Sulfonato Complex Formation Rather than Sulfonate Binding in the Extraction of Base Metals with 2,2′-Biimidazole: Extraction and Complexation Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instrumentation
2.3. Experimental Section
2.3.1. Preparation of 2,2′-Biimidazole
2.3.2. Preparation of 2,2′-Alkylbiimidazoles
- 1-Heptyl-2,2′-Biimidazole (HBIIMH)
- 1-Octyl-2,2′-Biimidazole (OBIIMH)
- 1-Decyl-2,2′-Biimidazole (DBIIMH)
- 1,1′-Bis-heptyl-2,2′-biimidazole (H2BIIM)
- 1,1′-Bis-octyl-2,2′-biimidazole (O2BIIM)
- 1,1′-Bis-decyl- 2,2′-biimidazole (D2BIIM)
2.3.3. Extraction Method
2.3.4. Syntheses of Metal Complexes
Sulfonate Salts
Preparation of Sulfonate Complexes
3. Results and Discussion
3.1. Synthesis and Characterization of 2,2′-Biimidazole and Extractants
3.2. Solvent Extraction Studies
3.3. Solution Complexation Studies
3.4. Synthesis and Characterization of Metal Complexes
3.4.1. Spectroscopic Characterization
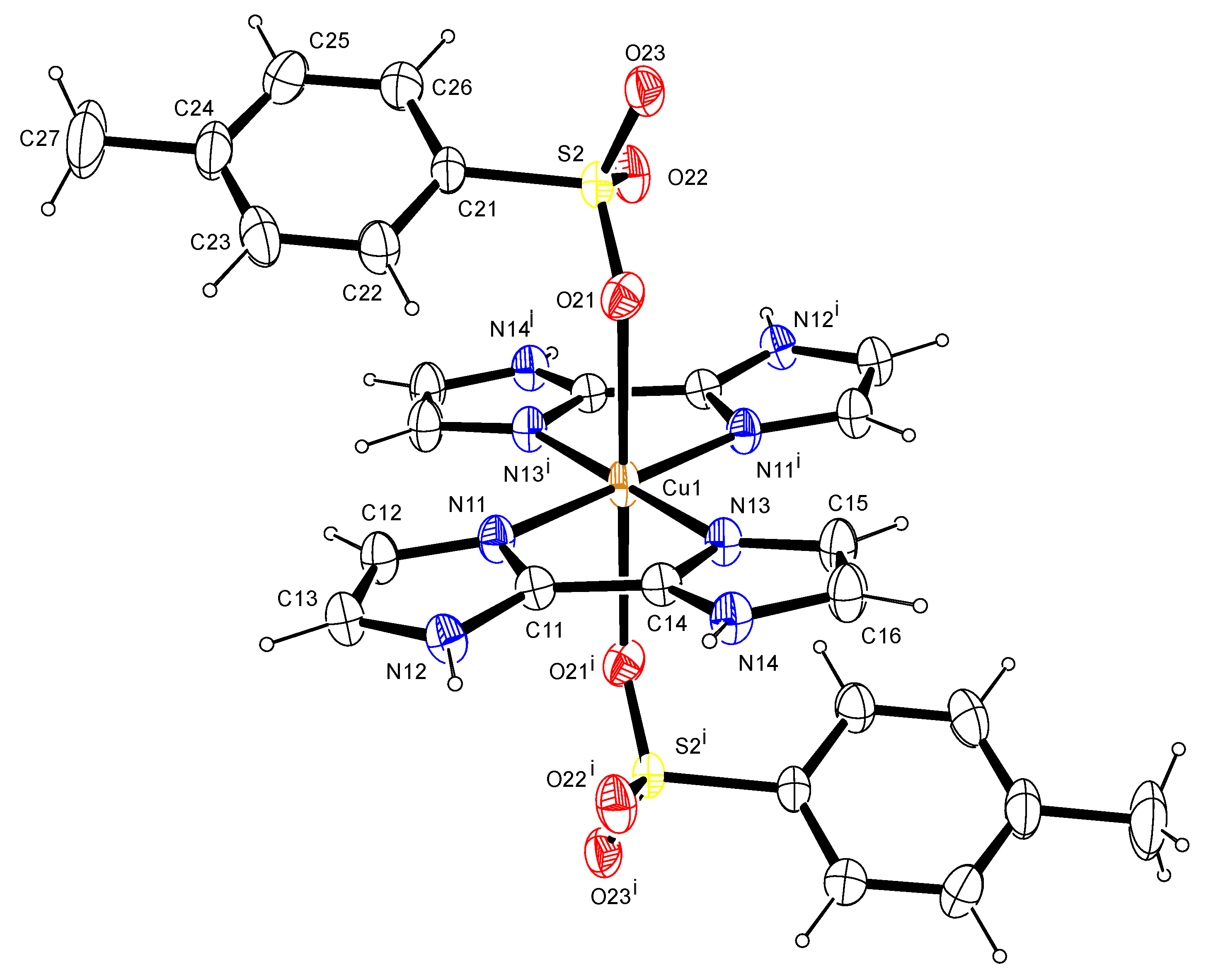
3.4.2. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du Preez, J.G.H. Recent advances in amines as separating agents for metal ions. Solvent Extr. Ion Exch. 2000, 18, 679–701. [Google Scholar] [CrossRef]
- Pearce, B.H.; Ogutu, H.F.; Luckay, R.C. Synthesis of pyrazole-based pyridine ligands and their use as extractants for nickel (II) and copper (II): Crystal structure of a copper (II)–ligand complex. Eur. J. Inorg. Chem. 2017, 8, 1189–1201. [Google Scholar] [CrossRef]
- Okewole, A.I.; Magwa, N.P.; Tshentu, Z.R. The separation of nickel(II) from base metal ions using 1-octyl-2-(2′-pyridyl)imidazole as extractant in a highly acidic sulfate medium. Hydrometallurgy 2012, 121–124, 81–89. [Google Scholar] [CrossRef]
- Roebuck, J.W.; Bailey, P.J.; Doidge, E.D.; Fischmann, A.J.; Healy, M.R.; Nichol, G.S.; O’Toole, N.; Pelser, M.; Sassi, T.; Sole, K.C.; et al. Strong and selective Ni(II) extractants based on synergistic mixtures of sulfonic acids and bidentate N-heterocycles. Solvent Extr. Ion Exch. 2018, 36, 437–458. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Okewole, A.I.; Walmsley, R.S.; Valtancoli, B.; Bianchi, A.; Tshentu, Z.R. Separation of copper(II) from a highly acidic synthetic sulfate leach solution using a novel 1-octylimidazole-2-aldoxime extractant. Solvent Extr. Ion Exch. 2013, 31, 61–78. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Moleko, P. The Coordination and Extractive Chemistry of the Later 3d Transition Metal Ions with N,N′-Donor Imidazole-Based Ligands. Master’s Dissertation, Nelson Mandela Metropolitan University, Gqeberha, South Africa, 2014; p. 37. [Google Scholar]
- Haring, M. A novel route to N-substituted heterocycles. Helv. Chim. Acta 1959, 42, 1845–1850. [Google Scholar]
- Allen, K.A. Equilibrium between didecylamine and sulphuric acid. J. Phys. Chem. 1956, 60, 943–946. [Google Scholar] [CrossRef]
- Flett, D.S. Solvent extraction in hydrometallurgy: The role of organophosphorus extractants. J. Organomet. Chem. 2005, 690, 2426–2438. [Google Scholar] [CrossRef]
- Magwa, N.P.; Hosten, E.; Watkins, G.M.; Tshentu, Z.R. An exploratory study of tridentate amine extractants-solvent extraction and coordination chemistry of base metals with bis((1R-benzimidazol-2-yl)methyl)amine. Int. J. Nonferrous Metall. 2012, 1, 49–58. [Google Scholar] [CrossRef]
- Magwa, N.P.; Hosten, E.; Watkins, G.M.; Tshentu, Z.R. The coordination and extractive chemistry of the later 3d transition metals with bis((1R-benzimidazol-2-yl)methyl)sulfide. J. Coord. Chem. 2013, 66, 114–125. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, Y.; Kida, S. Crystal structure of copper(II) complex with N,N-bis(2-benzimidazolylmethyl)benzylamine. Polyhedron 1984, 4, 113–116. [Google Scholar] [CrossRef]
- Pandiyan, T.; Hernandez, J.G. Geometrical isomers of bis(benzimidazol-2-ylethyl)sulfide)cobalt(II) diperchlorates: Synthesis, structure, spectra and redox behavior of pink-[Co(bbes)2](ClO4)2 and blue-[Co(bbes)2](ClO4)2. Inorg. Chim. Acta. 2004, 357, 2570–2578. [Google Scholar] [CrossRef]
- Hancock, R.D.; Martell, A.E. Ligand design for selective complexation of metal ions in aqueous solution. Chem. Rev. 1989, 89, 1875–1914. [Google Scholar] [CrossRef]
- Fewings, K.R.; Junk, P.C.; Georganopoulou, D.; Prince, P.D.; Steed, J.W. Supramolecular interactions in metal tosylate complexes. Polyhedron 2001, 20, 643–649. [Google Scholar] [CrossRef]
- Kosnic, J.E.; McClymont, L.; Hodder, R.A.; Squattrito, P.J. Synthesis and structures of layered metal sulfonate salts. Inorg. Chim. Acta 1996, 244, 253–254. [Google Scholar] [CrossRef]
- Cabaleiro-Martinez, S.; Castro, J.; Romero, J.; Garcia-Vazquez, J.; Sousa, A. Hexaaquacobalt(II) bis(4-toluene-sulfonate). Acta Cryst. 2000, C56, e249–e250. [Google Scholar]
- Rietmeijer, F.J.; Birker, P.J.M.W.L.; Gorter, S.; Reedijk, J. Copper(I) and copper(II) chelates containing imidazole and thioether groups; synthesis of the ligand 1,2-bis(benzimidazol-2′-ylmethylthio)-benzene (bbtb) and the X-ray crystal structure at 52 °C of [Cu-(bbtb)(H2O)][ClO4]2·5EtOH. J. Chem. Soc. 1982, 7, 1191–1198. [Google Scholar] [CrossRef]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2014, 4, 123–134. [Google Scholar] [CrossRef]
- de Sousa, A.S.; Fernandes, M.A.; Padayachy, K.; Marques, H.M. Amino-alcohol ligands: Synthesis and structure of N,N-bis(2-hydroxycyclopentyl)ethane-1,2-diamine and its salts, and an assessment of its fitness and that of related ligands for complexing metal ions. Inorg. Chem. 2010, 49, 8003–8011. [Google Scholar] [CrossRef]
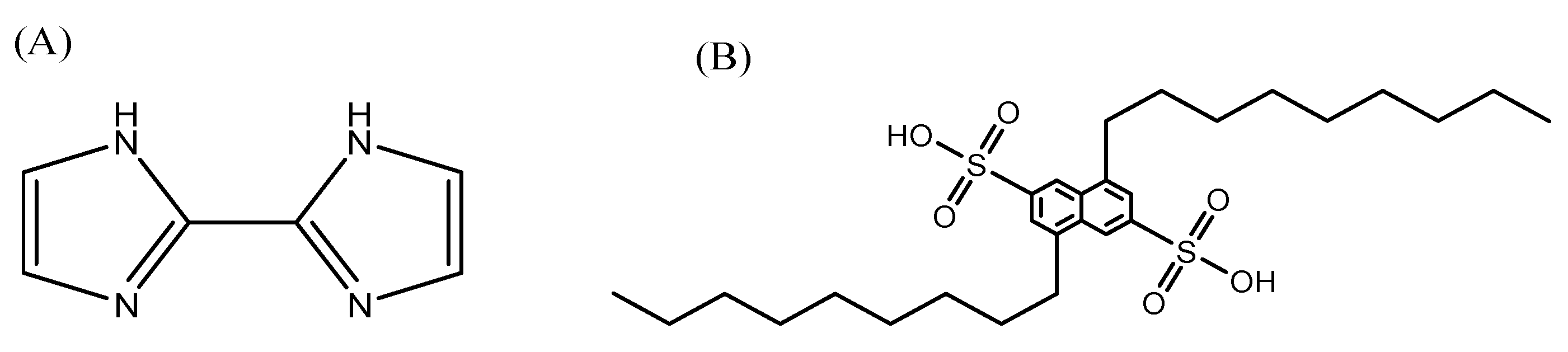
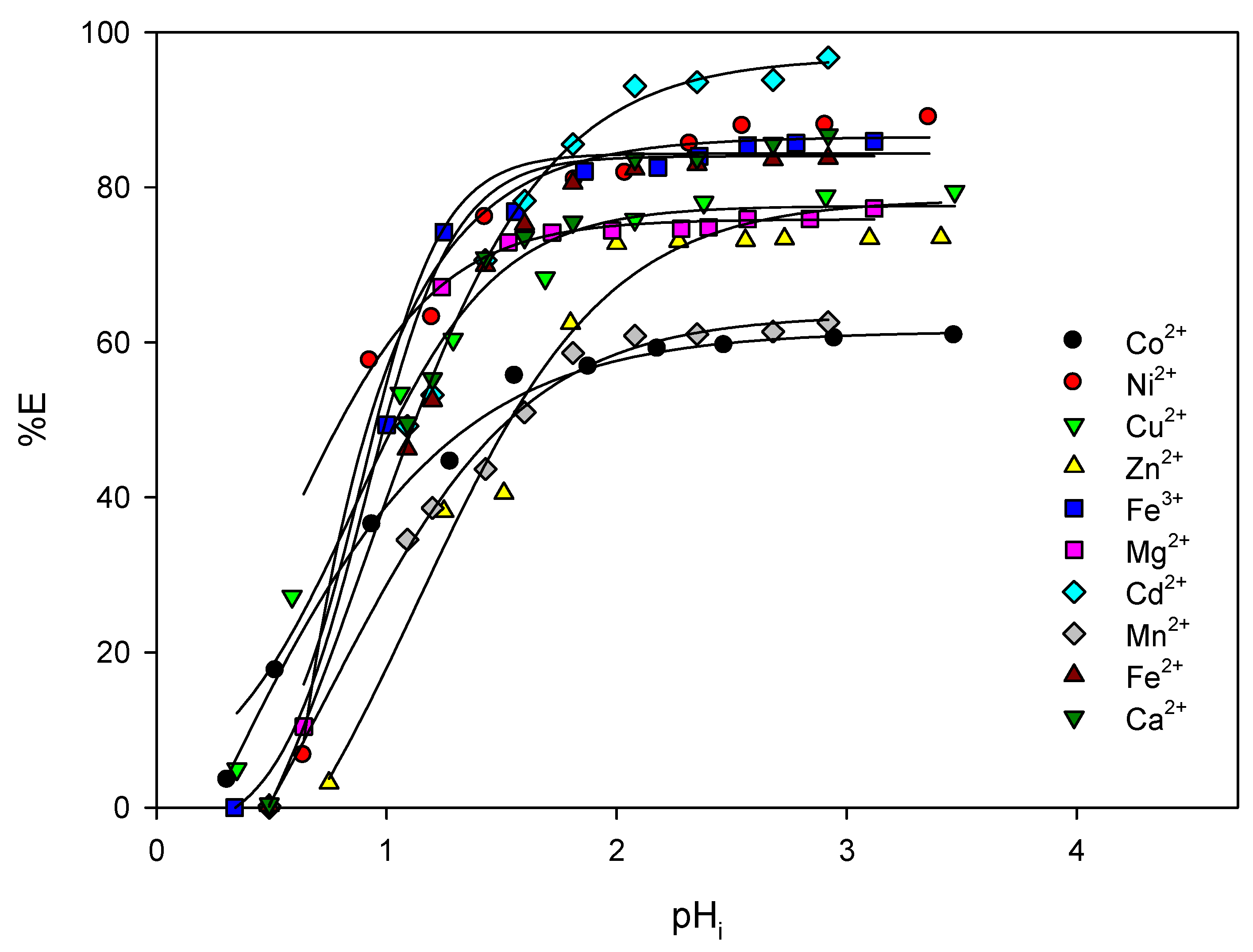

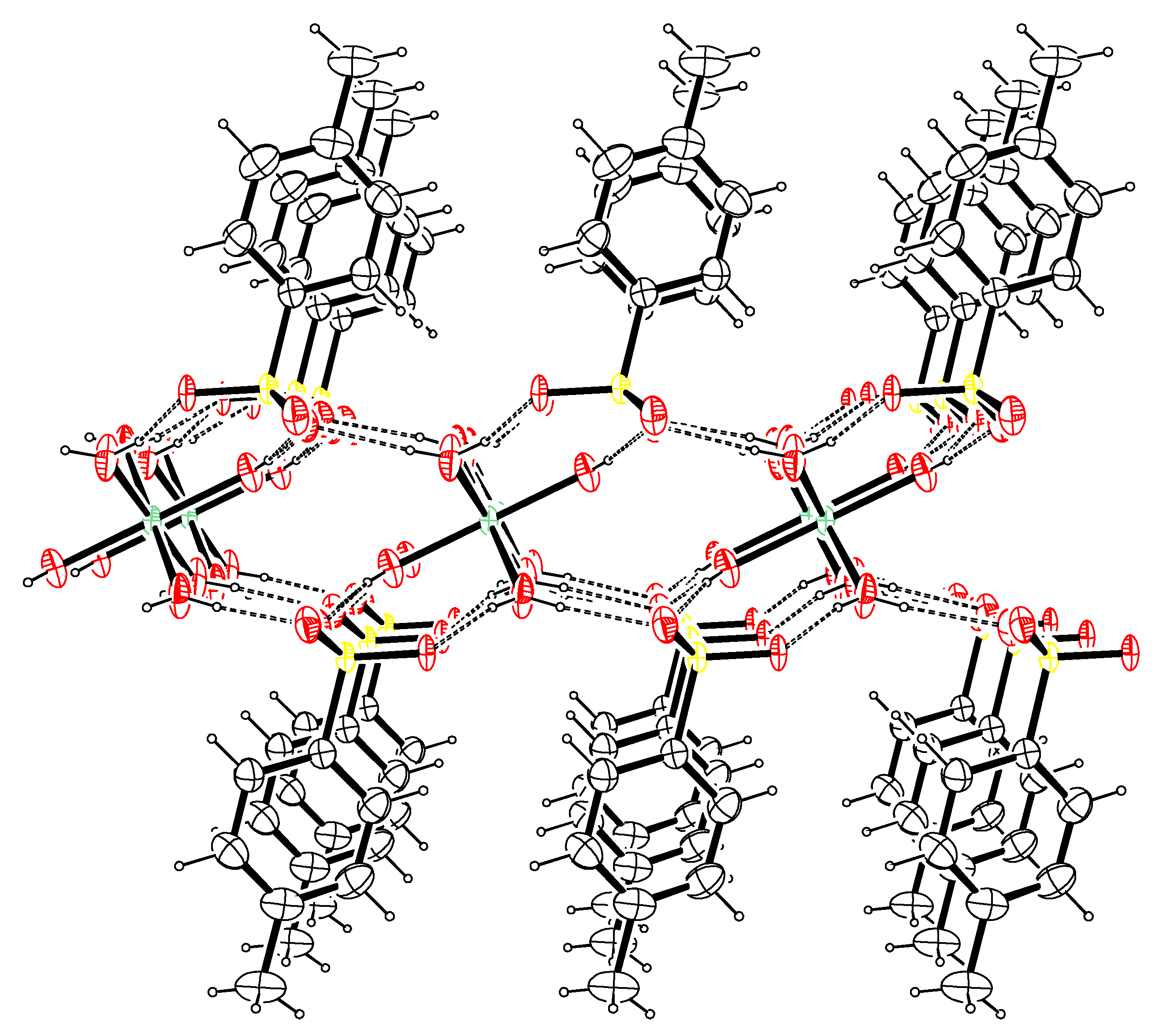
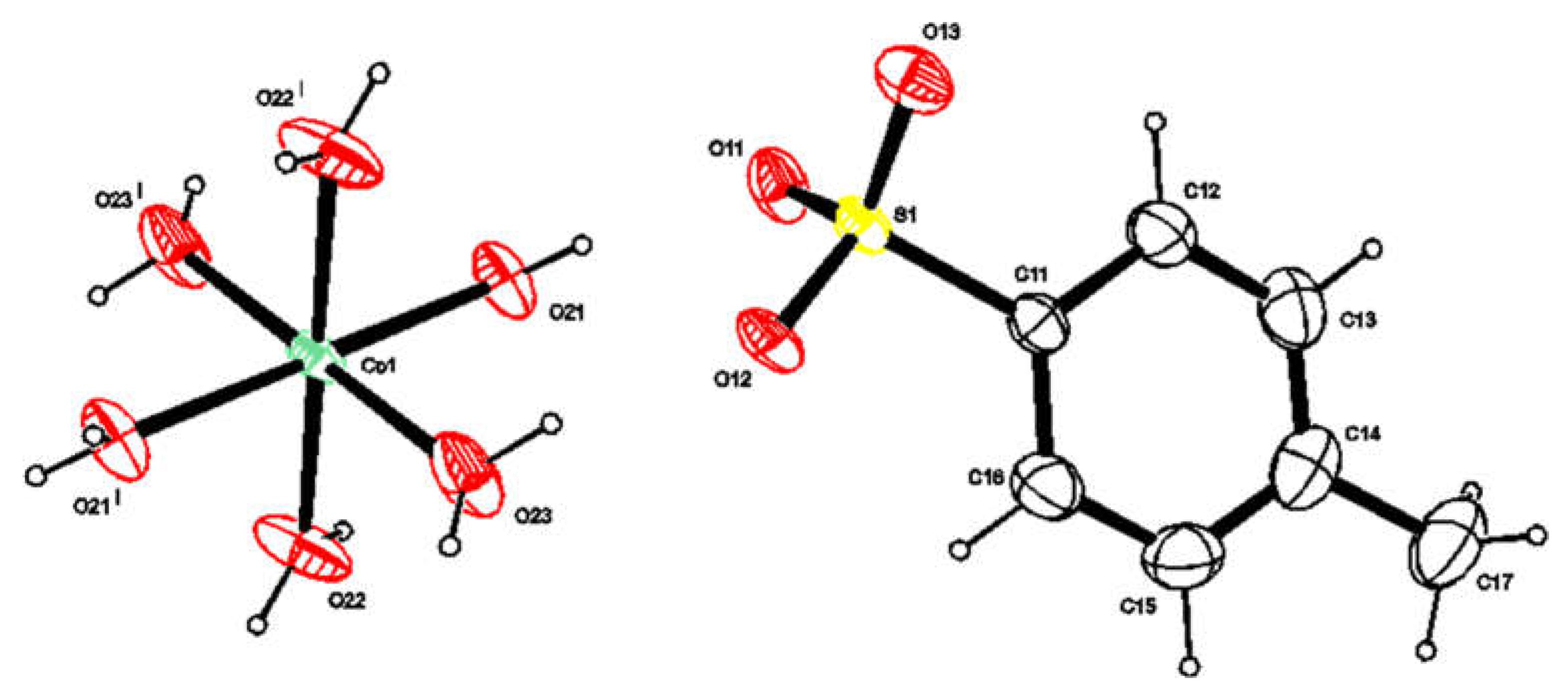
| Constant | Reaction | p q r | BIIMH2 | Ni2+ | Co2+ | Cu2+ | Zn2+ |
|---|---|---|---|---|---|---|---|
| logβ1 | LH+ = H+ + L | 0 1 1 | 5.96(5) | ||||
| logβ2 | LH22+ = 2H+ + L | 0 1 2 | 9.21(5) | ||||
| logβ110 | M2+ + L = [ML]2+ | 1 1 0 | 5.6(2) | 5.3(2) | # | 5.2(1) | |
| logβ120 | M2+ + 2L = [ML2]2+ | 1 2 0 | 10.7(1) | 10.3(3) | 10.9(2) | 10.6(1) |
| Compound | Co(RSO3)2·6H2O | [Cu(BIIM)2(RSO3)2] |
|---|---|---|
| Chemical formula | C14H26CoO12S2 | C26H26CuN8O6S2 |
| Formula weight | 509.40 | 674.24 |
| Crystal color | pink | green |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/n(14) | P21/c |
| Temperature (K) | 200 | 200 |
| Crystal size (mm−3) | 0.06 × 0.21 × 0.37 | 0.06 × 0.21 × 0.37 |
| ɑ (Å) | 6.9503(5) | 12.3968(4) |
| b (Å) | 6.2936(5) | 11.7452(3) |
| c (Å) | 25.030(2) | 9.7878(3) |
| ɑ (°) | 90 | 90 |
| β (°) | 90.944(3) | 91.721(2) |
| ɣ (°) | 90 | 90 |
| V (Å) | 1094.72(15) | 1424.49(7) |
| Z | 2 | 2 |
| Dcalc (g cm3) | 1.545 | 1.572 |
| μ/mm−1 | 1.031 | 0.970 |
| F (000) | 530 | 694 |
| Theta min–max (°) | 3.0, 28.4 | 2.4, 28.3 |
| S | 1.35 | 1.06 |
| Tot., Uniq. data, R(int) | 27465, 2747, 0.023 | 34565, 3551, 0.019 |
| Observed data [I > 2.0σ(I)] | 2638 | 3149 |
| R | 0.0653 | 0.0256 |
| Rw | 0.1486 | 0.0756 |
| Bond Lengths | |||
|---|---|---|---|
| Co(RSO3)2·6H2O | [Cu(BIIMH2)2(RSO3)2] | ||
| Co1-O21 | 2.046(3) | Cu1-O21 | 2.4302(11) |
| Co1-O22 | 2.077(4) | Cu1-N13 | 2.0216(12) |
| Co1-O23 | 2.076(4) | Cu1-N11′ | 2.0202(12) |
| S1-O11 | 1.455(4) | Cu1-N11 | 2.0202(12) |
| S1-O12 | 1.452(3) | Cu1-O21′ | 2.4302(11) |
| S1-O13 | 1.452(4) | Cu1-N13′ | 2.0216(12) |
| Bond angles | |||
| Co(RSO3)2·6H2O | [Cu(BIIMH2)2(RSO3)2] | ||
| O21-Co1-O23 | 90.81(14) | O21-Cu1-N11 | 91.34(4) |
| O21-Co1-O23_a | 89.19(14) | O21-Cu1-O21′ | 180.00 |
| O21-Co1-O21_a | 180.00 | O21-Cu1-N13′ | 91.39(4) |
| O21-Co1-O22_a | 88.90(14) | O21′-Cu1-N11 | 88.66(4) |
| O21_a-Co1-O22 | 88.90(14) | N11-Cu1-N13′ | 97.92(5) |
| O22-Co1-O22_a | 180.00 | N11′-Cu1-N13 | 97.92(5) |
| O22-Co1-O23 | 92.59(16) | O21′-Cu1-N11′ | 91.34(4) |
| O22-Co1-O23_a | 87.41(16) | N11′-Cu1-N13′ | 82.08(5) |
| O21_a-Co1-O23 | 89.19((14) | O21-Cu1-N11′ | 88.66(4) |
| O22_a-Co1-O23 | 87.41(16) | N11-Cu1-N13 | 82.08(5) |
| O23-Co1-O23_a | 180 | N11-Cu1-N11′ | 180.00 |
| O21_a-Co1-O22_a | 91.11(14) | O21′ -Cu1-N13 | 91.39(4) |
| O21_a-Co1-O23_a | 90.81(14) | N13-Cu1-N13′ | 180.00 |
| O22_a-Co1-O23_a | 92.59(16) | O21′-Cu1-N13′ | 88.61(4) |
| D―H…A | D―H (Å) | H…A (Å) | D…A (Å) | D―H…A (°) | Symmetry |
|---|---|---|---|---|---|
| O21―H21A…O11 | 0.81 | 1.92 | 2.731(5) | 173 | |
| O21―H21B…O13 | 0.77 | 1.98 | 2.752(5) | 173 | 1 + x, y, z |
| O22―H22A…O13 | 0.78 | 1.99 | 2.766(5) | 175 | 1 + x, −1 + y, z |
| O22―H22B…O12 | 0.90 | 1.93 | 2.803(5) | 165 | 1 + x, y, z |
| O23―H23A…O11 | 0.75 | 2.01 | 2.762(5) | 175 | x, −1 + y, z |
| O23―H23B…O12 | 0.85 | 1.95 | 2.790(5) | 170 |
| Cg…Cg (Å) | Dihedral Angle (°) | Symmetry | |
|---|---|---|---|
| Cg1…Cg1 | 4.924(3) | 37.5(3) | −1/2−X, −1/2 + Y, 1/2−Z |
| Cg1…Cg1 | 4.988(3) | 37.5(3) | 1/2−X, 1/2 + Y, 1/2−Z |
| D―H…A | D―H (Å) | H…A (Å) | D…A (Å) | D―H…A (°) | Symmetry |
|---|---|---|---|---|---|
| N12―H12…O23 | 0.90(2) | 1.91(2) | 2.8092(17) | 177.3(19) | 1−x, 1/2 + y, 1/2−z |
| N14―H14…O22 | 0.884(19) | 1.866(19) | 2.7332(16) | 166.7(17) | 1−x, 1/2 + y, 1/2−z |
| C13―H13…O21 | 0.95 | 2.51 | 3.1983(18) | 130 | x, 3/2−y, 1/2 + z |
| Cg…Cg (Å) | Dihedral Angle (°) | Symmetry | |
|---|---|---|---|
| Cg1…Cg2 | 3.8561(9) | 16.87(9) | X, 3/2−Y, −1/2 + Z |
| Cg1…Cg3 | 4.5989(9) | 35.97(8) | 1−X, 1−Y, 1−Z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moleko-Boyce, P.; Hosten, E.C.; Tshentu, Z.R. Sulfonato Complex Formation Rather than Sulfonate Binding in the Extraction of Base Metals with 2,2′-Biimidazole: Extraction and Complexation Studies. Crystals 2023, 13, 1350. https://doi.org/10.3390/cryst13091350
Moleko-Boyce P, Hosten EC, Tshentu ZR. Sulfonato Complex Formation Rather than Sulfonate Binding in the Extraction of Base Metals with 2,2′-Biimidazole: Extraction and Complexation Studies. Crystals. 2023; 13(9):1350. https://doi.org/10.3390/cryst13091350
Chicago/Turabian StyleMoleko-Boyce, Pulleng, Eric C. Hosten, and Zenixole R. Tshentu. 2023. "Sulfonato Complex Formation Rather than Sulfonate Binding in the Extraction of Base Metals with 2,2′-Biimidazole: Extraction and Complexation Studies" Crystals 13, no. 9: 1350. https://doi.org/10.3390/cryst13091350
APA StyleMoleko-Boyce, P., Hosten, E. C., & Tshentu, Z. R. (2023). Sulfonato Complex Formation Rather than Sulfonate Binding in the Extraction of Base Metals with 2,2′-Biimidazole: Extraction and Complexation Studies. Crystals, 13(9), 1350. https://doi.org/10.3390/cryst13091350






