Development of Cerium Oxide/Chitosan/Graphene Oxide Nanocomposite: An Investigation toward Its Biological Applications under In Vitro Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CeO2 NPs
2.3. Preparation of GO NPs
2.4. Preparation of Chitosan Nanoparticles (CS NPs)
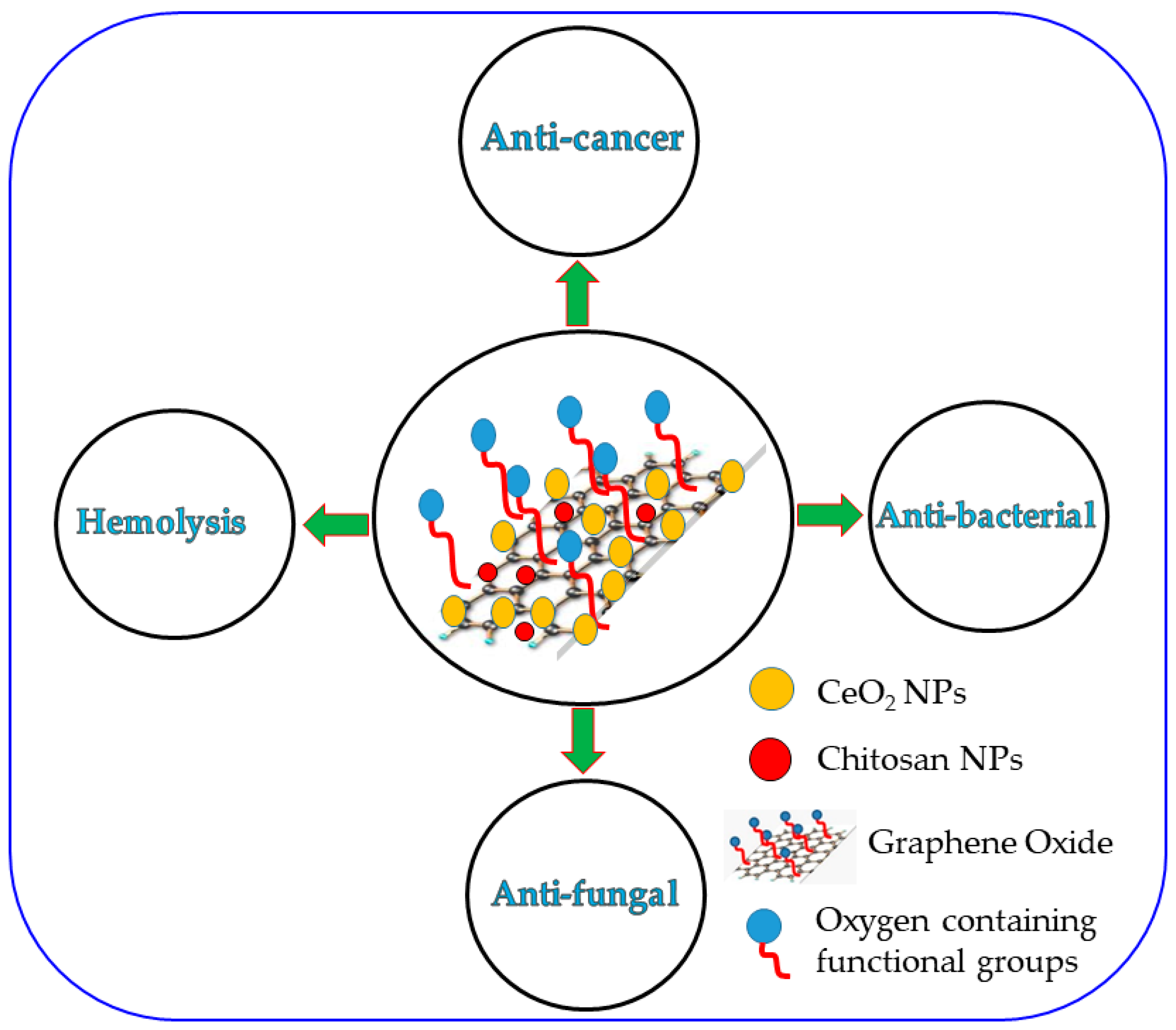
2.5. Preparation of the CeO2/CS/GO Ternary Nanocomposite
2.6. In Vitro Anti-Bacterial Assay Using the CeO2/CS/GO Ternary Nanocomposite
2.7. In Vitro Anti-Fungal Assay Using the CeO2/CS/GO Ternary Nanocomposite
2.8. In Vitro MTT Assay Using the CeO2/CS/GO Ternary Nanocomposite
2.9. In Vitro Hemolysis Assay Using the CeO2/CS/GO Ternary Nanocomposite
3. Results
3.1. XRD Analysis
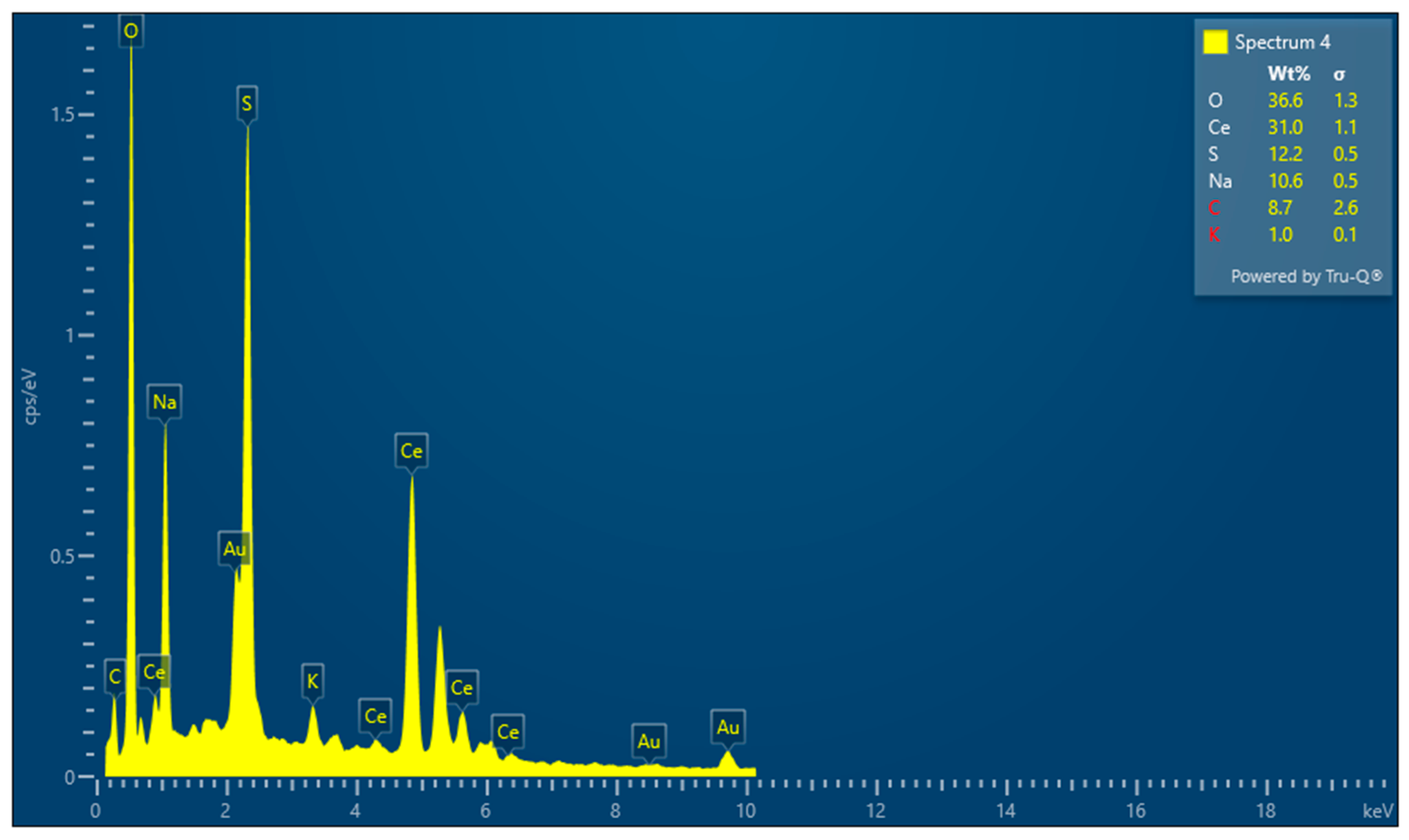
3.2. Surface Morphology
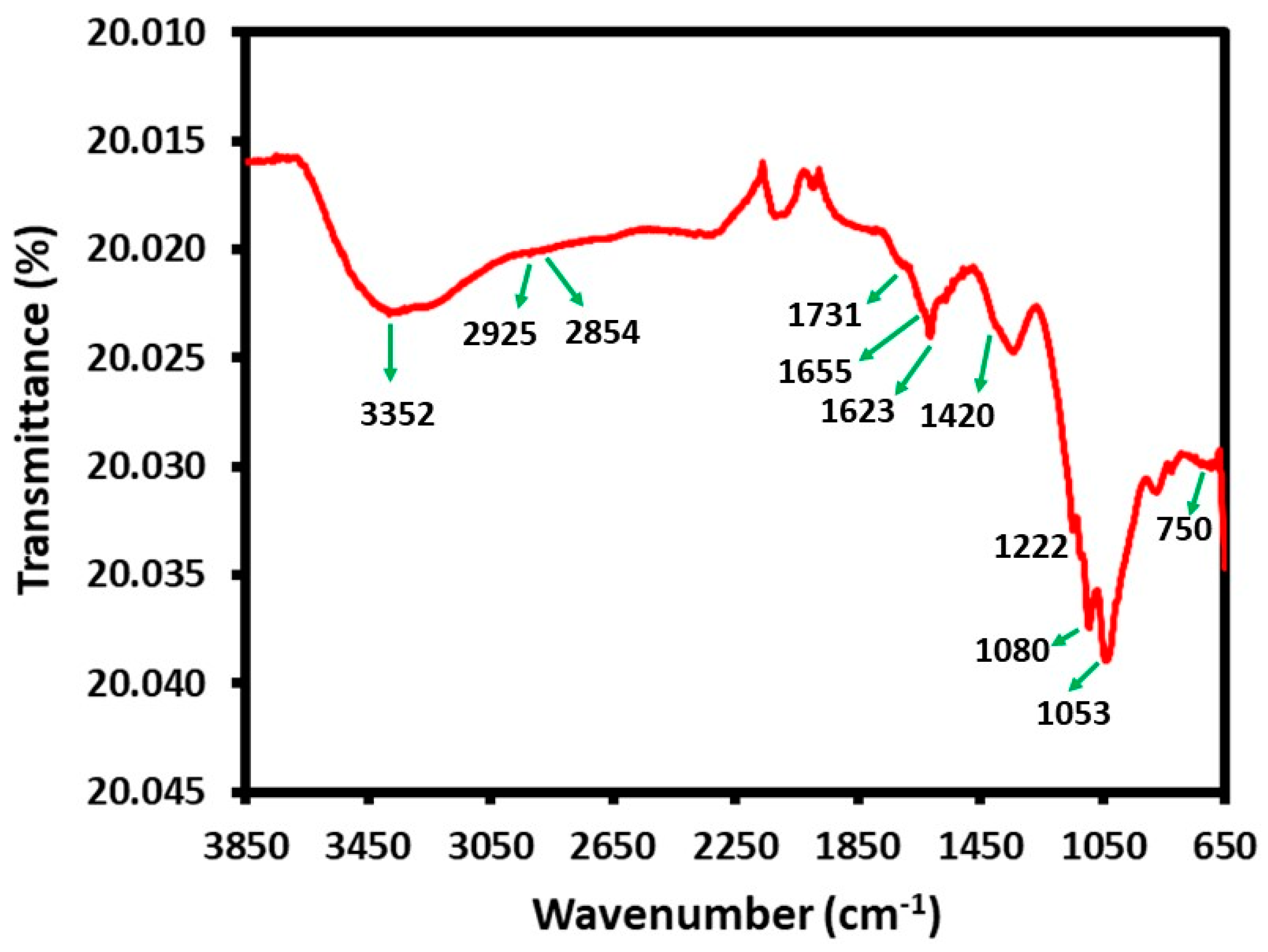
3.3. FT-IR Analysis
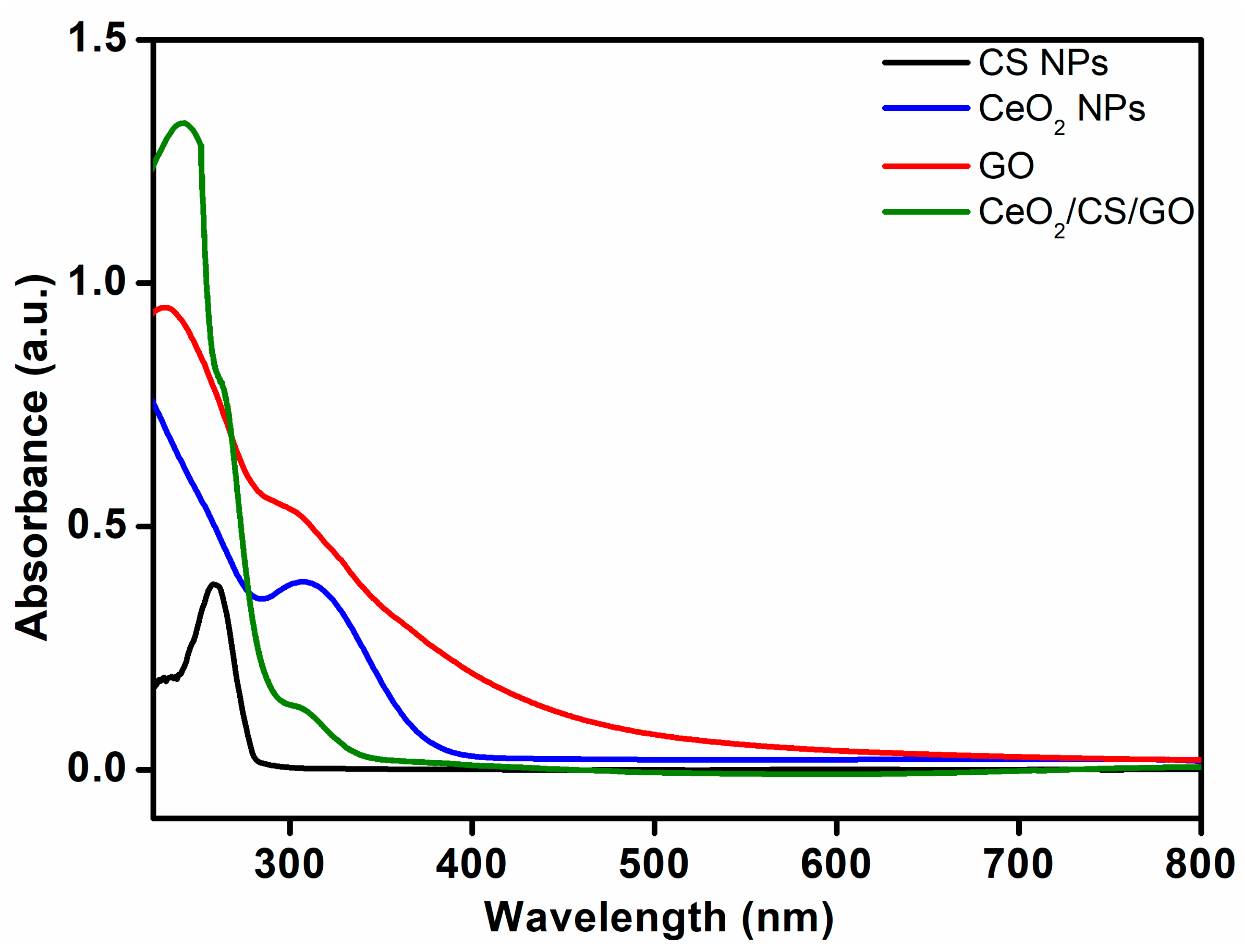
3.4. UV-Vis Analysis
3.5. Anti-Bacterial Assay
3.6. Anti-Fungal Assay
3.7. Hemolysis Assay
3.8. Anti-Cancer Activity of the CeO2/CS/GO Ternary Nanocomposite Using MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, M.; Vahidi, O.; Moghbeli, M.; Ahmadi, S.; Asadnia, M.; Akhavan, O.; Seidi, F.; Rabiee, M.; Saeb, M.R.; Webster, T.J. Customizing nano-chitosan for sustainable drug delivery. J. Control Release 2022, 350, 175–192. [Google Scholar] [CrossRef]
- Emilian Leucuta, S. Nanotechnology for delivery of drugs and biomedical applications. Curr. Clin. Pharmacol. 2010, 5, 257–280. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J. Cancer 2017, 8, 3131. [Google Scholar] [CrossRef] [PubMed]
- Maleki, P.; Nemati, F.; Gholoobi, A.; Hashemzadeh, A.; Sabouri, Z.; Darroudi, M. Green facile synthesis of silver-doped cerium oxide nanoparticles and investigation of their cytotoxicity and antibacterial activity. Inorg. Chem. Commun. 2021, 131, 108762. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium oxide nanoparticles: A brief review of their synthesis methods and biomedical applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Sivamohan, R.; Ohsuna, T.; Takahashi, H.; Tohji, K. Ultraviolet absorption spectra of CeO2 nano-particles. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 1999; pp. 439–445. [Google Scholar]
- Saranya, J.; Sreeja, B.; Padmalaya, G.; Radha, S.; Manikandan, T. Ultrasonic assisted cerium oxide/graphene oxide hybrid: Preparation, anti-proliferative, apoptotic induction and G2/M cell cycle arrest in HeLa cell lines. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2666–2676. [Google Scholar] [CrossRef]
- Çiplak, Z.; Yildiz, N.; Çalimli, A. Investigation of graphene/Ag nanocomposites synthesis parameters for two different synthesis methods. Fuller. Nanotub. Carbon Nanostructures 2015, 23, 361–370. [Google Scholar] [CrossRef]
- Saranya, J.; Saminathan, P.; Ankireddy, S.R.; Shaik, M.R.; Khan, M.; Khan, M.; Shaik, B. Cerium Oxide/Graphene Oxide Hybrid: Synthesis, Characterization, and Evaluation of Anticancer Activity in a Breast Cancer Cell Line (MCF-7). Biomedicines 2023, 11, 531. [Google Scholar] [CrossRef]
- Pang, L.; Dai, C.; Bi, L.; Guo, Z.; Fan, J. Biosafety and antibacterial ability of graphene and graphene oxide in vitro and in vivo. Nanoscale Res. Lett. 2017, 12, 564. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Kayani, F.B.; Rafique, S.; Akram, R.; Hussain, M.; Raja, K.; Khan, J.S. Fabrication of novel chitosan@Ag/CeO2 hybrid nanocomposites for the study of antibacterial activity. Phys. E Low-Dimens. Syst. Nanostructures 2023, 149, 115683. [Google Scholar] [CrossRef]
- Panda, P.K.; Dash, P.; Yang, J.-M.; Chang, Y.-H. Development of chitosan, graphene oxide, and cerium oxide composite blended films: Structural, physical, and functional properties. Cellulose 2022, 29, 2399–2411. [Google Scholar] [CrossRef]
- Mali, S.; Nune, K.; Misra, R. Biomimetic nanostructured hydroxyapatite coatings on metallic implant materials. Mater. Technol. 2016, 31, 782–790. [Google Scholar] [CrossRef]
- Pimenta, J.A.; Zaparolli, D.; Pécora, J.D.; Cruz-Filho, A.M. Chitosan: Effect of a new chelating agent on the microhardness of root dentin. Braz. Dent. J. 2012, 23, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Alimirzaei, F.; Vasheghani-Farahani, E.; Ghiaseddin, A.; Soleimani, M. pH-Sensitive Chitosan Hydrogel with Instant Gelation for Myocardial Regeneration. J. Tissue Sci. Eng. 2017, 8, 3. [Google Scholar]
- de Sousa Victor, R.; Marcelo da Cunha Santos, A.; Viana de Sousa, B.; de Araújo Neves, G.; Navarro de Lima Santana, L.; Rodrigues Menezes, R. A review on Chitosan’s uses as biomaterial: Tissue engineering, drug delivery systems and cancer treatment. Materials 2020, 13, 4995. [Google Scholar]
- Anjum, S.; Arora, A.; Alam, M.; Gupta, B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int. J. Pharm. 2016, 508, 92–101. [Google Scholar] [CrossRef]
- Zaboon, M.H.; Saleh, A.A.; Al-Lami, H.S. Synthesis, characterization and cytotoxicity investigation of chitosan-amino acid derivatives nanoparticles in human breast cancer cell lines. J. Mex. Chem. Soc. 2021, 65, 178–188. [Google Scholar] [CrossRef]
- Appu, M.; Wu, H.; Chen, H.; Huang, J. Tea polyphenols mediated biogenic synthesis of chitosan-coated cerium oxide (CS/CeO2) nanocomposites and their potent antimicrobial capabilities. Environ. Sci. Pollut. Res. 2023, 30, 42575–42586. [Google Scholar] [CrossRef]
- Kasinathan, K.; Murugesan, B.; Pandian, N.; Mahalingam, S.; Selvaraj, B.; Marimuthu, K. Synthesis of biogenic chitosan-functionalized 2D layered MoS2 hybrid nanocomposite and its performance in pharmaceutical applications: In-Vitro antibacterial and anticancer activity. Int. J. Biol. Macromol. 2020, 149, 1019–1033. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Homayouni Tabrizi, M.; Ardalan, T.; Roumi, S. Cerium oxide nanoparticles-loaded on chitosan for the investigation of anticancer properties. Mater. Technol. 2022, 37, 1439–1449. [Google Scholar] [CrossRef]
- Adil, S.F.; Shaik, M.R.; Nasr, F.A.; Alqahtani, A.S.; Ahmed, M.Z.; Qamar, W.; Kuniyil, M.; Almutairi, A.; Alwarthan, A.; Siddiqui, M.R.H. Enhanced apoptosis by functionalized highly reduced graphene oxide and gold nanocomposites in MCF-7 breast cancer cells. ACS Omega 2021, 6, 15147–15155. [Google Scholar] [CrossRef] [PubMed]
- Ioelovich, M. Crystallinity and hydrophility of chitin and chitosan. J. Chem. 2014, 3, 7–14. [Google Scholar]
- Nurhasanah, I.; Safitri, W.; Arifin, Z.; Subagio, A.; Windarti, T. Antioxidant Activity and Dose Enhancement Factor of CeO2 Nanoparticles Synthesized by Precipitation Method; IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2018; p. 012031. [Google Scholar]
- Wang, S.; Zhang, Y.; Ma, H.-L.; Zhang, Q.; Xu, W.; Peng, J.; Li, J.; Yu, Z.-Z.; Zhai, M. Ionic-liquid-assisted facile synthesis of silver nanoparticle-reduced graphene oxide hybrids by gamma irradiation. Carbon 2013, 55, 245–252. [Google Scholar] [CrossRef]
- Sanmugam, A.; Abbishek, S.; Kumar, S.L.; Sairam, A.B.; Palem, V.V.; Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Vikraman, D. Synthesis of chitosan based reduced graphene oxide-CeO2 nanocomposites for drug delivery and antibacterial applications. J. Mech. Behav. Biomed. Mater. 2023, 145, 106033. [Google Scholar] [CrossRef]
- Senthilkumar, R.; Bhuvaneshwari, V.; Ranjithkumar, R.; Sathiyavimal, S.; Malayaman, V.; Chandarshekar, B. Synthesis, characterization and antibacterial activity of hybrid chitosan-cerium oxide nanoparticles: As a bionanomaterials. Int. J. Biol. Macromol. 2017, 104, 1746–1752. [Google Scholar] [CrossRef]
- Manikandan, A.; Manikandan, E.; Meenatchi, B.; Vadivel, S.; Jaganathan, S.; Ladchumananandasivam, R.; Henini, M.; Maaza, M.; Aanand, J.S. Rare earth element (REE) lanthanum doped zinc oxide (La: ZnO) nanomaterials: Synthesis structural optical and antibacterial studies. J. Alloys Compd. 2017, 723, 1155–1161. [Google Scholar] [CrossRef]
- Gudata, L.; Saka, A.; Tesfaye, J.L.; Shanmugam, R.; Dwarampudi, L.P.; Nagaprasad, N.; Stalin, B.; Krishnaraj, R. Investigation of TiO2 nanoparticles using leaf extracts of Lippia adoensis (Kusaayee) for antibacterial activity. J. Nanomater. 2022, 2022, 3881763. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef]
- Dutta, T.; Sarkar, R.; Pakhira, B.; Ghosh, S.; Sarkar, R.; Barui, A.; Sarkar, S. ROS generation by reduced graphene oxide (rGO) induced by visible light showing antibacterial activity: Comparison with graphene oxide (GO). RSC Adv. 2015, 5, 80192–80195. [Google Scholar] [CrossRef]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the mechanism of antibacterial activity of ZnO: Surface defects mediated reactive oxygen species even in the dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical applications of reactive oxygen species generation by metal nanoparticles. Materials 2020, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon 2012, 50, 1853–1860. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.-H.; Liu, Y.; Wang, H. Superior antibacterial activity of zinc oxide/graphene oxide composites originating from high zinc concentration localized around bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- Jannesari, M.; Akhavan, O.; Hosseini, H.R.M.; Bakhshi, B. Oxygen-rich graphene/ZnO2-Ag nanoframeworks with pH-switchable catalase/peroxidase activity as O2 nanobubble-self generator for bacterial inactivation. J. Colloid Interface Sci. 2023, 637, 237–250. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; Zhai, X.; Luo, F.; Du, Y.; Yan, C. Antibacterial mechanism and activity of cerium oxide nanoparticles. Sci. China Mater. 2019, 62, 1727–1739. [Google Scholar] [CrossRef]
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci. Rep. 2016, 6, 28443. [Google Scholar] [CrossRef]
- Sawai, J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. J. Microbiol. Methods 2003, 54, 177–182. [Google Scholar] [CrossRef]
- Meng, D.; Garba, B.; Ren, Y.; Yao, M.; Xia, X.; Li, M.; Wang, Y. Antifungal activity of chitosan against Aspergillus ochraceus and its possible mechanisms of action. Int. J. Biol. Macromol. 2020, 158, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Egorova, M.N.; Tarasova, L.A.; Vasilieva, F.D.; Akhremenko, Y.A.; Smagulova, S.A. Antimicrobial activity of graphene oxide sheets. In Proceedings of the AIP Conference Proceedings, Yakutsk, Russia, 18–23 June 2018; AIP Publishing: Long Island, NY, USA. [Google Scholar]
- Cao, M.; Wang, P.; Ao, Y.; Wang, C.; Hou, J.; Qian, J. Visible light activated photocatalytic degradation of tetracycline by a magnetically separable composite photocatalyst: Graphene oxide/magnetite/cerium-doped titania. J. Colloid Interface Sci. 2016, 467, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Taran, M. Fabrication, characterization, and antifungal activity of sodium hyaluronate-TiO2 bionanocomposite against Aspergillus niger. Mater. Lett. 2017, 207, 113–116. [Google Scholar] [CrossRef]
- Hasanin, M.; Hashem, A.H.; Lashin, I.; Hassan, S.A. In vitro improvement and rooting of banana plantlets using antifungal nanocomposite based on myco-synthesized copper oxide nanoparticles and starch. Biomass Convers. Biorefin. 2021, 13, 8865–8875. [Google Scholar] [CrossRef]
- Roopan, S.M.; Priya, D.D.; Shanavas, S.; Acevedo, R.; Al-Dhabi, N.A.; Arasu, M.V. CuO/C nanocomposite: Synthesis and optimization using sucrose as carbon source and its antifungal activity. Mater. Sci. Eng. C 2019, 101, 404–414. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Physicochemical and antifungal properties of bio-nanocomposite film based on gelatin-chitin nanoparticles. Int. J. Biol. Macromol. 2017, 97, 373–381. [Google Scholar] [CrossRef]
- Anitha, P.; Sakthivel, P. Microwave Assisted Synthesis and Characterization of Silver Nanoparticles Using Citrullus Lanatus Leaf Extract and Its Anti-Inflammatory Activity Against Human Blood Cells. Int. J. Adv. Eng. Nano Technol. 2016, 3, 3. [Google Scholar]
- Rajeshkumar, S.; Menon, S.; Ponnanikajamideen, M.; Ali, D.; Arunachalam, K. Anti-inflammatory and antimicrobial potential of Cissus quadrangularis-assisted copper oxide nanoparticles. J. Nanomater. 2021, 2021, 5742981. [Google Scholar] [CrossRef]
- Kermani, G.; Karimi, E.; Tabrizi, M.H. Hybrid nanoarchitectonics of chitosan-cerium oxide nanoparticles for anticancer potentials. J. Inorg. Organomet. Polym. Mater. 2022, 32, 2591–2599. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.-T.; Liu, Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef]
- Jermy, R.; Ravinayagam, V.; Alamoudi, W.; Almohazey, D.; Elanthikkal, S.; Dafalla, H.; Rehman, S.; Chandrasekar, G.; Baykal, A. Tuning pH sensitive chitosan and cisplatin over spinel ferrite/silica nanocomposite for anticancer activity in MCF-7 cell line. J. Drug Deliv. Sci. Technol. 2020, 57, 101711. [Google Scholar] [CrossRef]
- Doghish, A.S.; El-Sayyad, G.S.; Sallam, A.-A.M.; Khalil, W.F.; El Rouby, W.M. Graphene oxide and its nanocomposites with EDTA or chitosan induce apoptosis in MCF-7 human breast cancer. RSC Adv. 2021, 11, 29052–29064. [Google Scholar] [CrossRef] [PubMed]
- Atif, M.; Iqbal, S.; Fakhar-E-Alam, M.; Ismail, M.; Mansoor, Q.; Mughal, L.; Aziz, M.H.; Hanif, A.; Farooq, W. Manganese-doped cerium oxide nanocomposite induced photodynamic therapy in MCF-7 cancer cells and antibacterial activity. Biomed Res. Int. 2019, 2019, 7156828. [Google Scholar] [CrossRef] [PubMed]



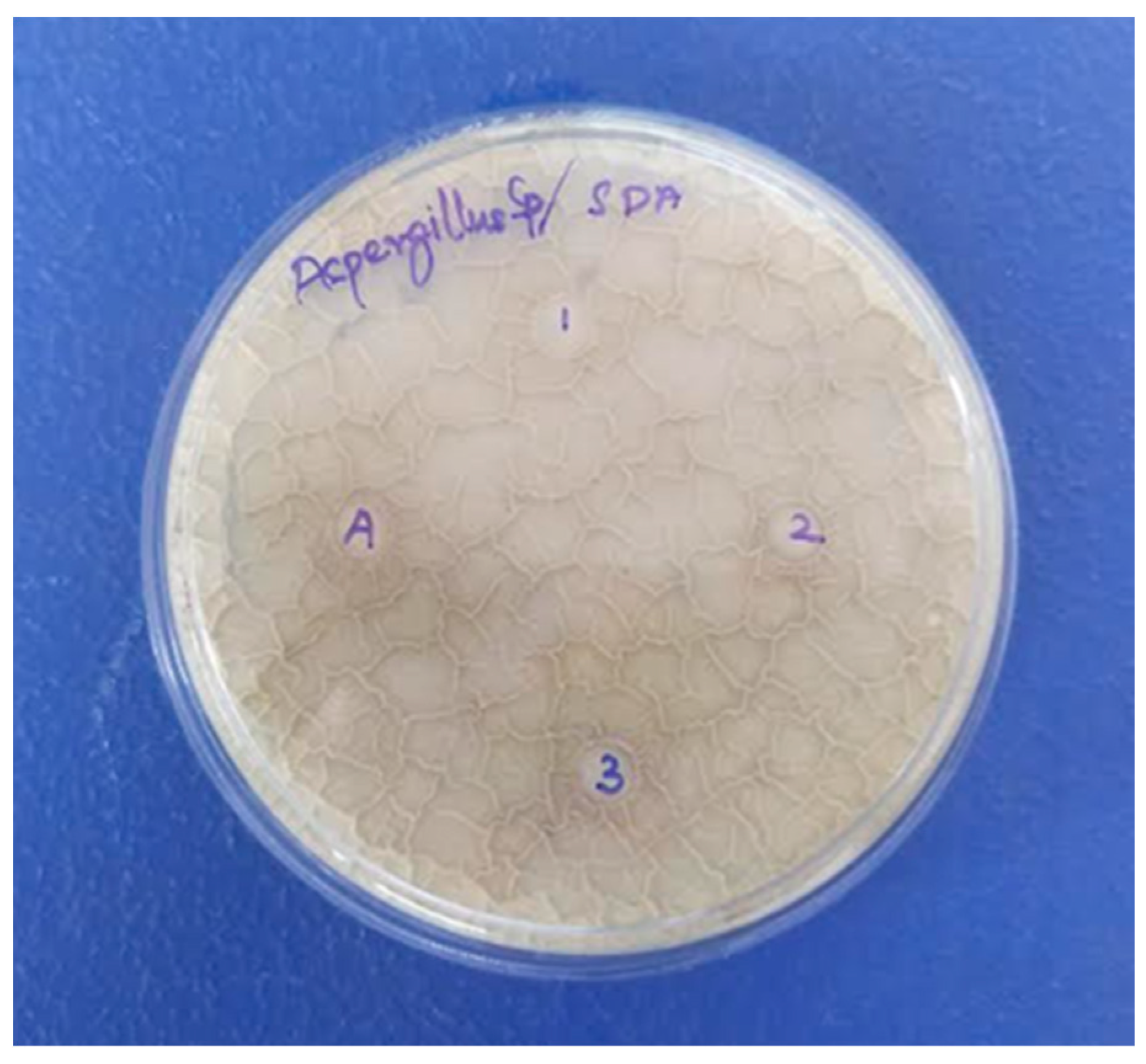

| S. No. | Microorganisms | Proteus Species (ZOI in mm) | Salmonella Species (ZOI in mm) | E. coli Species (ZOI in mm) | Staphylococcus Species (ZOI in mm) | Bacillus Species (ZOI in mm) |
|---|---|---|---|---|---|---|
| 1 | CeO2/CS/GO ternary nanocomposite (1000 µg/disc) | 10 | 10 | 10 | 10 | 8 |
| 2 | CeO2/CS/GO ternary nanocomposite (750 µg/disc) | 7 | 9 | 9 | 9 | 6 |
| 3 | CeO2/CS/GO ternary nanocomposite (500 µg/disc) | 5 | 8 | 8 | 7 | 5 |
| A | Ampicillin (antibiotic) | 15 | 20 | 15 | 15 | 13 |
| S. No. | Maximum Concentration of Nanocomposite (1000 µg/disc) | Aspergillus Species (ZOI in mm) | References |
|---|---|---|---|
| 1 | Sodium hydranate titanium dioxide bio-nanocomposite | 13.3 | [46] |
| 2 | Polymer nanocomposite | 15 | [47] |
| 3 | CuO/C nanocomposite | 12 | [48] |
| 4 | Gelatin chitin nanocomposite | 10 | [49] |
| 5 | CeO2/GO/CS ternary nanocomposite | 10 | This work |
| S. No. | Nanocomposite | % Live Cells at 1000 µg/mL | References |
|---|---|---|---|
| 1 | CeO2/GO nanocomposite | 19.44 | [9] |
| 2 | Cu Fe2O4/HYPS nanocomposite | 21.23 | [54] |
| 3 | GO/CS nanocomposite | 23.8 | [55] |
| 4 | Mn/CeO2 nanocomposite | 22.58 | [56] |
| 5 | CeO2/GO/CS ternary nanocomposite | 18.84 | This work |
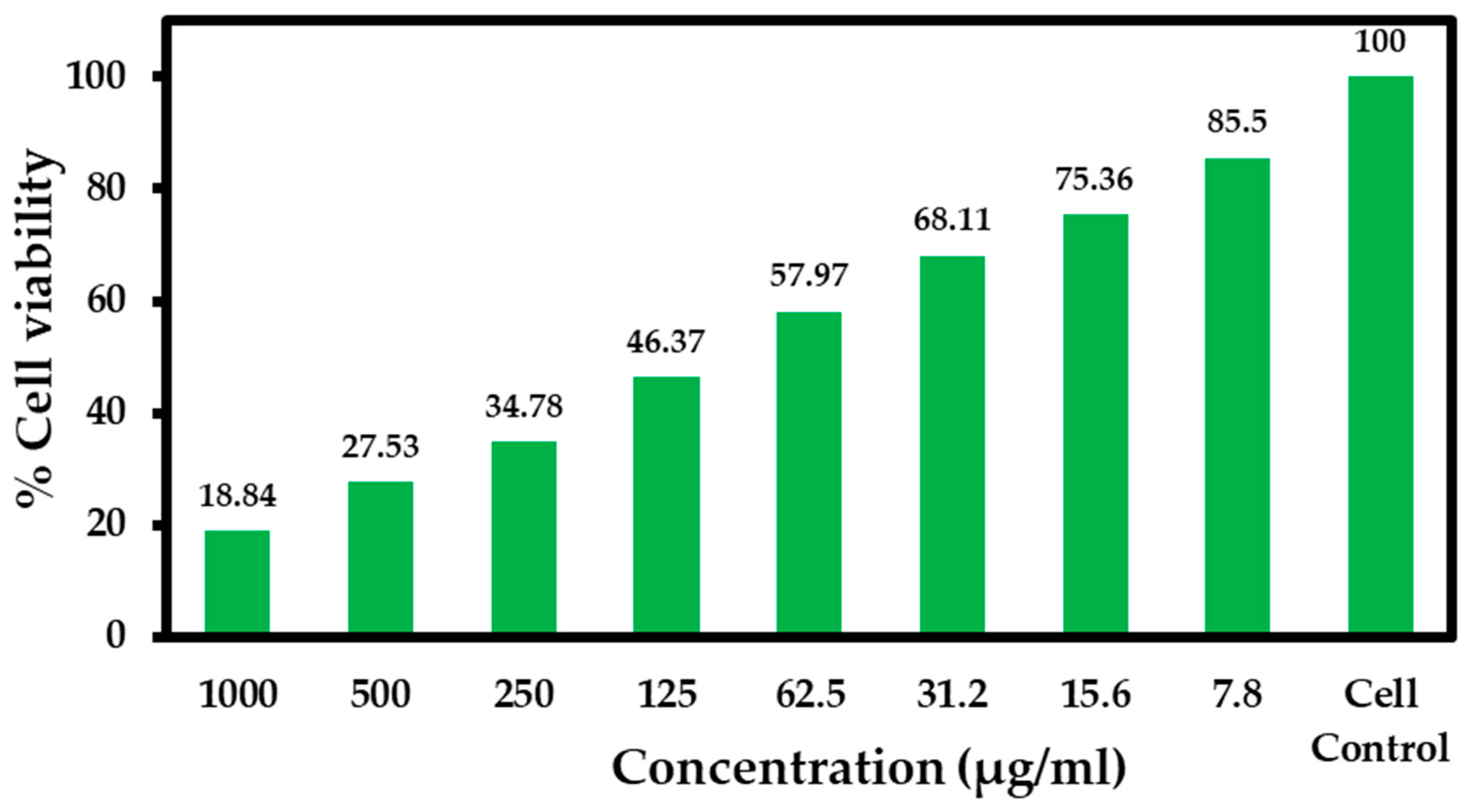
| S. No | Concentration (µg/mL) | Cell Viability (% Live Cells) |
|---|---|---|
| 1. | 1000 | 18.84 ± 0.48 |
| 2. | 500 | 27.53 ± 0.31 |
| 3. | 250 | 34.78 ± 0.35 |
| 4. | 125 | 46.37 ± 0.12 |
| 5. | 62.5 | 57.97 ± 0.26 |
| 6. | 31.2 | 68.11 ± 0.27 |
| 7. | 15.6 | 75.36 ± 1.19 |
| 8. | 7.8 | 85.50 ± 0.34 |
| 9. | Cell control | 100 ± 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saranya, J.; Preethi, S.; Shaik, M.R.; Khan, M.; Khan, M.; Shaik, B. Development of Cerium Oxide/Chitosan/Graphene Oxide Nanocomposite: An Investigation toward Its Biological Applications under In Vitro Conditions. Crystals 2023, 13, 1393. https://doi.org/10.3390/cryst13091393
Saranya J, Preethi S, Shaik MR, Khan M, Khan M, Shaik B. Development of Cerium Oxide/Chitosan/Graphene Oxide Nanocomposite: An Investigation toward Its Biological Applications under In Vitro Conditions. Crystals. 2023; 13(9):1393. https://doi.org/10.3390/cryst13091393
Chicago/Turabian StyleSaranya, J., S. Preethi, Mohammed Rafi Shaik, Merajuddin Khan, Mujeeb Khan, and Baji Shaik. 2023. "Development of Cerium Oxide/Chitosan/Graphene Oxide Nanocomposite: An Investigation toward Its Biological Applications under In Vitro Conditions" Crystals 13, no. 9: 1393. https://doi.org/10.3390/cryst13091393








