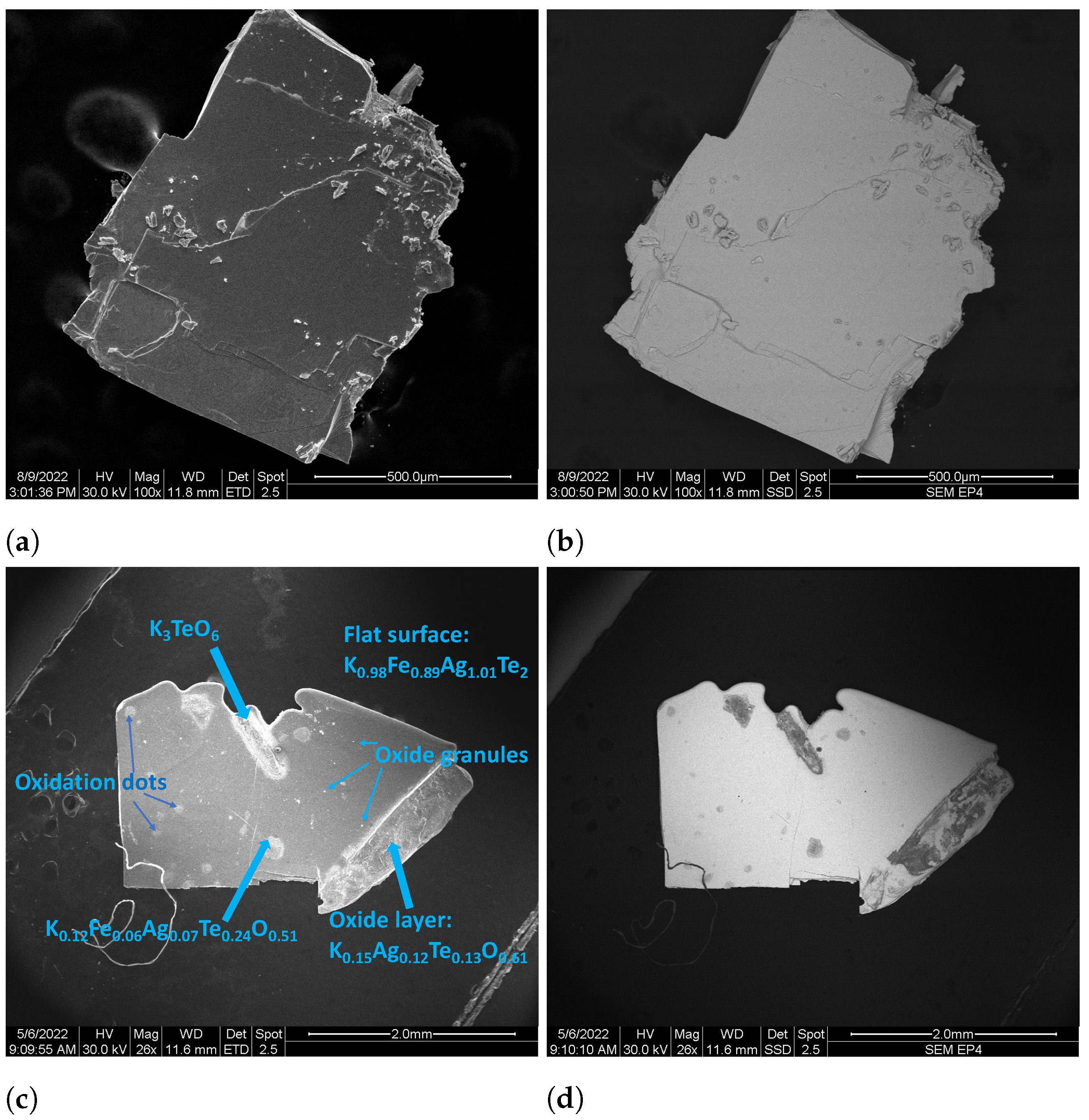
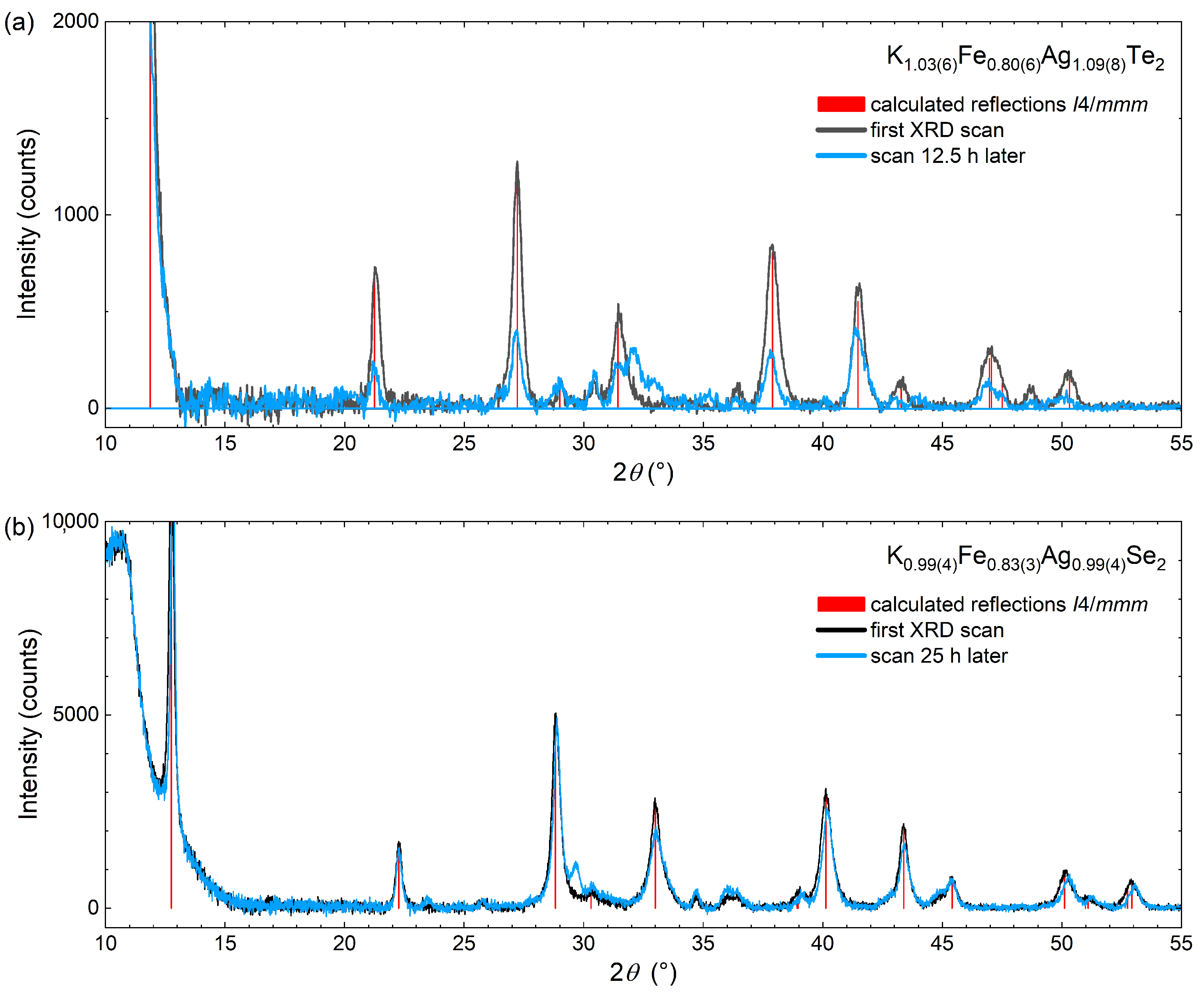
3.2. Characterization of Air-Sensitivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canfield, P.C.; Fisher, I.R. High-temperature solution growth of intermetallic single crystals and quasicrystals. J. Cryst. Growth 2001, 225, 155–161. [Google Scholar] [CrossRef]
- Canfield, P. Solution growth of intermetallic single crystals: A beginner’s guide. In Properties and Applications of Complex Intermetallics; World Scientific: Hackensack, NJ, USA, 2009; pp. 93–111. [Google Scholar]
- Jesche, A.; Canfield, P. Single crystal growth from light, volatile and reactive materials using lithium and calcium flux. Philos. Mag. 2014, 94, 2372–2402. [Google Scholar] [CrossRef]
- Tachibana, M. Beginner’s Guide to Flux Crystal Growth; Springer: Tokyo, Japan, 2017; pp. 82, 87. [Google Scholar]
- Kihou, K.; Saito, T.; Ishida, S.; Nakajima, M.; Tomioka, Y.; Fukazawa, H.; Kohori, Y.; Ito, T.; Uchida, S.I.; Iyo, A.; et al. Single Crystal Growth and Characterization of the Iron-Based Superconductor KFe2As2 Synthesized by KAs Flux Method. J. Phys. Soc. Jpn. 2010, 79, 124713. [Google Scholar] [CrossRef]
- EN 1.4841. Available online: https://ucpcdn.thyssenkrupp.com/_legacy/UCPthyssenkruppBAMXUK/assets.files/material-data-sheets/stainless-steel/stainless-steel-1.4841.pdf (accessed on 18 August 2023).
- Canfield, P.; Kong, T.; Kaluarachchi, U.; Jo, N. Use of frit-disc crucibles for routine and exploratory solution growth of single crystalline samples. Philos. Mag. 2016, 96, 84–92. [Google Scholar] [CrossRef]
- Lei, H.; Bozin, E.S.; Wang, K.; Petrovic, C. Antiferromagnetism in semiconducting KFe0.85Ag1.15Te2 single crystals. Phys. Rev. B 2011, 84, 060506. [Google Scholar] [CrossRef]
- Song, Y.; Cao, H.; Chakoumakos, B.C.; Zhao, Y.; Wang, A.; Lei, H.; Petrovic, C.; Birgeneau, R.J. Intertwined Magnetic and Nematic Orders in Semiconducting KFe0.8Ag1.2Te2. Phys. Rev. Lett. 2019, 122, 087201. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Lei, H.; Klobes, B.; Warren, J.B.; Hermann, R.P.; Petrovic, C. Spin glass in semiconducting KFe1.05Ag0.88Te2 single crystals. Phys. Rev. B 2015, 91, 174517. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Z.; Sun, F.; Deng, J.; Lin, J.; Wu, D.; Yuan, W. The transition between antiferromagnetic order and spin-glass state in layered chalcogenides KFeAgCh2 (Ch = Se, S). J. Solid State Chem. 2019, 272, 126–130. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Püttmann, J.; Sangeetha, N.S.; Thomas, T.R.; Meermann, J.; Kreyssig, A.; Böhmer, A.E. Robust Conveniently Sealable Container for High-Temperature Single-Crystal Growth Out of Reactive Melts with High Vapor Pressure. Crystals 2023, 13, 1332. https://doi.org/10.3390/cryst13091332
Püttmann J, Sangeetha NS, Thomas TR, Meermann J, Kreyssig A, Böhmer AE. Robust Conveniently Sealable Container for High-Temperature Single-Crystal Growth Out of Reactive Melts with High Vapor Pressure. Crystals. 2023; 13(9):1332. https://doi.org/10.3390/cryst13091332
Chicago/Turabian StylePüttmann, Jutta, N. S. Sangeetha, Teslin R. Thomas, Jörg Meermann, Andreas Kreyssig, and Anna E. Böhmer. 2023. "Robust Conveniently Sealable Container for High-Temperature Single-Crystal Growth Out of Reactive Melts with High Vapor Pressure" Crystals 13, no. 9: 1332. https://doi.org/10.3390/cryst13091332



