Controlling Crystal Growth of a Rare Earth Element Scandium Salt in Antisolvent Crystallization
Abstract
:1. Introduction
1.1. Controlling Crystal Size Distribution in Antisolvent Crystallization
1.2. Crystal Size Measurement
2. Materials and Methods
2.1. Preparation of the Solution
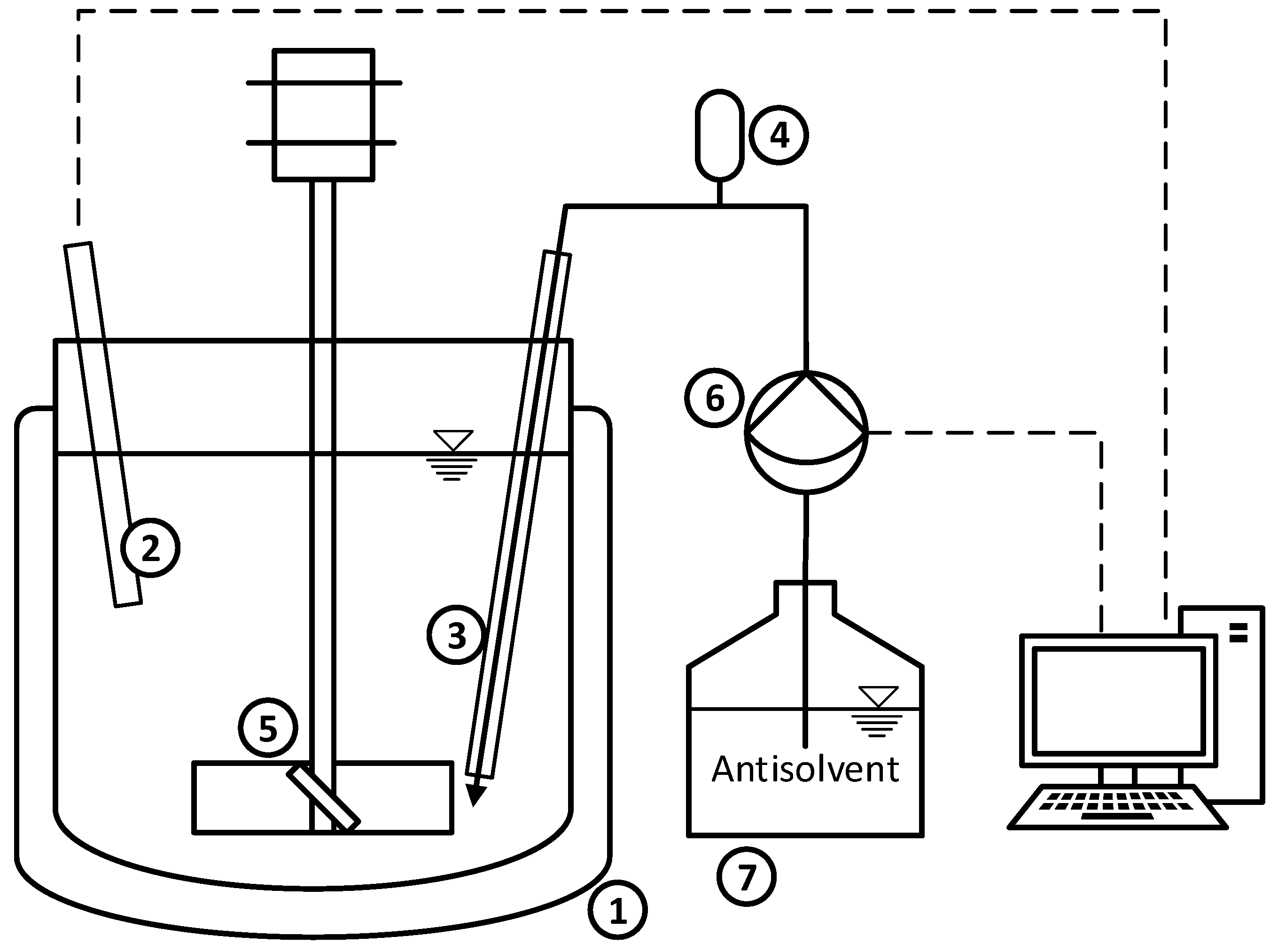
2.2. Setup and Sampling
2.3. Seeding Method
2.4. Theory
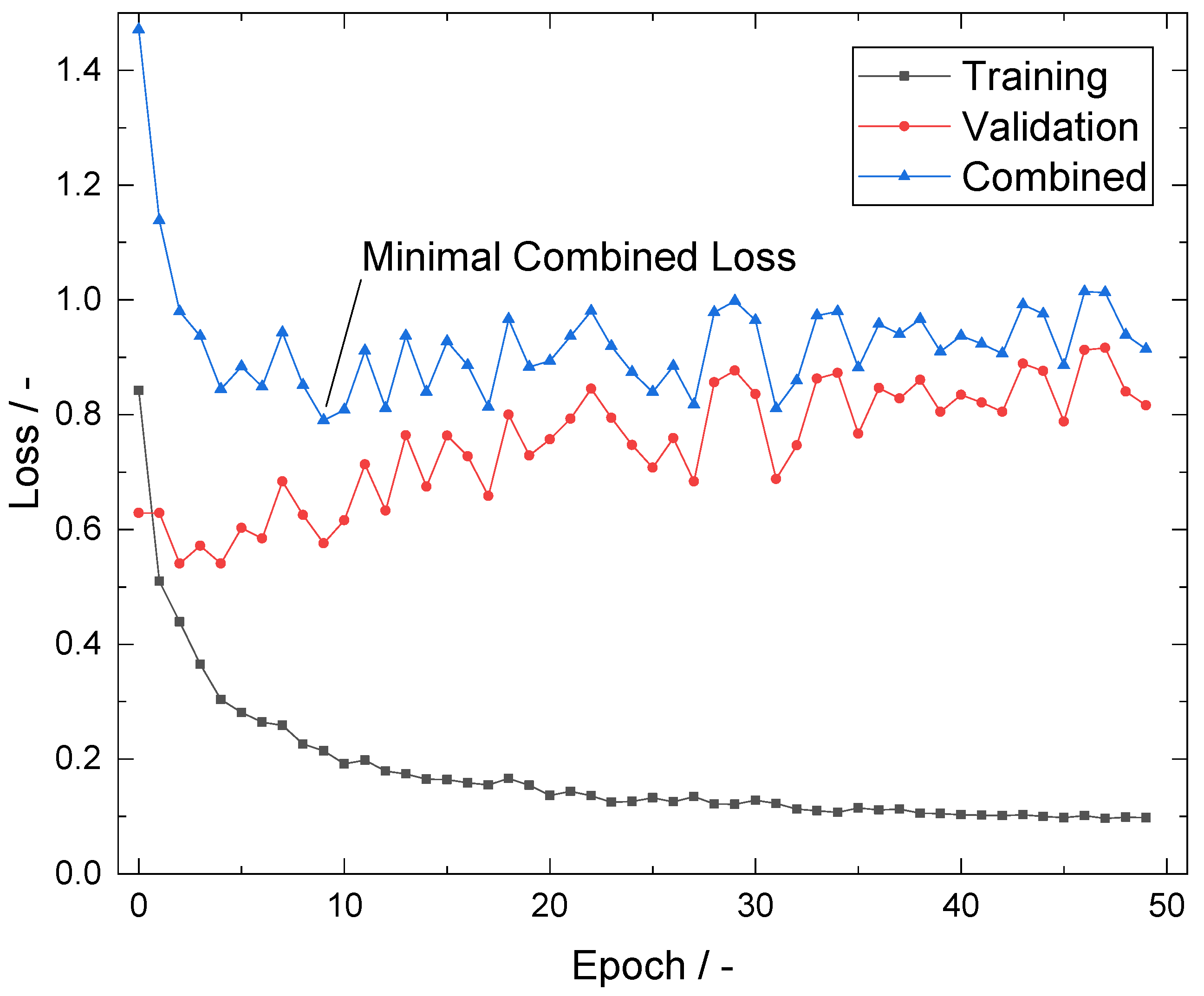
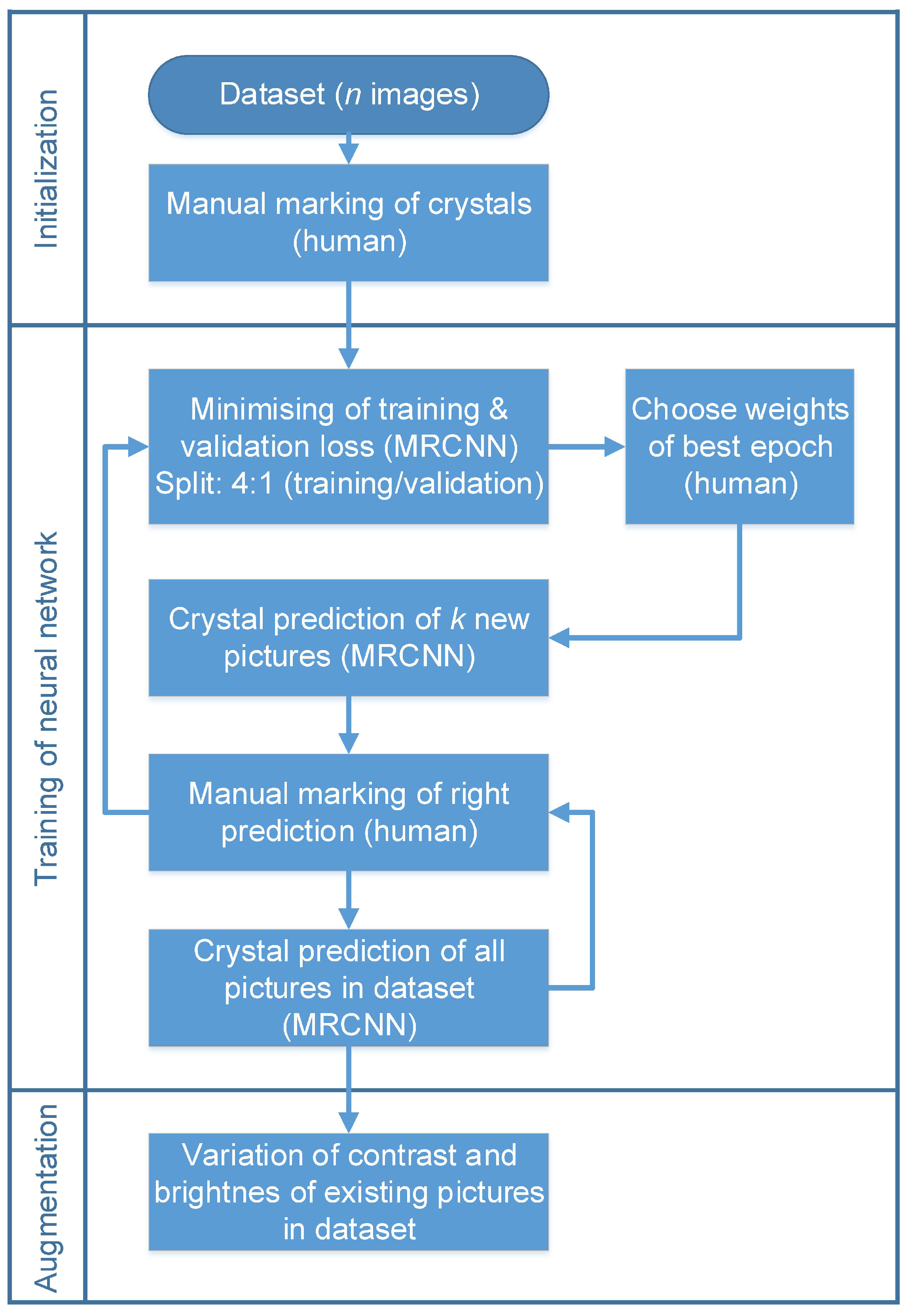
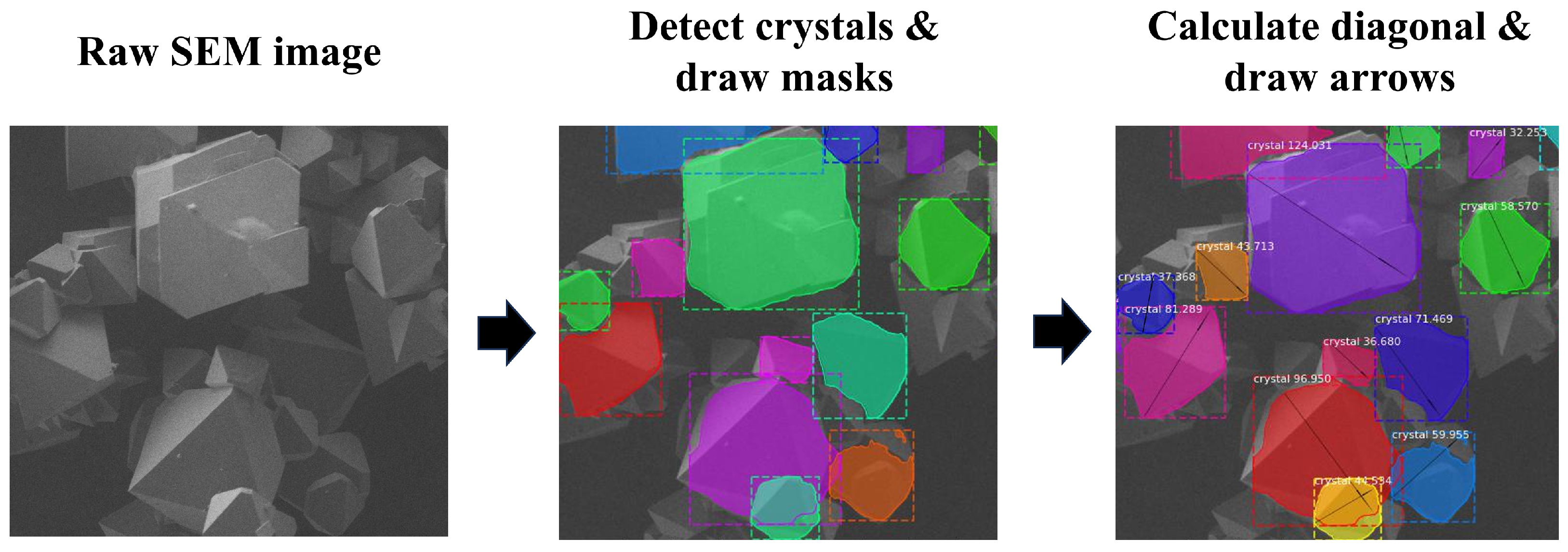
2.5. Automated Crystal Detection
3. Results
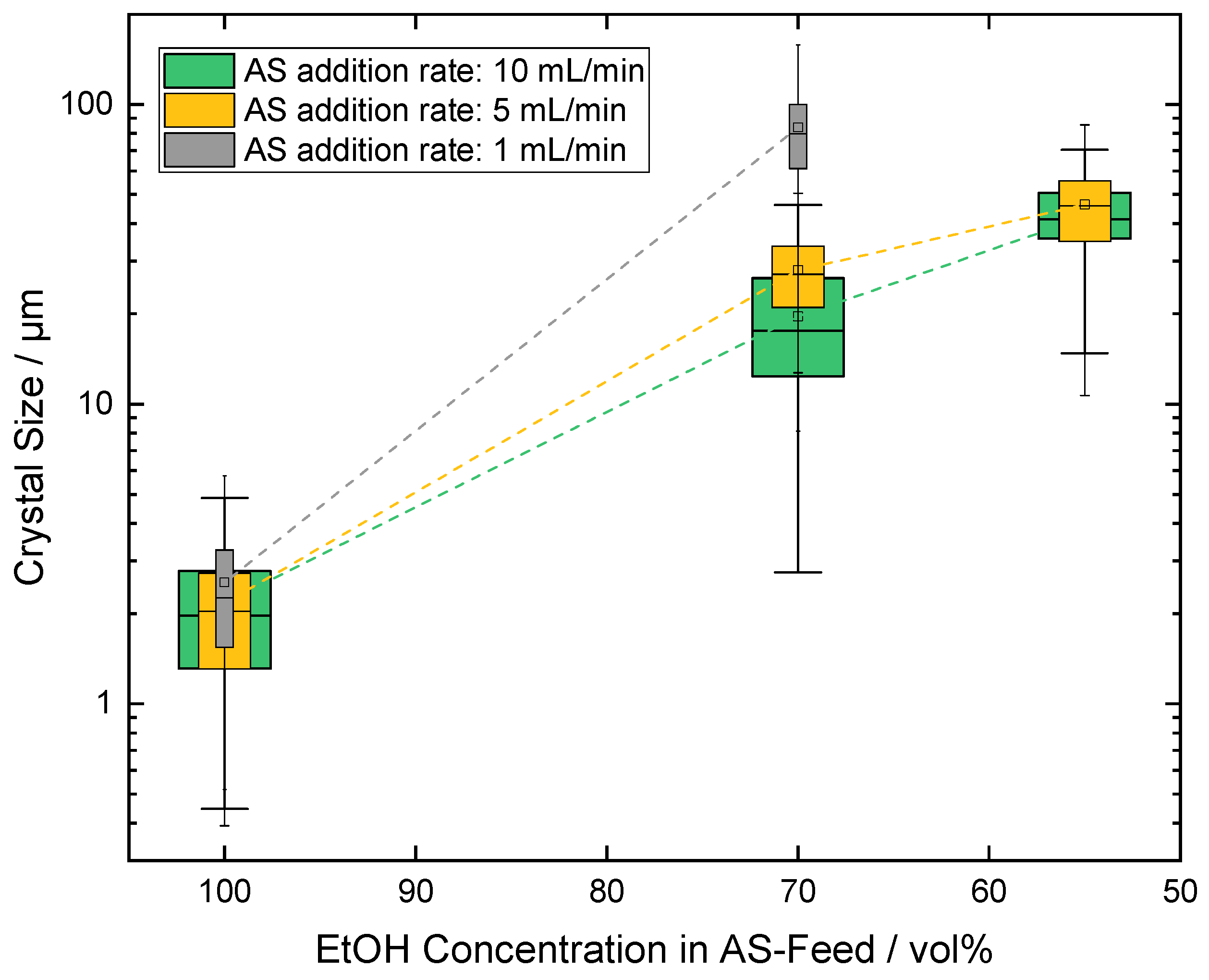
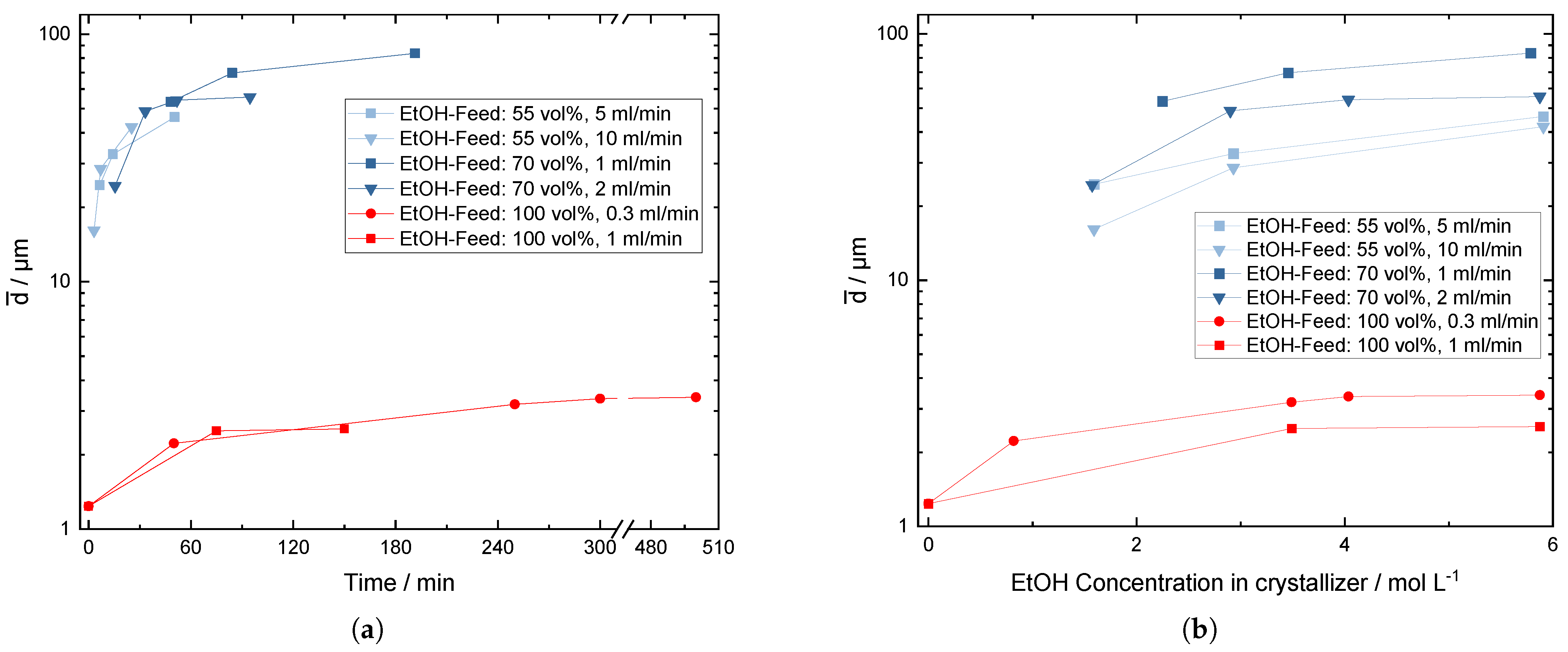
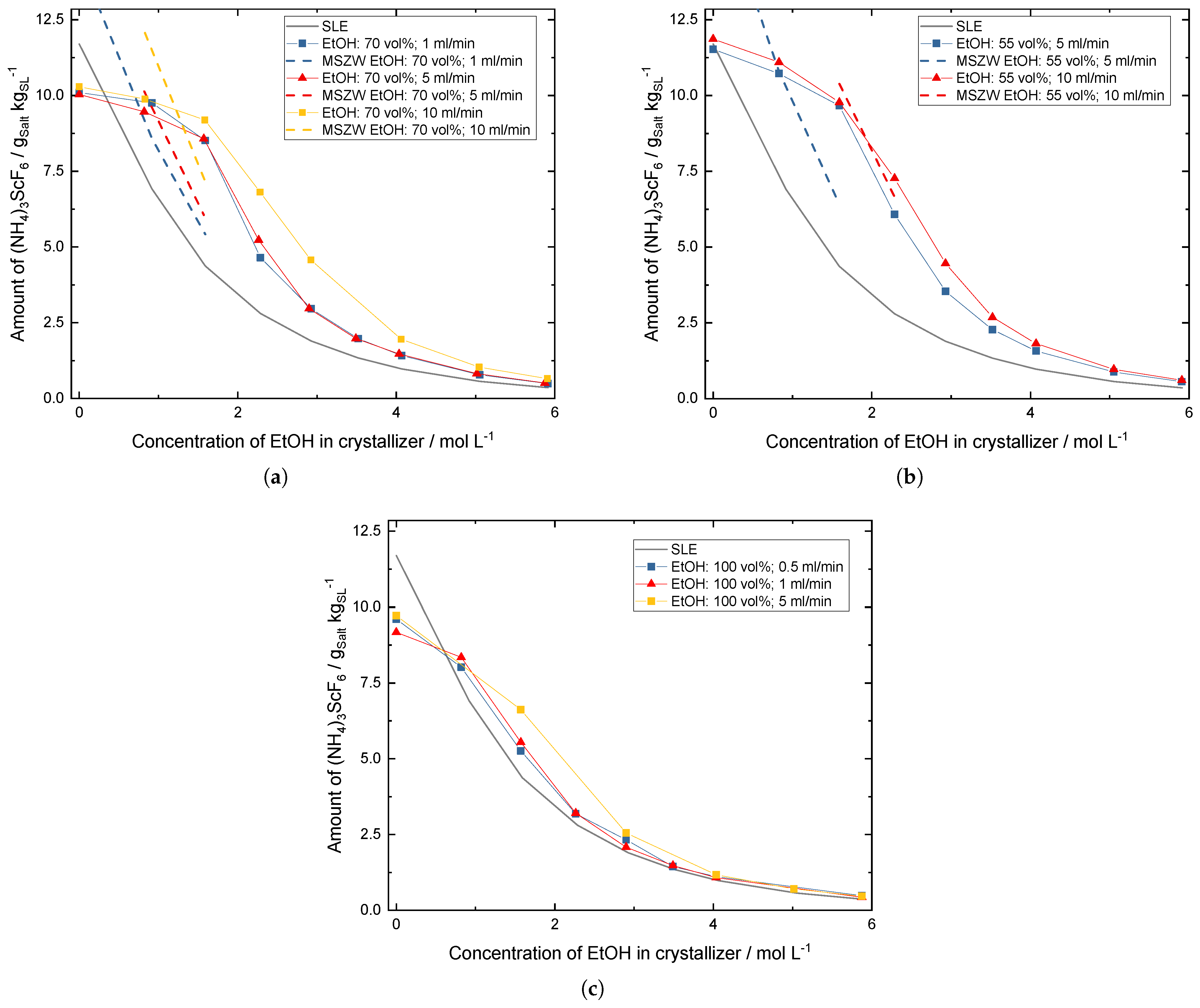
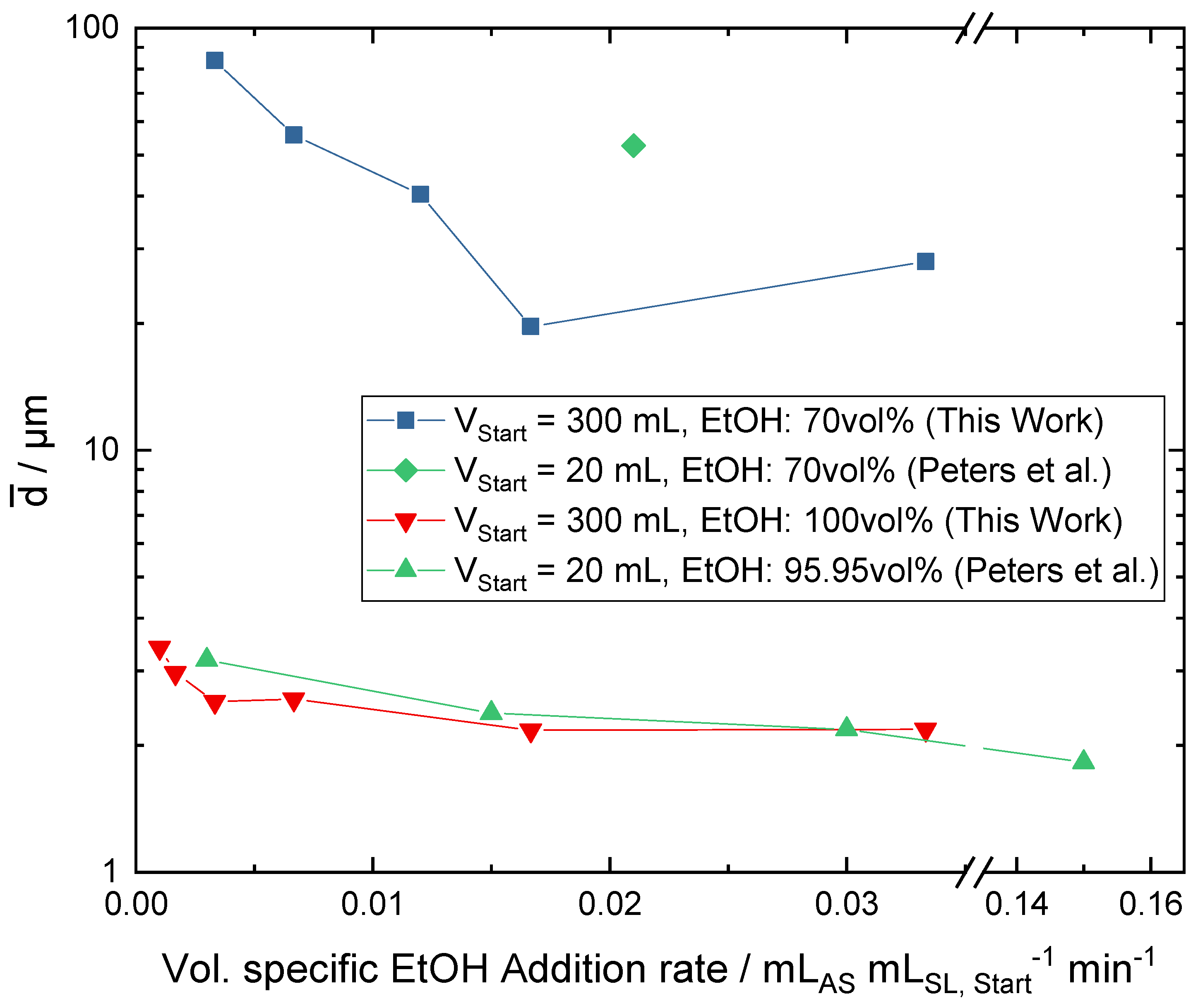
3.1. Influence of the Addition Rate and the Dilution of the Antisolvent
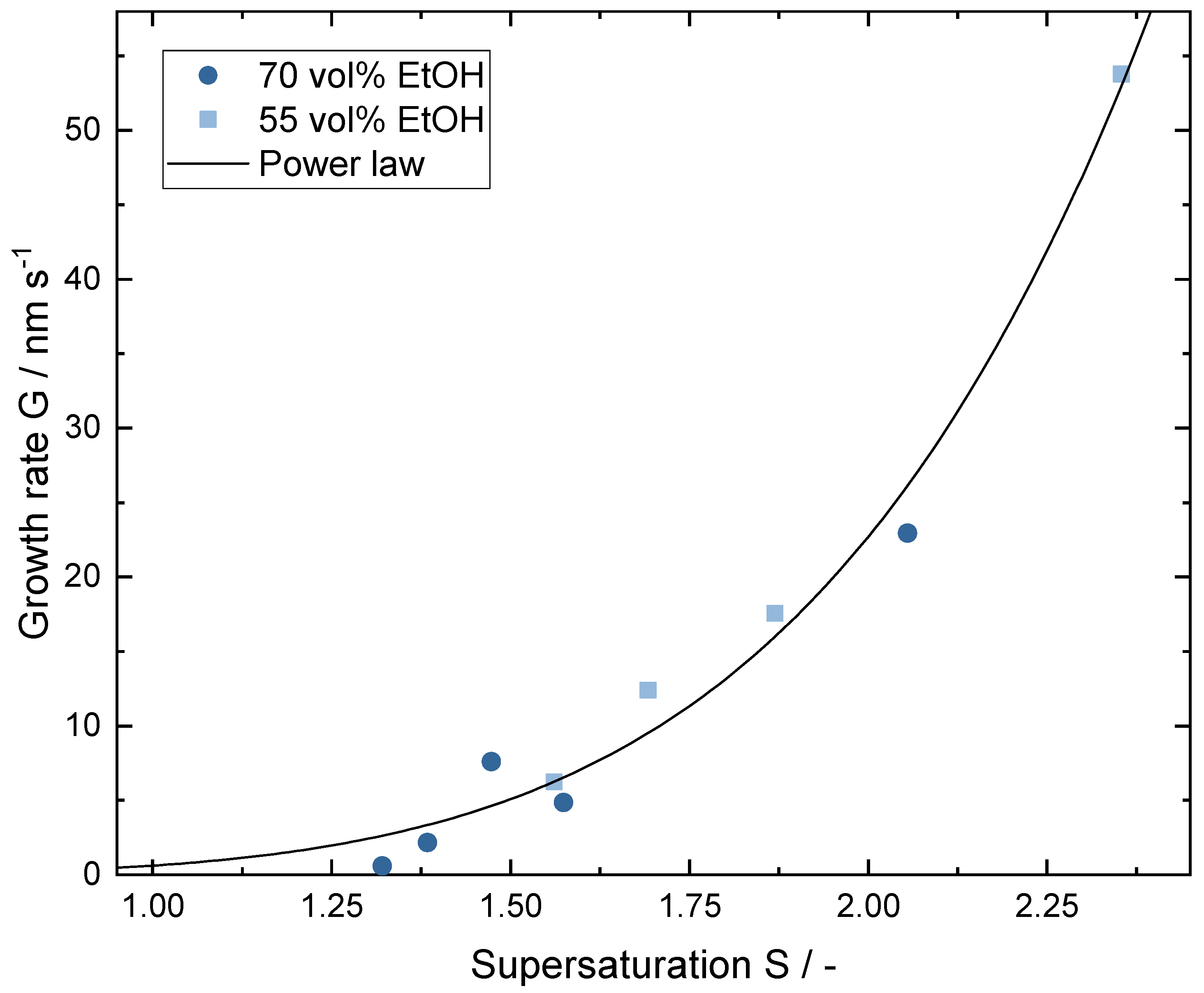
3.2. Crystal Growth during the Crystallization Process
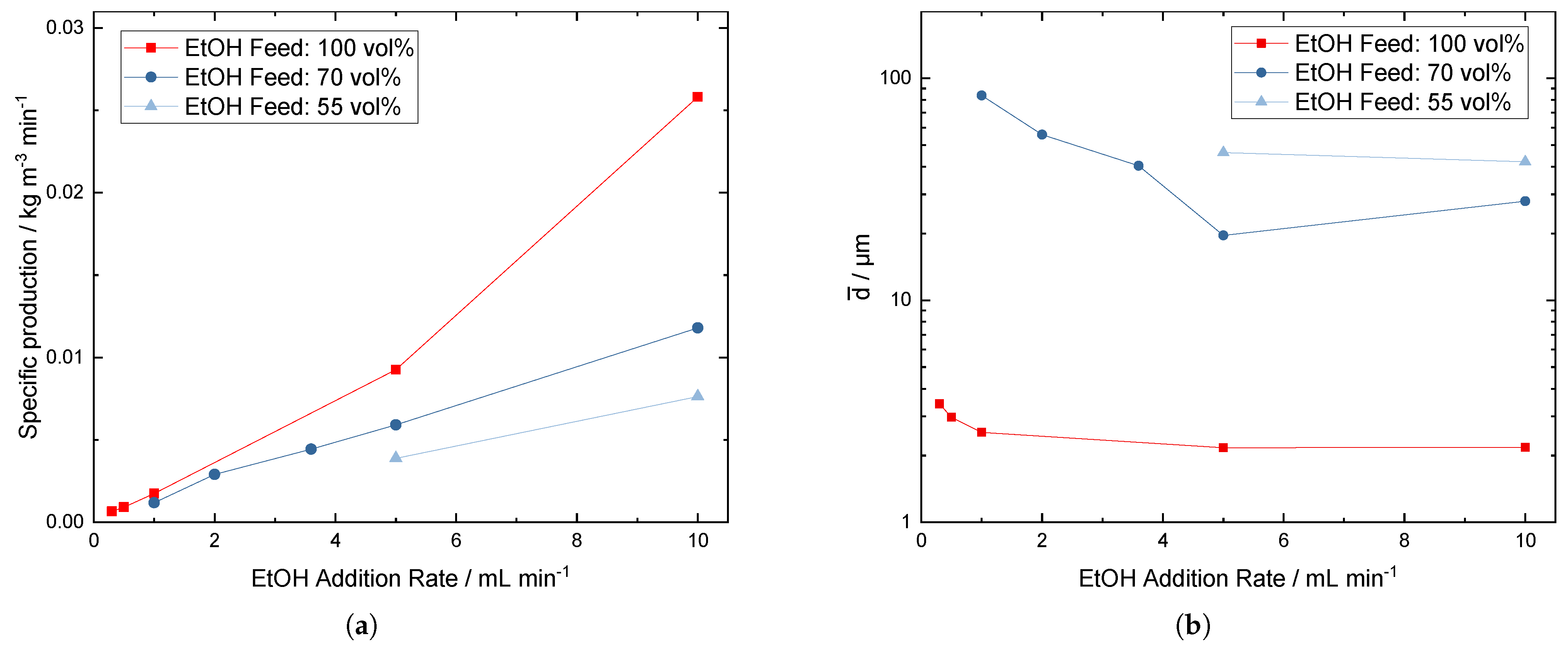
3.3. Specific Production
3.4. Comparison of Scale
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| REE | rare earth element |
| MRCNN | mask region-based convolutional neural network |
| CSD | crystal size distribution |
| SEM | scanning electron microscopy |
| MSZW | metastable zone width |
| AS | antisolvent |
| SL | crystallization solution |
| STY | space-time yield |
| SLE | solid-liquid equilibrium |
Appendix A. CSD of Seed Crystals

Appendix B. CSD of MRCNN vs. Human Measurement

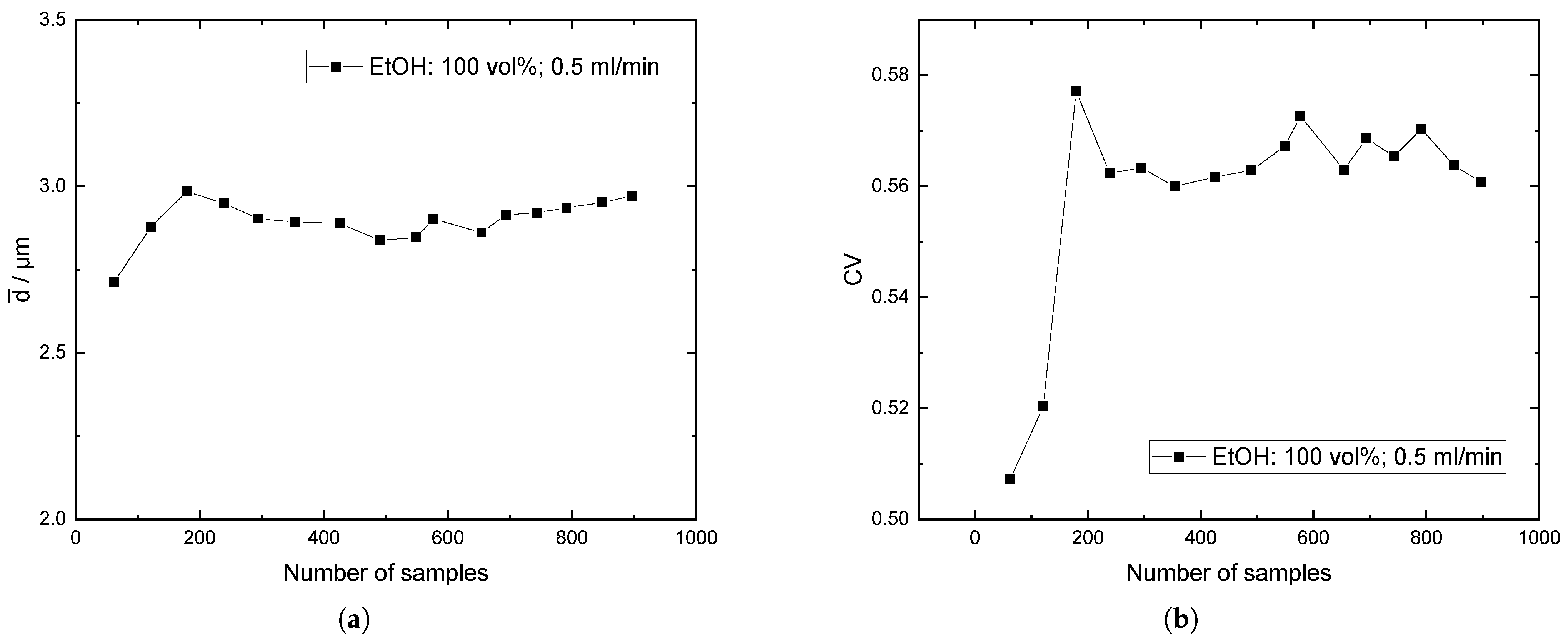
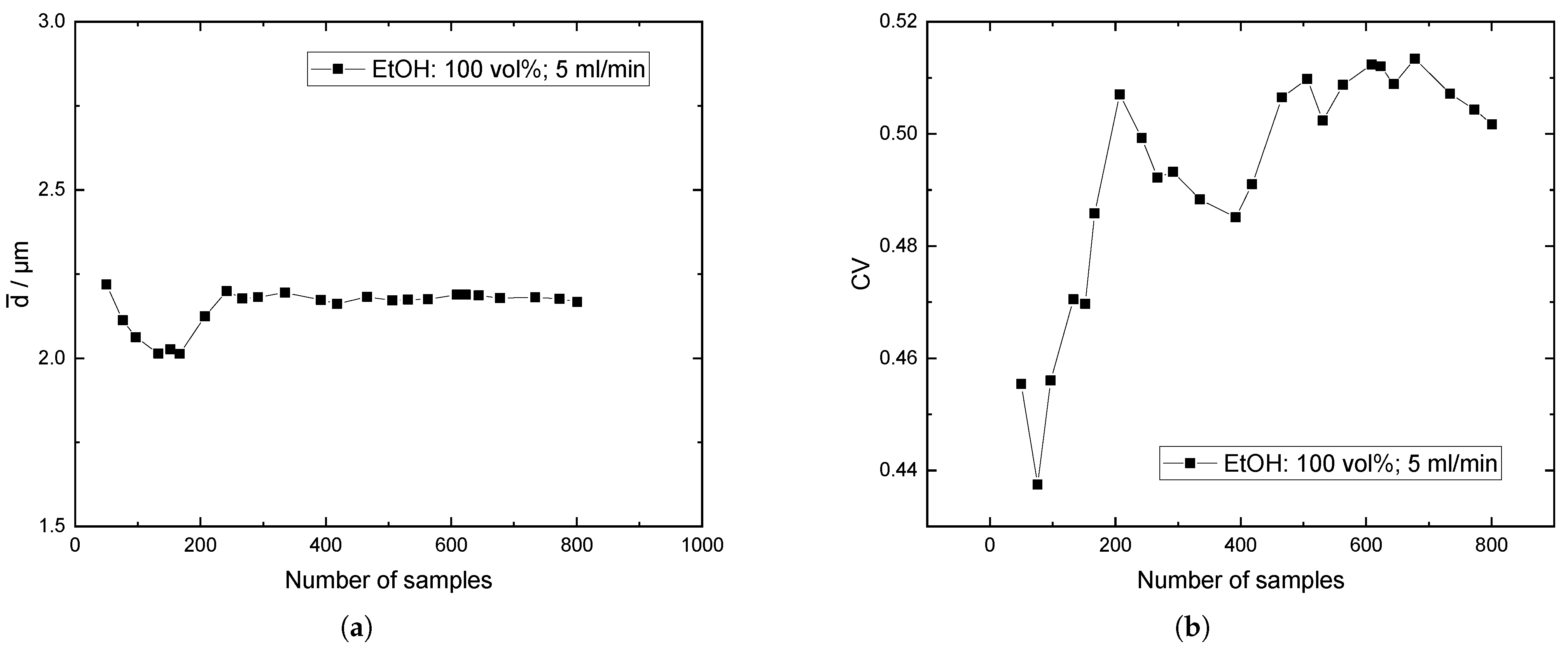

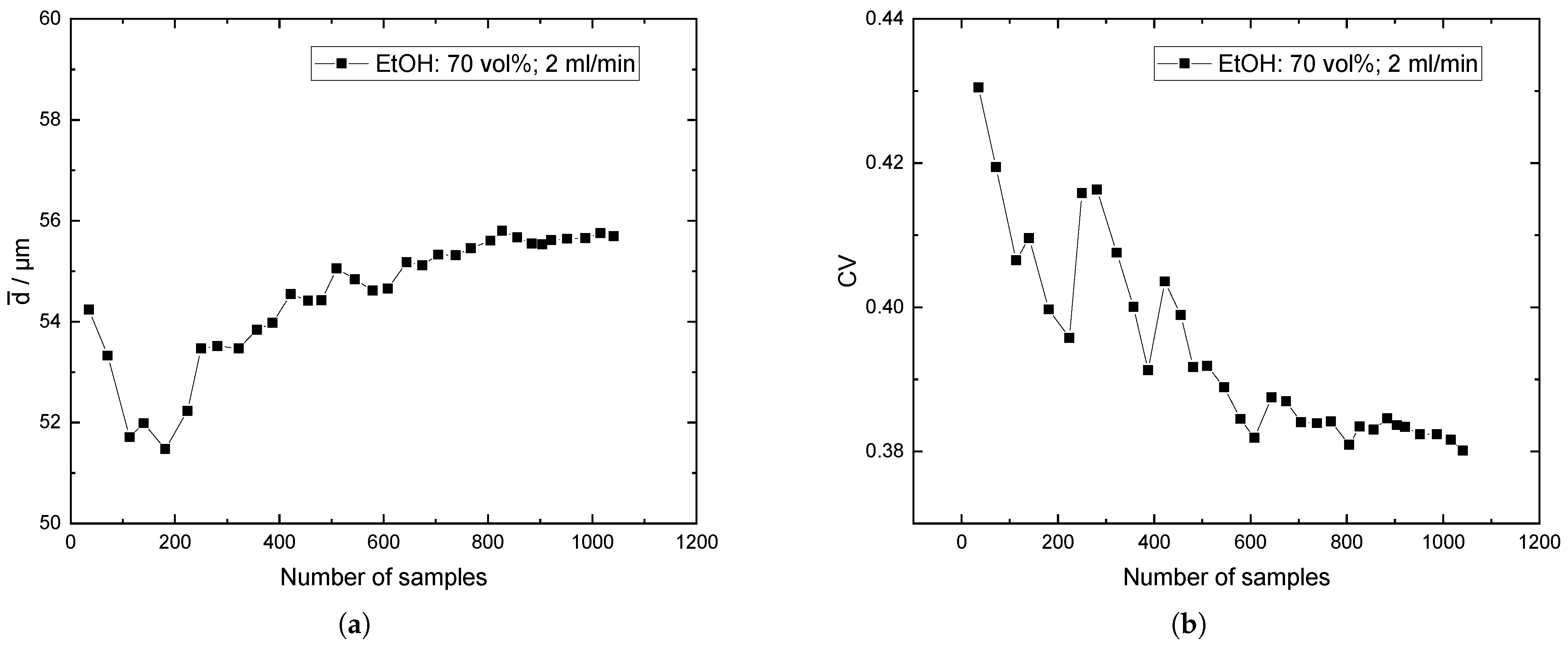
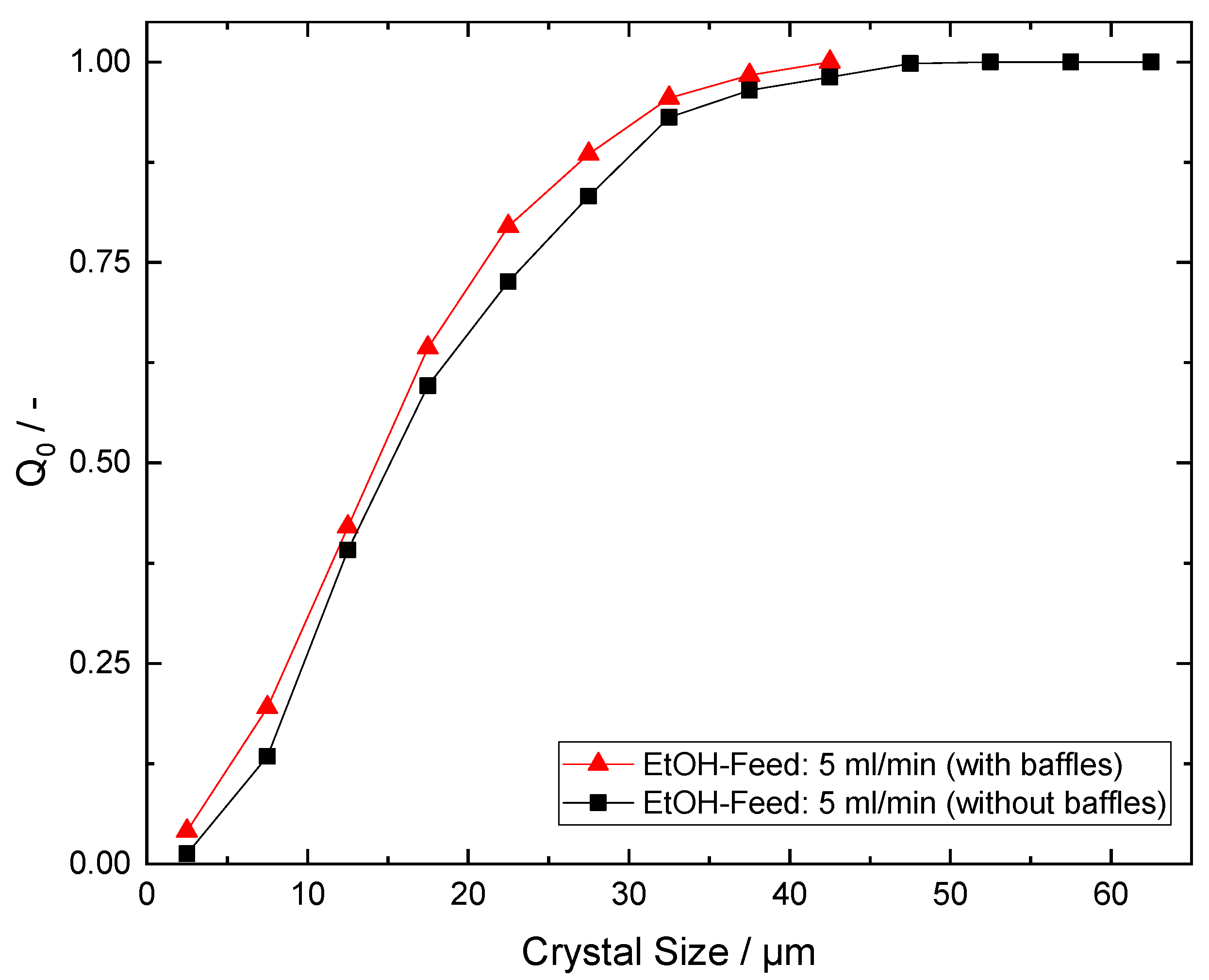
Appendix C. Baffles vs. No Baffles
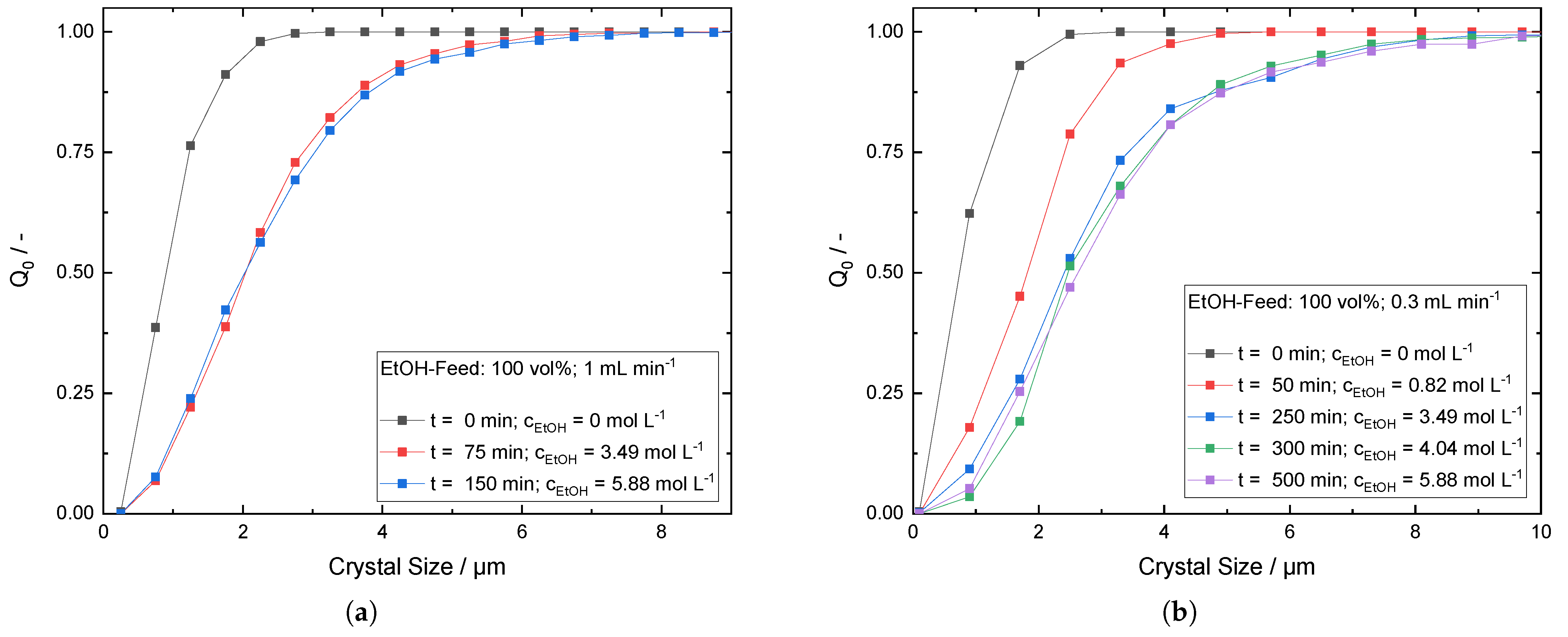
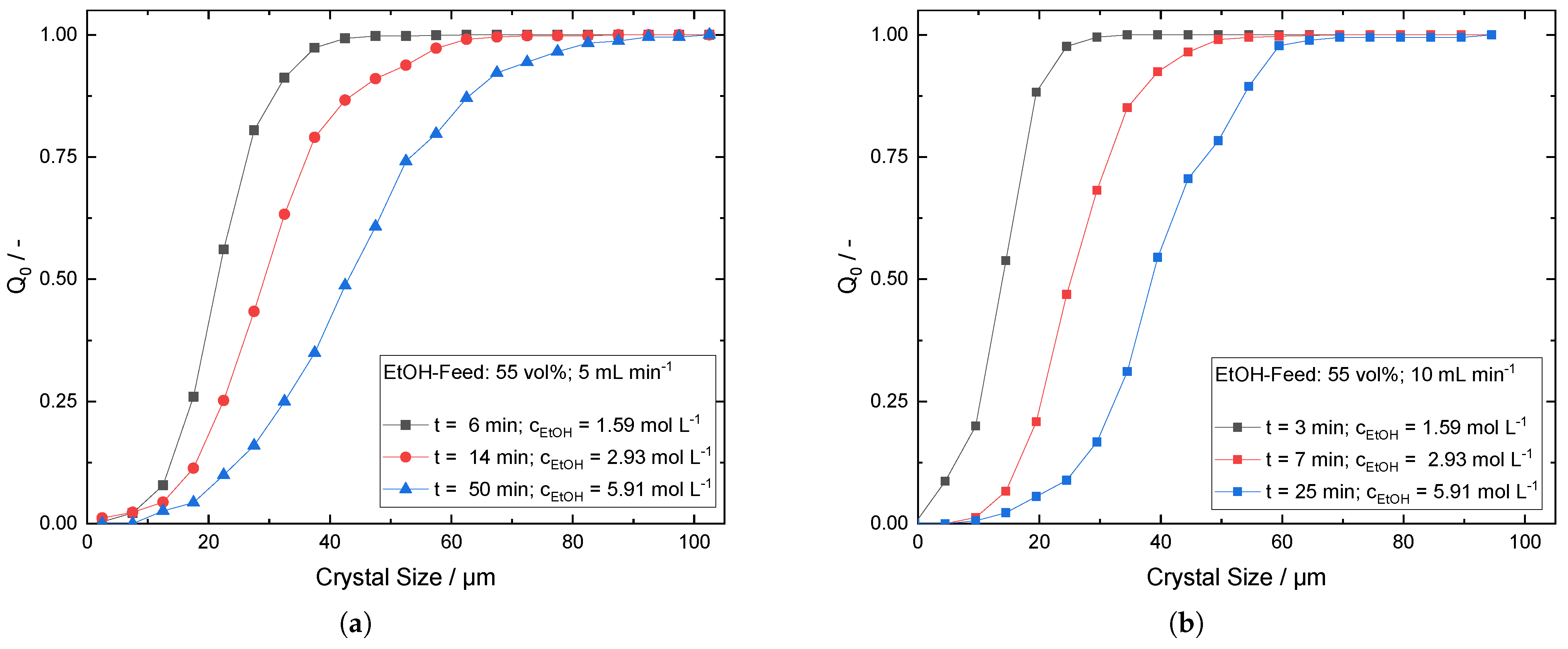
Appendix D. Cumulative Number Frequency Plots for Progressive CSD during Antisolvent Addition

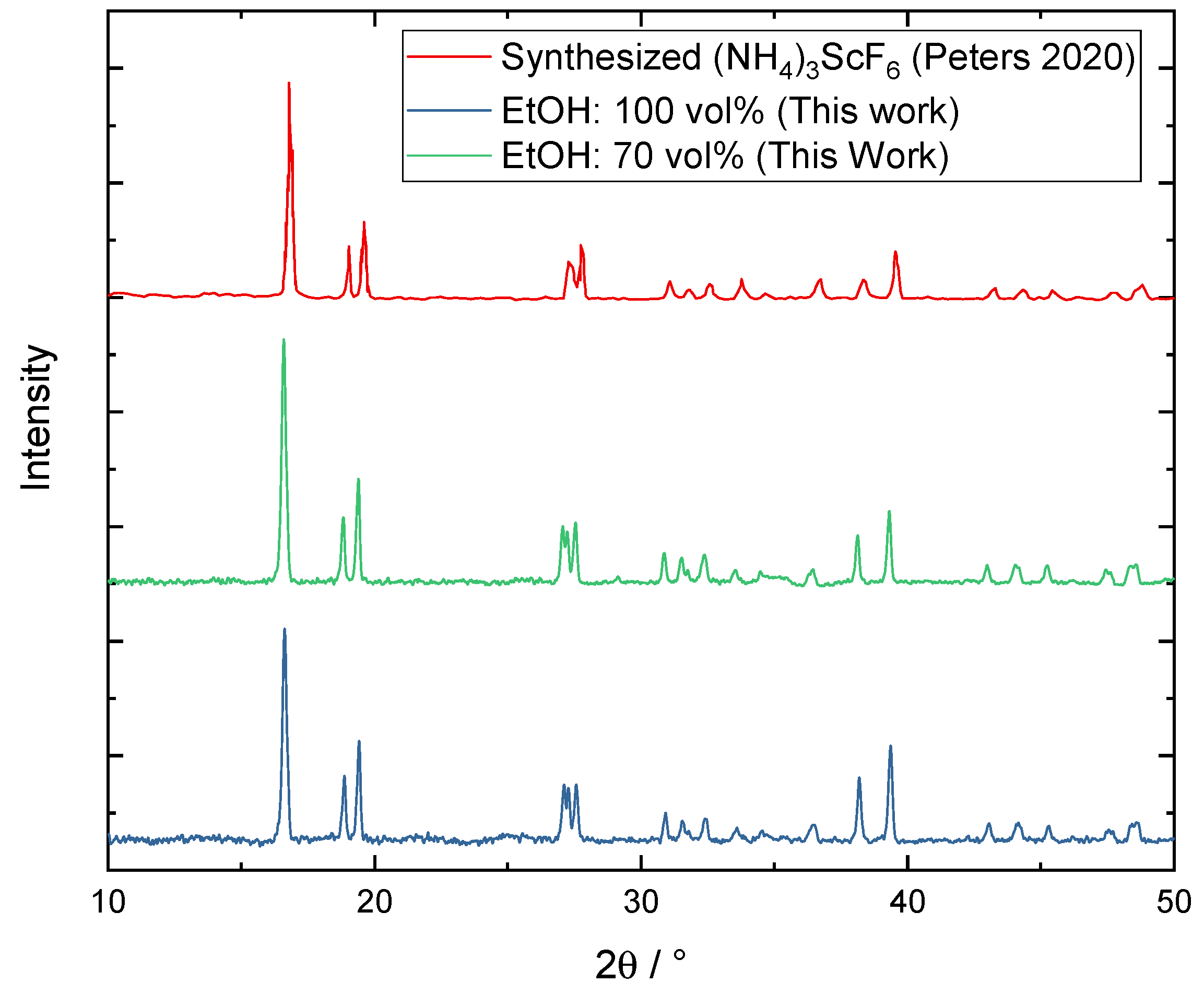
Appendix E. XRD Measurement
References
- Sibanda, J.; Chivavava, J.; Lewis, A.E. Crystal Engineering in Antisolvent Crystallization of Rare Earth Elements (REEs). Minerals 2022, 12, 1554. [Google Scholar] [CrossRef]
- Ma, Y.; Svärd, M.; Xiao, X.; Gardner, J.M.; Olsson, R.T.; Forsberg, K. Precipitation and Crystallization Used in the Production of Metal Salts for Li-Ion Battery Materials: A Review. Metals 2020, 10, 1609. [Google Scholar] [CrossRef]
- Riva, S.; Yusenko, K.V.; Lavery, N.P.; Jarvis, D.J.; Brown, S.G.R. The scandium effect in multicomponent alloys. Int. Mater. Rev. 2016, 61, 203–228. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Müller, T.; Yurramendi, L. Rare Earths and the Balance Problem: How to Deal with Changing Markets? J. Sustain. Metall. 2018, 4, 126–146. [Google Scholar] [CrossRef]
- European Commission. Critical Raw Materials Resilience: Charting a Path towards Greater Security and Sustainability; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Department of the Interior. Draft List of Critical Minerals; Federal Register the Daily Journal of the United States Government: Washington, DC, USA, 2018. [Google Scholar]
- Alkan, G.; Yagmurlu, B.; Cakmakoglu, S.; Hertel, T.; Kaya, Ş.; Gronen, L.; Stopic, S.; Friedrich, B. Novel Approach for Enhanced Scandium and Titanium Leaching Efficiency from Bauxite Residue with Suppressed Silica Gel Formation. Sci. Rep. 2018, 8, 5676. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, C.Y. Separation and purification of scandium by solvent extraction and related technologies: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1237–1246. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Yagmurlu, B.; Dittrich, C.; Friedrich, B. Effect of Aqueous Media on the Recovery of Scandium by Selective Precipitation. Metals 2018, 8, 314. [Google Scholar] [CrossRef]
- Peters, E.; Dittrich, C.; Kaya, S.; Forsberg, K. Crystallization of a Pure Scandium Phase from Solvent Extraction Strip Liquors. In Extraction 2018; Davis, B.R., Moats, M.S., Wang, S., Gregurek, D., Kapusta, J., Battle, T.P., Schlesinger, M.E., Alvear Flores, G.R., Jak, E., Goodall, G., et al., Eds.; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, Switzerland, 2018; Volume 7, pp. 2707–2713. [Google Scholar] [CrossRef]
- Peters, E.M.; Kaya, Ş.; Dittrich, C.; Forsberg, K. Recovery of Scandium by Crystallization Techniques. J. Sustain. Metall. 2019, 5, 48–56. [Google Scholar] [CrossRef]
- Peters, E.M.; Svärd, M.; Forsberg, K. Phase equilibria of ammonium scandium fluoride phases in aqueous alcohol mixtures for metal recovery by anti-solvent crystallization. Sep. Purif. Technol. 2020, 252, 117449. [Google Scholar] [CrossRef]
- Kaya, Ş.; Peters, E.; Forsberg, K.; Dittrich, C.; Stopic, S.; Friedrich, B. Scandium Recovery from an Ammonium Fluoride Strip Liquor by Anti-Solvent Crystallization. Metals 2018, 8, 767. [Google Scholar] [CrossRef]
- Tonn, J.; Grotjohann, A.; Kocks, C.; Jupke, A. Nucleation Kinetics of Rare Earth Scandium Salt: An Experimental Investigation of the Metastable Zone Width. Crystals 2023, 13, 1074. [Google Scholar] [CrossRef]
- Kodera, T.; Kobari, M.; Hirasawa, I. Modeling and Growth Kinetics of Antisolvent Crystallization Applied to the Pharmaceutical Industry. Chem. Eng. Technol. 2019, 42, 1458–1465. [Google Scholar] [CrossRef]
- Nowee, S.M.; Abbas, A.; Romagnoli, J.A. Model-Based Optimal Strategies for Controlling Particle Size in Antisolvent Crystallization Operations. Cryst. Growth Des. 2008, 8, 2698–2706. [Google Scholar] [CrossRef]
- Lindenberg, C.; Krättli, M.; Cornel, J.; Mazzotti, M.; Brozio, J. Design and Optimization of a Combined Cooling/Antisolvent Crystallization Process. Cryst. Growth Des. 2009, 9, 1124–1136. [Google Scholar] [CrossRef]
- O’Grady, D.; Barrett, M.; Casey, E.; Glennon, B. The Effect of Mixing on the Metastable Zone Width and Nucleation Kinetics in the Anti-Solvent Crystallization of Benzoic Acid. Chem. Eng. Res. Des. 2007, 85, 945–952. [Google Scholar] [CrossRef]
- Barrett, M.; O’Grady, D.; Casey, E.; Glennon, B. The role of meso-mixing in anti-solvent crystallization processes. Chem. Eng. Sci. 2011, 66, 2523–2534. [Google Scholar] [CrossRef]
- Alvarez, A.J.; Myerson, A.S. Continuous Plug Flow Crystallization of Pharmaceutical Compounds. Cryst. Growth Des. 2010, 10, 2219–2228. [Google Scholar] [CrossRef]
- Ridder, B.J.; Majumder, A.; Nagy, Z.K. Population Balance Model-Based Multiobjective Optimization of a Multisegment Multiaddition (MSMA) Continuous Plug-Flow Antisolvent Crystallizer. Ind. Eng. Chem. Res. 2014, 53, 4387–4397. [Google Scholar] [CrossRef]
- Lee, J.; Ashokkumar, M.; Kentish, S.E. Influence of mixing and ultrasound frequency on antisolvent crystallisation of sodium chloride. Ultrason. Sonochem. 2014, 21, 60–68. [Google Scholar] [CrossRef]
- Chen, D.; Wang, B.; Sirkar, K.K. Hydrodynamic modeling of porous hollow fiber anti-solvent crystallizer for continuous production of drug crystals. J. Membr. Sci. 2018, 556, 185–195. [Google Scholar] [CrossRef]
- Tuo, L.; Ruan, X.; Xiao, W.; Li, X.; He, G.; Jiang, X. A novel hollow fiber membrane-assisted antisolvent crystallization for enhanced mass transfer process control. AIChE J. 2019, 65, 734–744. [Google Scholar] [CrossRef]
- Zarkadas, D.M.; Sirkar, K.K. Antisolvent crystallization in porous hollow fiber devices. Chem. Eng. Sci. 2006, 61, 5030–5048. [Google Scholar] [CrossRef]
- Baldyga, J.; Bourne, J.R. Interactions between mixing on various scales in stirred tank reactors. Chem. Eng. Sci. 1992, 47, 1839–1848. [Google Scholar] [CrossRef]
- Demirel, H.S.; Svärd, M.; Uysal, D.; Doğan, Ö.M.; Uysal, B.Z.; Forsberg, K. Antisolvent crystallization of battery grade nickel sulphate hydrate in the processing of lateritic ores. Sep. Purif. Technol. 2022, 286, 120473. [Google Scholar] [CrossRef]
- Peters, E.M.; Svärd, M.; Forsberg, K. Impact of process parameters on product size and morphology in hydrometallurgical antisolvent crystallization. CrystEngComm 2022, 24, 2851–2866. [Google Scholar] [CrossRef]
- He, K.; Gkioxari, G.; Dollár, P.; Girshick, R. Mask R-CNN. arXiv 2017, arXiv:1703.06870. [Google Scholar] [CrossRef]
- Sibirtsev, S.; Zhai, S.; Neufang, M.; Seiler, J.; Jupke, A. Mask R-CNN based droplet detection in liquid–liquid systems, Part 2: Methodology for determining training and image processing parameter values improving droplet detection accuracy. Chem. Eng. J. 2023, 473, 144826. [Google Scholar] [CrossRef]
- MacTaggart, R.S.; Nasr-El-Din, H.A.; Masliyah, J.H. Sample withdrawal from a slurry mixing tank. Chem. Eng. Sci. 1993, 48, 921–931. [Google Scholar] [CrossRef]
- Mersmann, A. Crystallization Technology Handbook, 2nd ed.; revised and expanded ed.; Dekker: New York, NY, USA, 2001. [Google Scholar]
- Mostafa Nowee, S.; Abbas, A.; Romagnoli, J.A. Antisolvent crystallization: Model identification, experimental validation and dynamic simulation. Chem. Eng. Sci. 2008, 63, 5457–5467. [Google Scholar] [CrossRef]
- Sviridova, T.A.; Sokolova, Y.V.; Pirozhenko, K.Y. Crystal structure of (NH4)5Sc3F14. Crystallogr. Rep. 2013, 58, 220–225. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonn, J.; Fuchs, A.R.; Libuda, L.; Jupke, A. Controlling Crystal Growth of a Rare Earth Element Scandium Salt in Antisolvent Crystallization. Crystals 2024, 14, 94. https://doi.org/10.3390/cryst14010094
Tonn J, Fuchs AR, Libuda L, Jupke A. Controlling Crystal Growth of a Rare Earth Element Scandium Salt in Antisolvent Crystallization. Crystals. 2024; 14(1):94. https://doi.org/10.3390/cryst14010094
Chicago/Turabian StyleTonn, Josia, Andreas Roman Fuchs, Leon Libuda, and Andreas Jupke. 2024. "Controlling Crystal Growth of a Rare Earth Element Scandium Salt in Antisolvent Crystallization" Crystals 14, no. 1: 94. https://doi.org/10.3390/cryst14010094




