Production of (Fe,Co)Si2 and (Fe.Mn)Si2 Thermoelectric Materials by Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mechanical Alloying (MA)
2.2. Spark Plasma Sintering
2.3. Characterization
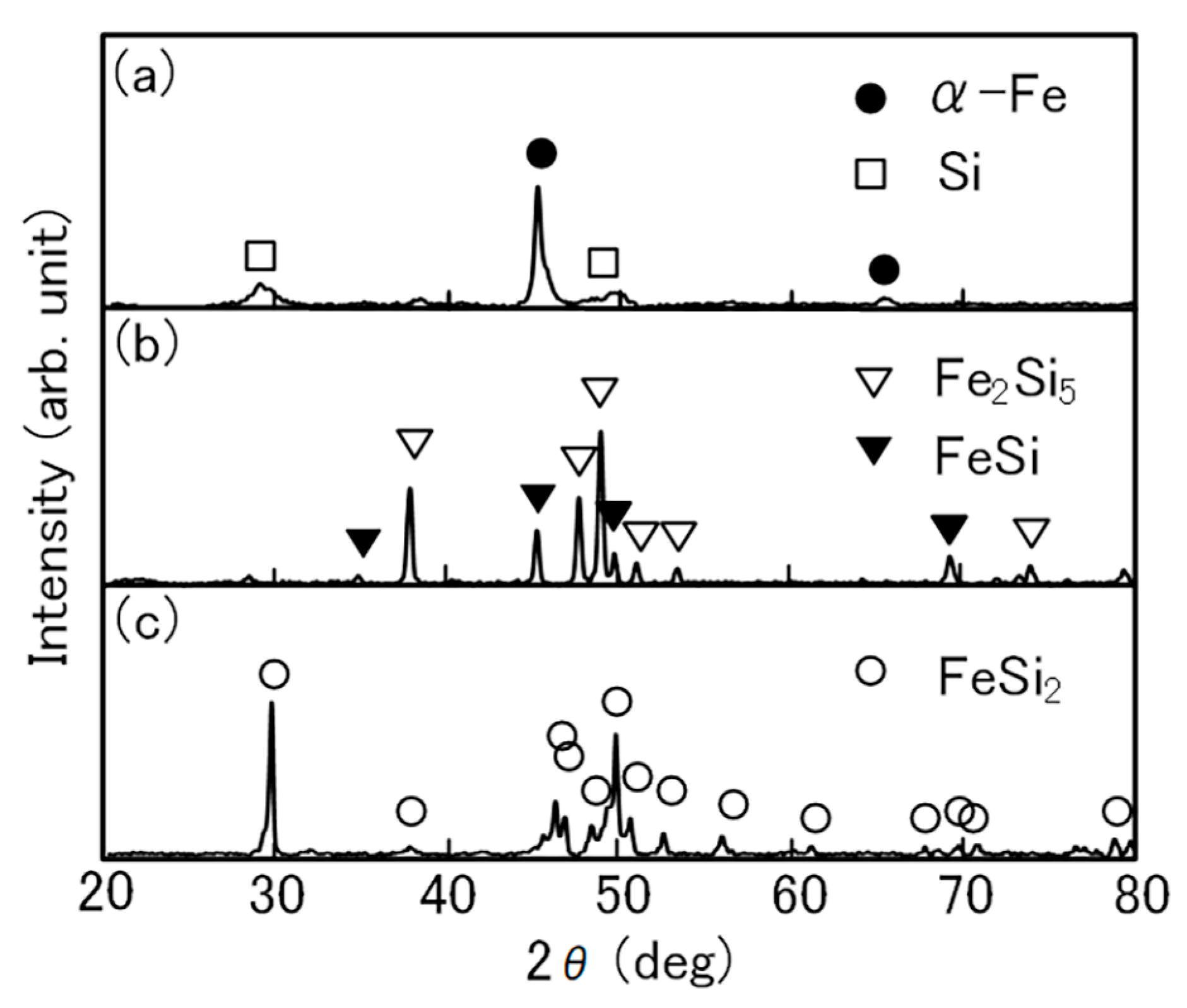
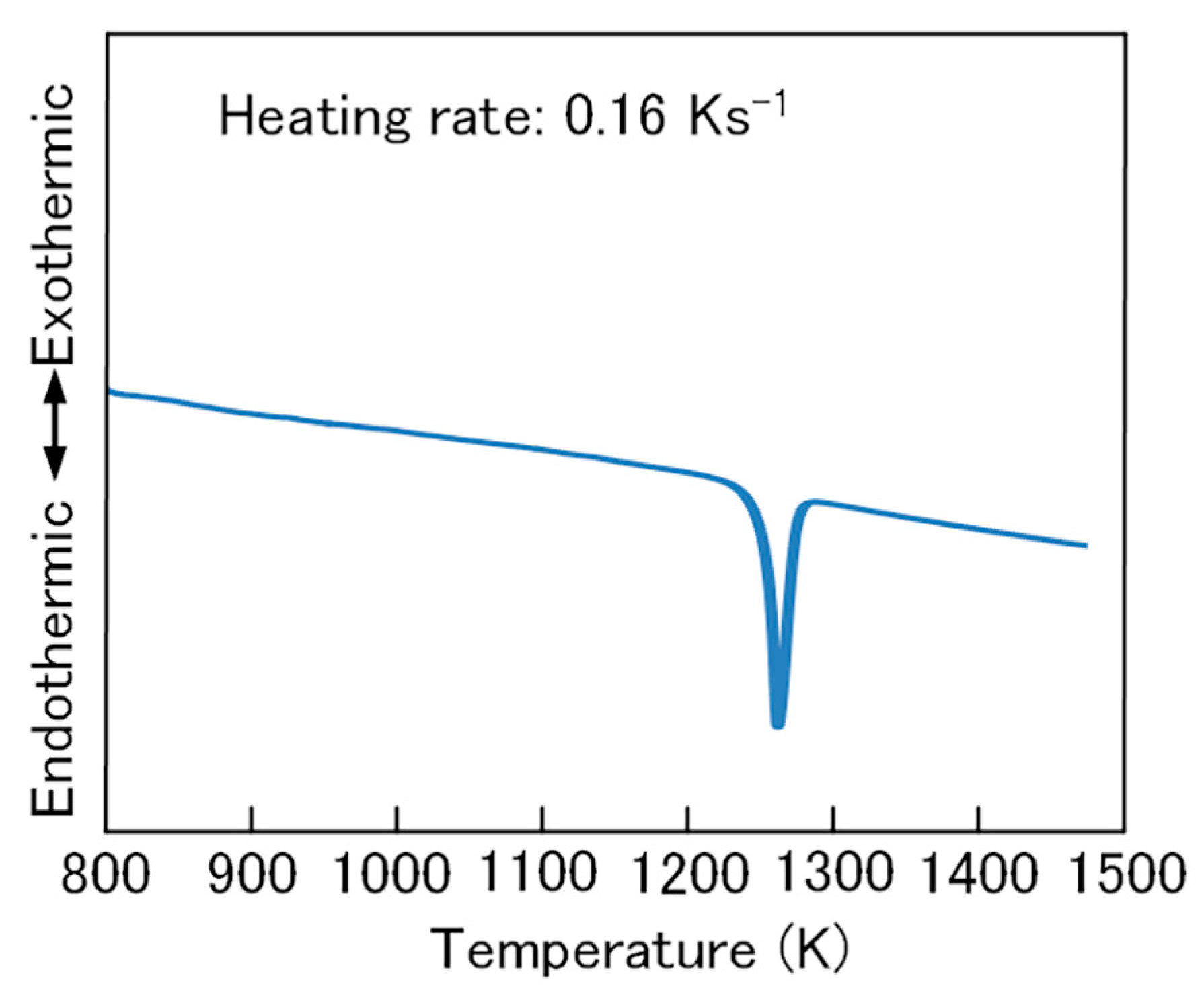
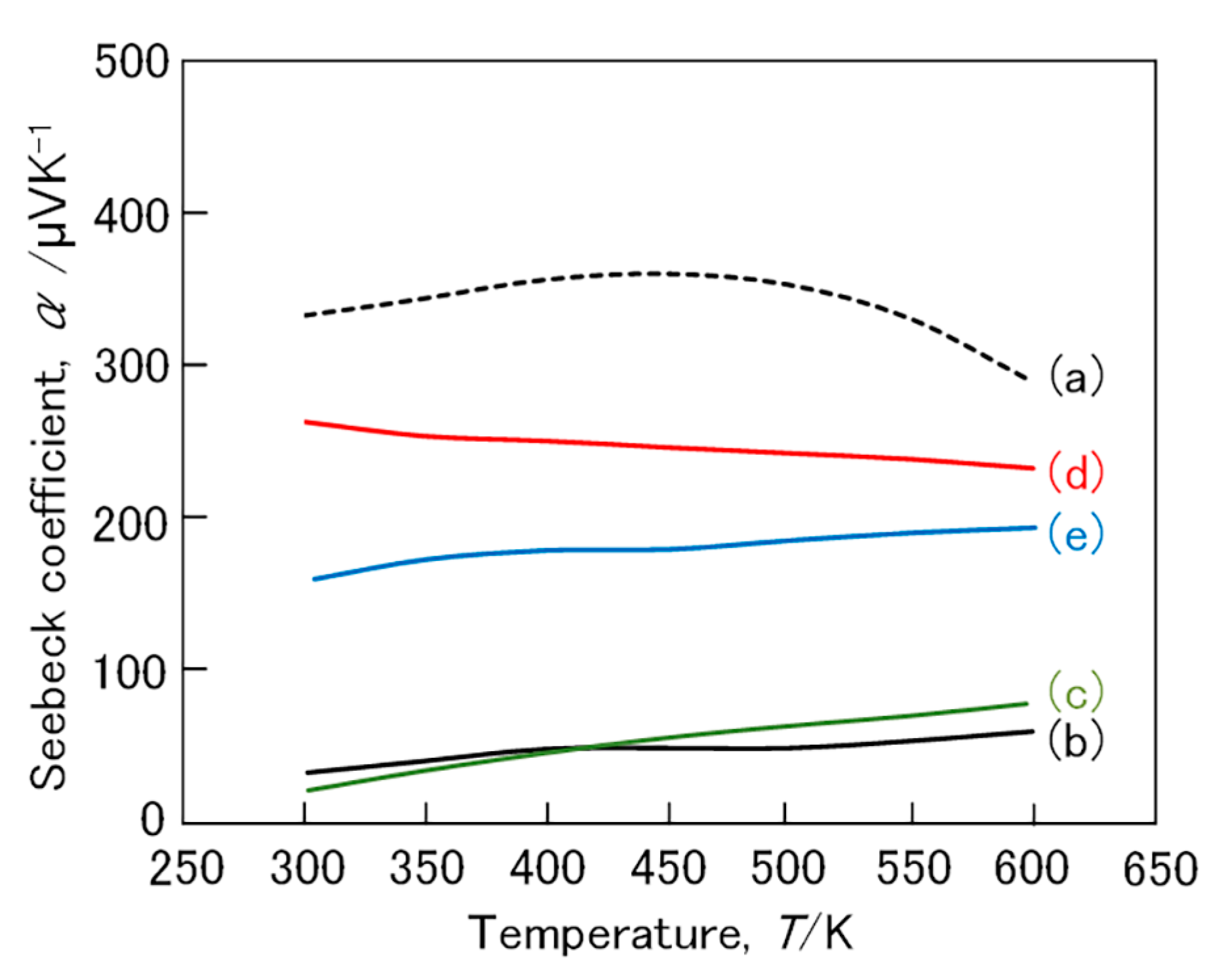
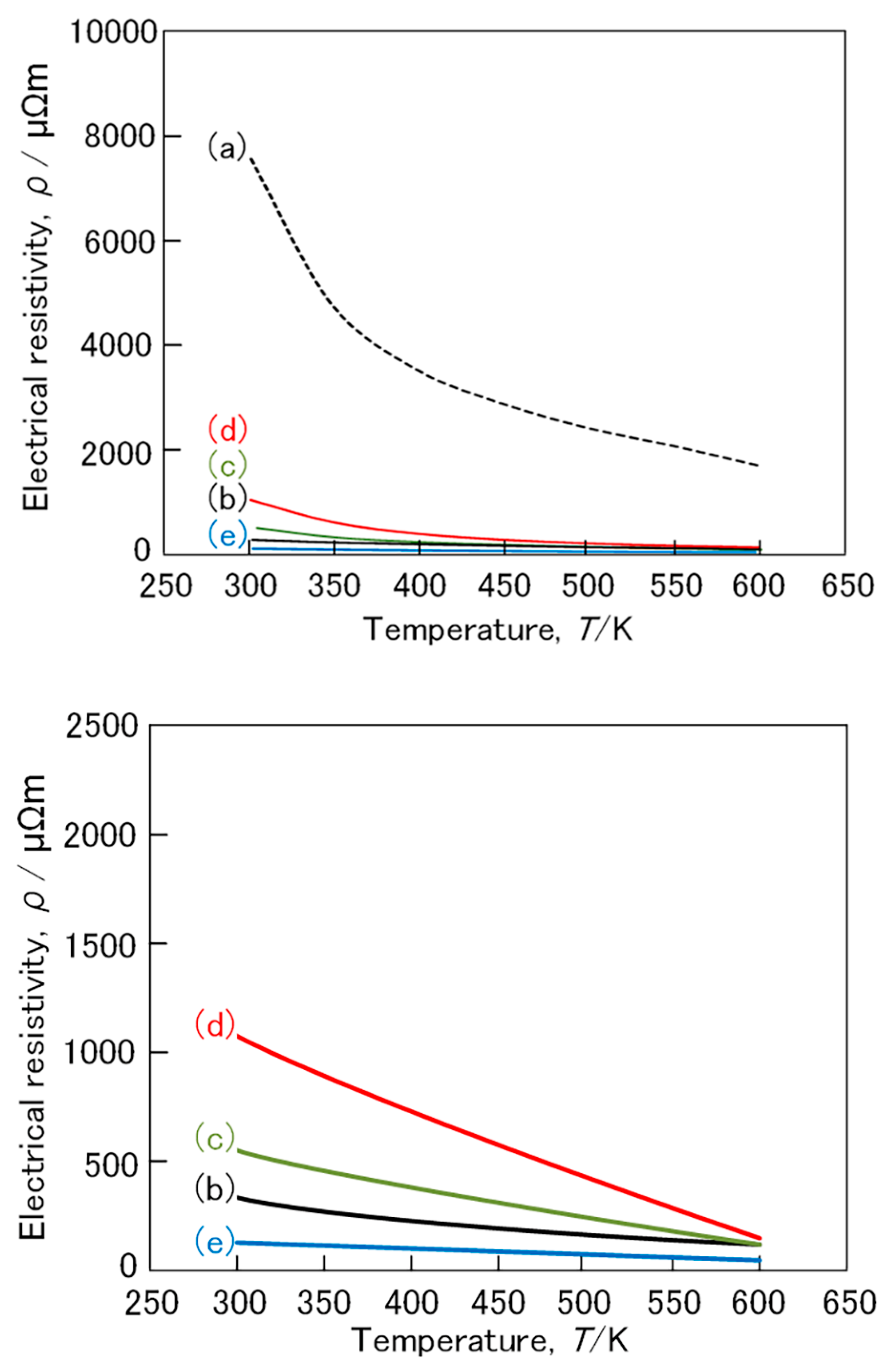
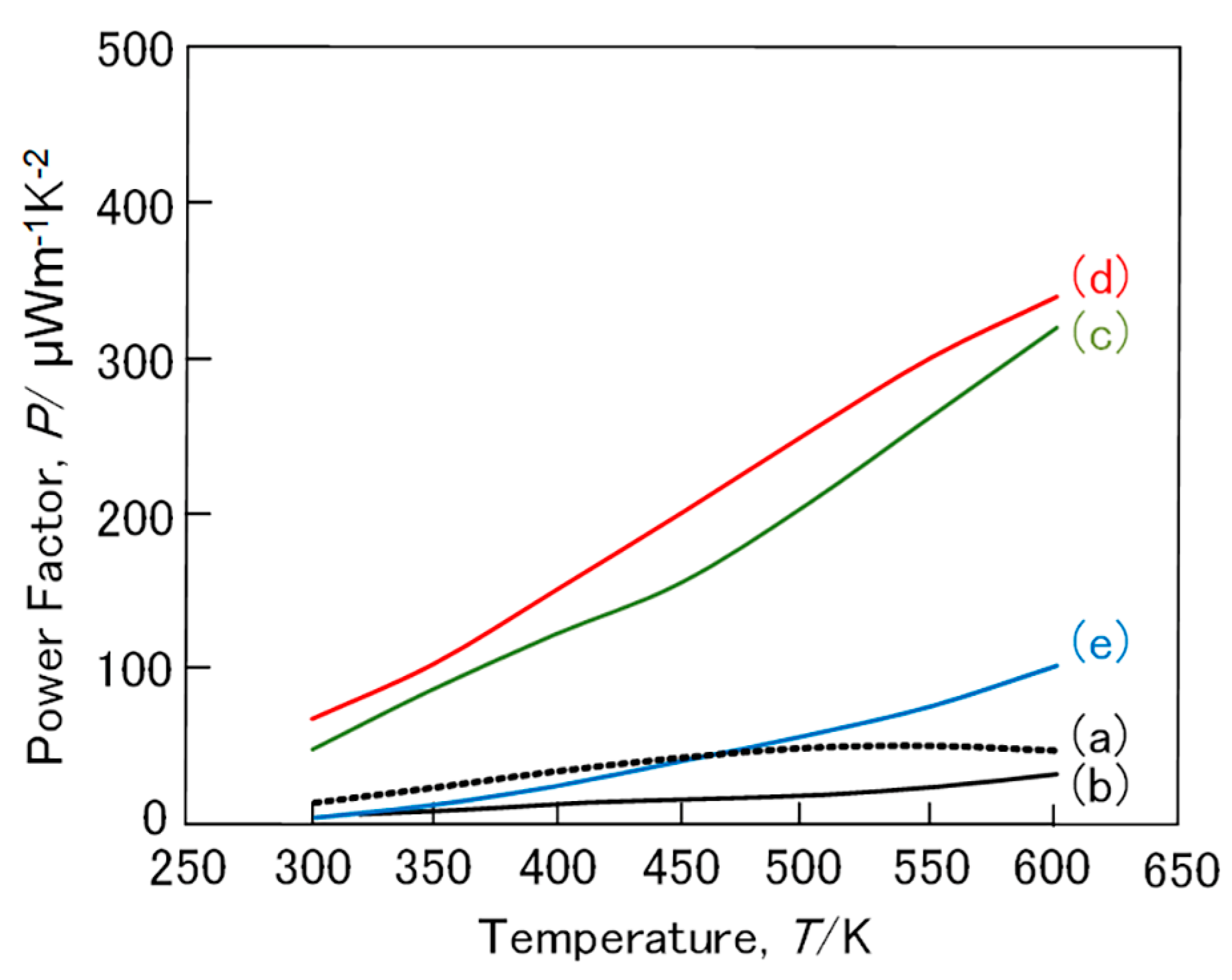
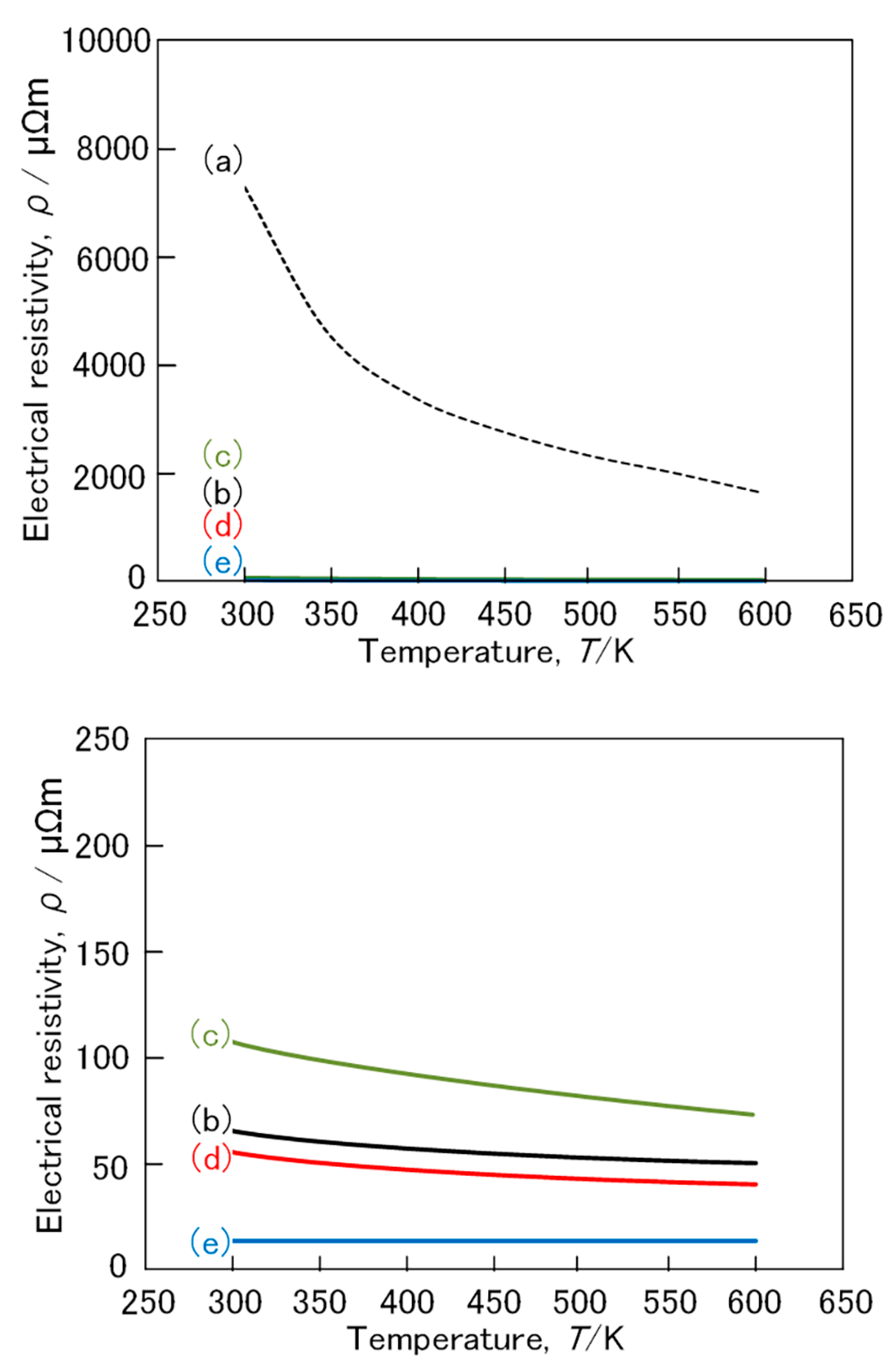
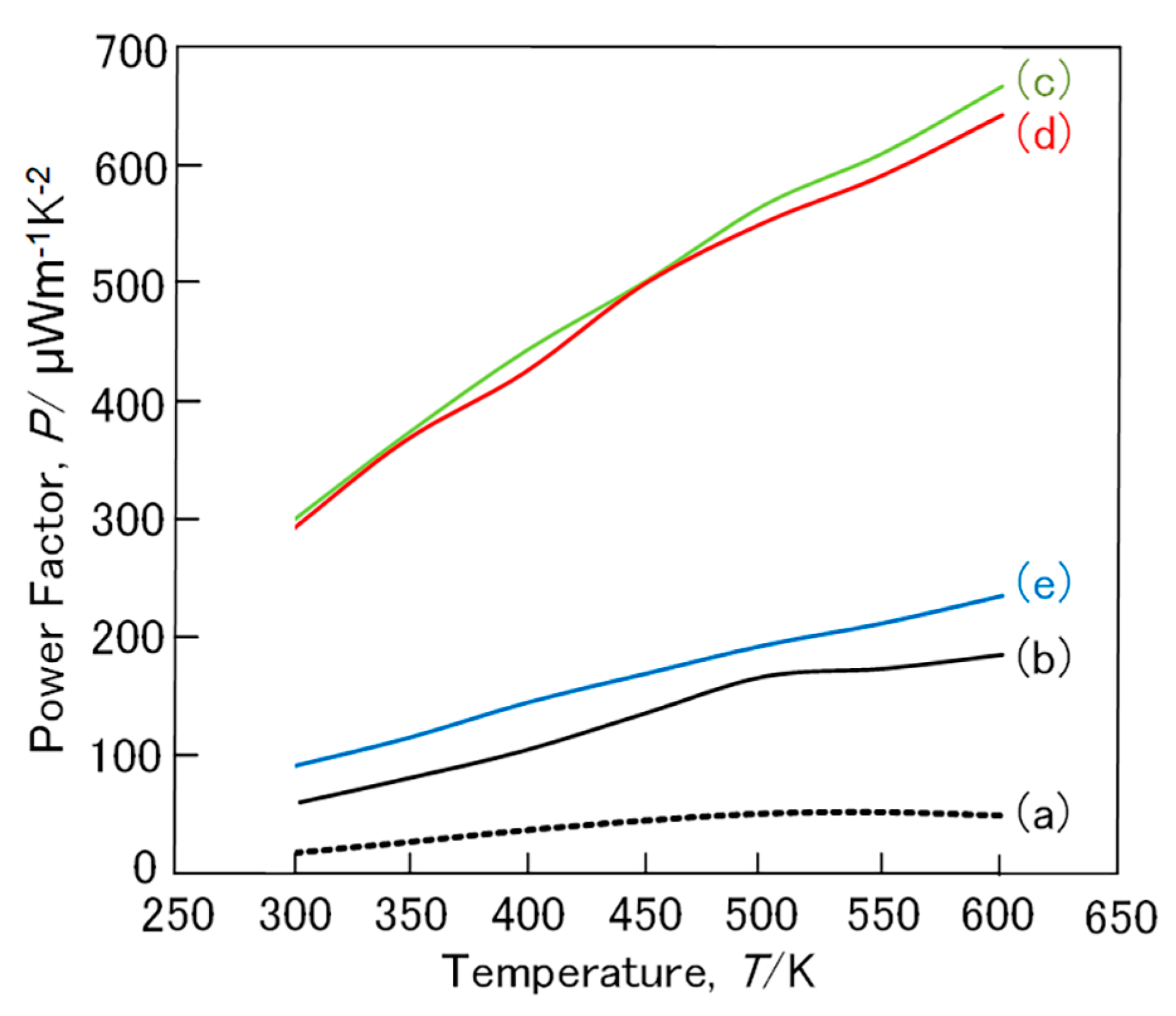
3. Results and Discussion
3.1. FeSi2 Compound
3.2. (Fe,Mn)Si2 and (Fe,Co)Si2 Compound
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zhao, L.D. Thermoelectric materials: Energy conversion between heat and electricity. J. Mater. 2015, 1, 92–105. [Google Scholar] [CrossRef]
- Jouhara, H.; Olabi, A.G. Editorial: Industrial waste heat recovery. Energy 2018, 160, 1–2. [Google Scholar] [CrossRef]
- Brückner, S.; Liu, S.; Miró, L.; Radspieler, M.; Cabeza, L.F.; Lävemann, E. ndustrial waste heat recovery technologies: An economic analysis of heat transformation technologies. Appl. Energy 2015, 151, 157–167. [Google Scholar] [CrossRef]
- Law, R.; Harvey, A.; Reay, D. A knowledge-based system for low-grade waste heat recovery in the process industries. Appl. Therm. Eng. 2016, 94, 590–599. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, J.; Baleynaud, J.M.; Lu, J. Heat recovery potentials and technologies in industrial zones. J. Energy Inst. 2017, 90, 951–961. [Google Scholar] [CrossRef]
- Jouhara, H.; Khordehgah, N.; Almahmoud, S.; Delpech, B.; Chauhan, A.; Tassou, S.A. Waste Heat Recovery Technologies and Applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar] [CrossRef]
- Satterthwaite, C.B.; Ure, R.W., Jr. Electrical and Thermal Properties of Bi2Te3. Phys. Rev. 1957, 108, 1164–1170. [Google Scholar] [CrossRef]
- Rosi, F.D.; Abeles, B.; Jensen, R.V. Materials for thermoelectric refrigeration. J. Phys. Chem. Solids 1959, 10, 191–200. [Google Scholar] [CrossRef]
- Wada, H.; Sato, T.; Takahashi, K.; Nakatsukasa, K. The anisotropic powder metallurgy of n-type Bi2Te2.85Se0.15 thermoelectric material. J. Mater. Res. 1990, 5, 1052–1057. [Google Scholar] [CrossRef]
- Goldsmid, H.J. Bismuth Telluride and Its Alloys as Materials for Thermoelectric Generation. Materials 2014, 7, 2577–2592. [Google Scholar] [CrossRef]
- Mamur, H.; Bhuiyan, M.R.A.; Korkmaz, F.; Nil, M. A review on bismuth telluride (Bi2Te3) nanostructure for thermoelectric applications. Renew. Sust. Energ. Rev. 2018, 82, 4159–4169. [Google Scholar] [CrossRef]
- Thakur, V.; Upadhyay, K.; Kaur, R.; Goyal, N.; Gautam, S. Investigating phase transition and morphology of Bi-Te thermoelectric system. Mater. Today Adv. 2020, 8, 100082. [Google Scholar] [CrossRef]
- Ware, M.; McNeill, D.J. Iron disilicide as a thermoelectric generator materials. Proc. Inst. Electr. Eng. 1964, 111, 178–182. [Google Scholar] [CrossRef]
- Birkholz, U.; Schelm, J. Electrical Investigation of the Semiconductor-to-Metal Transition in FeSi2. Phys. Stat. Sol. B 1969, 34, K177–K180. [Google Scholar] [CrossRef]
- Nishida, I. Study of Semiconductor-to-Metal Transition in Mn-Doped FeSi2. Phys. Rev. B 1973, 7, 2710–2713. [Google Scholar] [CrossRef]
- Piton, J.P.; Fay, M.F. Phase changes in iron–silicon alloys around the composition FeSi2. Acad. C. R. Sci. 1968, C266, 514–516. [Google Scholar]
- Kojima, T. Semiconducting and Thermoelectric Properties of Sintered Iron Disilicide. Phys. Stat. Sol. A 1989, 111, 233–242. [Google Scholar] [CrossRef]
- Dąbrowski, F.; Ciupiński, Ł.; Zdunek, J.; Kruszewski, J.; Zybała, R.; Michalski, A.; Kurzydłowski, K.J. Microstructure and thermoelectric properties of p and n type doped β-FeSi2 fabricated by mechanical alloying and pulse plasma sintering. Mater. Today Proc. 2019, 8, 531–539. [Google Scholar] [CrossRef]
- Ohta, Y.; Miura, S.; Mishima, Y. Thermoelectric semiconductor iron disilicides produced by sintering elemental powders. Intermetallics 1999, 7, 1203–1210. [Google Scholar] [CrossRef]
- Qu, X.; Lü, S.; Hu, J.; Meng, Q. Microstructure and thermoelectric properties of β-FeSi2 ceramics fabricated by hot-pressing and spark plasma sintering. J. Alloys Compd. 2011, 509, 10217–10221. [Google Scholar] [CrossRef]
- Nogi, K.; Kita, T. Rapid production of β-FeSi2 by spark-plasma sintering. J. Mater. Sci. 2000, 35, 5845–5849. [Google Scholar] [CrossRef]
- Ito, M.; Nagai, H.; Tanaka, T.; Katsuyama, S.; Majima, K. Thermoelectric performance of n-type and p-type β-FeSi2 prepared by pressureless sintering with Cu addition. J. Alloys Compd. 2001, 319, 303–311. [Google Scholar] [CrossRef]
- Kim, S.W.; Cho, M.K.; Mishima, Y.; Choi, D.C. High temperature thermoelectric properties of p- and n-type β-FeSi2 with some dopants. Intermetallics 2003, 11, 399–405. [Google Scholar] [CrossRef]
- Pandey, T.; Singh, D.J.; Parker, D.; Singh, A.K. Thermoelectric properties of β-FeSi2. J. Appl. Phys. 2013, 114, 153704. [Google Scholar] [CrossRef]
- Abbassi, L.; Mesguich, D.; Coulomb, L.; Chevallier, G.; Aries, R.; Estournès, C.; Flahaut, E.; Viennois, R.; Beaudhuina, M. In-situ reactive synthesis of dense nanostructured β-FeSi2 by Spark Plasma Sintering. J. Alloys Compd. 2022, 902, 163683. [Google Scholar] [CrossRef]
- Yamada, H.; Katsumata, H.; Yuasa, D.; Uekusa, S.; Ishiyama, M.; Souma, H.; Azumaya, I. Structural and electrical properties of β-FeSi2 bulk materials for thermoelectric applications. Phys. Procedia 2012, 23, 13–16. [Google Scholar] [CrossRef]
- Broseghini, M.; Gelisioa, L.; D’Incaua, M.; Azanza Ricardoa, C.L.; Pugno, N.M.; Scardi, P. Modeling of the planetary ball-milling process: The case study of ceramic powders. J. Eur. Cerm. Soc. 2016, 9, 2205–2212. [Google Scholar] [CrossRef]
- Kubaschewski, O. Iron-Binary Phase Diagram; Springer: New York, NY, USA, 1982; pp. 136–137. [Google Scholar]
- Le Caër, G.; Bauer-Grosse, E.; Pianelli, A.; Bouzy, E.; Matteazzi, P. Mechanically driven syntheses of carbides and silicides. J. Mater. Sci. 1990, 25, 4726–4731. [Google Scholar] [CrossRef]
- Umemoto, M. Preparation of Thermoelectric β-FeSi2 Doped with Al and Mn by Mechanical Alloying (Overview). Mater. Trans. JIM 1995, 36, 373–383. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, T.; Asakawa, R. Production of (Fe,Co)Si2 and (Fe.Mn)Si2 Thermoelectric Materials by Spark Plasma Sintering. Crystals 2024, 14, 56. https://doi.org/10.3390/cryst14010056
Saito T, Asakawa R. Production of (Fe,Co)Si2 and (Fe.Mn)Si2 Thermoelectric Materials by Spark Plasma Sintering. Crystals. 2024; 14(1):56. https://doi.org/10.3390/cryst14010056
Chicago/Turabian StyleSaito, Tetsuji, and Ryoki Asakawa. 2024. "Production of (Fe,Co)Si2 and (Fe.Mn)Si2 Thermoelectric Materials by Spark Plasma Sintering" Crystals 14, no. 1: 56. https://doi.org/10.3390/cryst14010056



