Pressure Effects on the Thermodynamic Properties of MgSiO3 Akimotoite
Abstract
1. Introduction
2. Methods
2.1. Calculation Procedure
2.2. Thermoelastic Data of MgSiO3 Akimotoite
3. Results
3.1. High Temperature and High Pressure Unit-Cell Volume of MgSiO3 Akimotoite
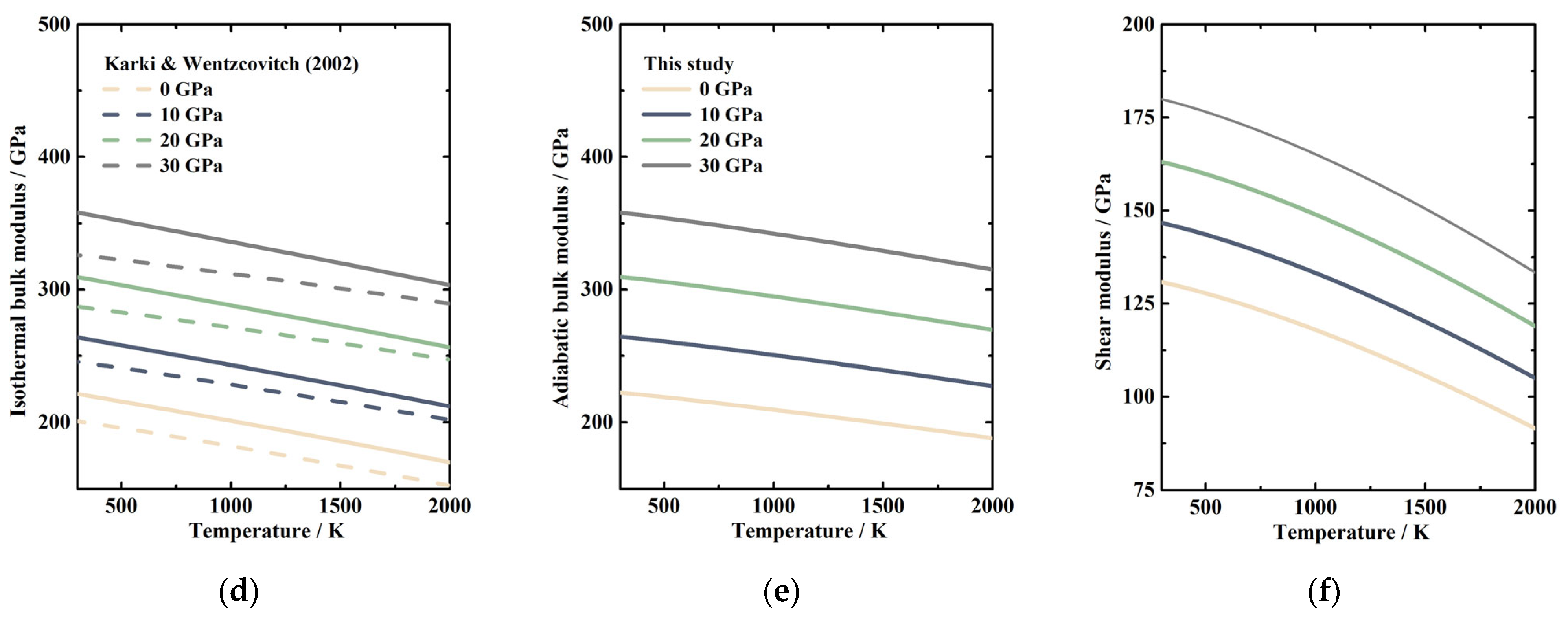
3.2. High Temperature and High-Pressure Elastic Properties of MgSiO3 Akimotoite
3.3. High Temperature and High-Pressure Thermodynamic Properties of MgSiO3 Akimotoite
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irifune, T.; Ringwood, A.E. Phase transformations in a harzburgite composition to 26 GPa: Implications for dynamical behavior of the subducting slab. Earth Planet. Sci. Lett. 1987, 86, 365–376. [Google Scholar] [CrossRef]
- Ringwood, A.E.; Irifune, T. Nature of the 650–km seismic discontinuity: Implications for mantle dynamics and differentiation. Nature 1988, 331, 131–136. [Google Scholar] [CrossRef]
- Zhou, C.; Gréaux, S.; Nishiyama, N.; Irifune, T.; Higo, Y. Sound velocities measurement on MgSiO3 akimotoite at high pressures and high temperatures with simultaneous in situ X-ray diffraction and ultrasonic study. Phys. Earth Planet. Inter. 2014, 228, 97–105. [Google Scholar] [CrossRef]
- Siersch, N.C.; Kurnosov, A.; Criniti, G.; Ishii, T.; Boffa Ballaran, T.; Frost, D.J. The elastic properties and anisotropic behavior of MgSiO3 akimotoite at transition zone pressures. Phys. Earth Planet. Inter. 2021, 320, 106786. [Google Scholar] [CrossRef]
- Ishii, T.; Kojitani, H.; Akaogi, M. Phase relations of harzburgite and MORB up to the uppermost lower mantle conditions: Precise comparison with pyrolite by multisample cell high-pressure experiments with implication to dynamics of subducted slabs. J. Geophys. Res. Solid Earth 2019, 124, 3491–3507. [Google Scholar] [CrossRef]
- Gasparik, T. Phase relations in the transition zone. J. Geophys. Res. Solid Earth 1990, 95, 15751–15769. [Google Scholar] [CrossRef]
- Chanyshev, A.; Ishii, T.; Bondar, D.; Bhat, S.; Kim, E.J.; Farla, R.; Nishida, K.; Liu, Z.; Wang, L.; Nakajima, A.; et al. Depressed 660-km discontinuity caused by akimotoite–bridgmanite transition. Nature 2022, 601, 69–73. [Google Scholar] [CrossRef]
- Weidner, D.; Ito, E. Elasticity of MgSiO3 in the ilmenite phase. Phys. Earth Planet. Inter. 1985, 40, 65–70. [Google Scholar] [CrossRef]
- Reynard, B.; Fiquet, G.; Itie, J.-P.; Rubie, D.C. High-pressure X-ray diffraction study and equation of state of MgSiO3 ilmenite. Am. Mineral. 1996, 81, 45–50. [Google Scholar] [CrossRef]
- Wang, Y.; Uchida, T.; Zhang, J.; Rivers, M.L.; Sutton, S.R. Thermal equation of state of akimotoite MgSiO3 and effects of the akimotoite–garnet transformation on seismic structure near the 660 km discontinuity. Phys. Earth Planet. Inter. 2004, 143–144, 57–80. [Google Scholar] [CrossRef]
- Karki, B.; Wentzcovitch, R. First-principles lattice dynamics and thermoelasticity of MgSiO3 ilmenite at high pressure. J. Geophys. Res. 2002, 107, ECV 2-1–ECV 2-6. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, D.; Matsui, M. Anisotropy of akimotoite: A molecular dynamics study. Phys. Earth Planet. Inter. 2005, 151, 309–319. [Google Scholar] [CrossRef]
- Li, L.; Weidner, D.J.; Brodholt, J.; Alfè, D.; Price, G.D. Ab initio molecular dynamics study of elasticity of akimotoite MgSiO3 at mantle conditions. Phys. Earth Planet. Inter. 2009, 173, 115–120. [Google Scholar] [CrossRef]
- Hao, S.; Wang, W.; Qian, W.; Wu, Z. Elasticity of akimotoite under the mantle conditions: Implications for multiple discontinuities and seismic anisotropies at the depth of ∼600–750 km in subduction zones. Earth Planet. Sci. Lett. 2019, 528, 115830. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, Z.; Hao, S.; Wang, W.; Deng, X.; Song, J. Elastic properties of Fe-bearing Akimotoite at mantle conditions: Implications for composition and temperature in lower mantle transition zone. Fundam. Res. 2022, 2, 570–577. [Google Scholar] [CrossRef]
- Ashida, T.; Kume, S.; Ito, E.; Navrotsky, A. MgSiO3 ilmenite: Heat capacity, thermal expansivity, and enthalpy of transformation. Phys. Chem. Miner. 1988, 16, 239–245. [Google Scholar] [CrossRef]
- Kojitani, H.; Yamazaki, M.; Tsunekawa, Y.; Katsuragi, S.; Noda, M.; Inoue, T.; Inaguma, Y.; Akaogi, M. Enthalpy, heat capacity and thermal expansivity measurements of MgSiO3 akimotoite: Reassessment of its self-consistent thermodynamic data set. Phys. Earth Planet. Inter. 2022, 333, 106937. [Google Scholar] [CrossRef]
- Akaogi, M.; Kojitani, H.; Morita, T.; Kawaji, H.; Atake, T. Low-temperature heat capacities, entropies and high-pressure phase relations of MgSiO3 ilmenite and perovskite. Phys. Chem. Miner. 2008, 35, 287–297. [Google Scholar] [CrossRef]
- Hernández, E.; Brodholt, J.; Alfè, D. Structural, vibrational and thermodynamic properties of Mg2SiO4 and MgSiO3 minerals from first-principles simulations. Phys. Earth Planet. Inter. 2014, 240, 1–24. [Google Scholar] [CrossRef]
- Davis, L.A.; Gordon, R.B. Compression of mercury at high pressure. J. Chem. Phys. 1967, 46, 2650–2660. [Google Scholar] [CrossRef]
- Su, C.; Liu, Y.; Fan, D.; Song, W.; Yang, G. Self-consistent thermodynamic parameters of pyrope and almandine at high-temperature and high-pressure conditions: Implication on the adiabatic temperature gradient. Phys. Earth Planet. Inter. 2022, 322, 106789. [Google Scholar] [CrossRef]
- Qiu, W.; Su, C.; Liu, Y.; Song, W. Thermodynamic properties of MgAl2O4 spinel at high temperatures and high pressures. Crystals 2023, 13, 240. [Google Scholar] [CrossRef]
- Johari, G.P. Entropy, enthalpy and volume of perfect crystals at limiting high pressure and the third law of thermodynamics. Thermochim. Acta 2021, 698, 178891. [Google Scholar] [CrossRef]
- Gonzalez-Platas, J.; Alvaro, M.; Nestola, F.; Angel, R. EosFit7-GUI: A new graphical user interface for equation of state calculations, analyses and teaching. J. Appl. Crystallogr. 2016, 49, 1377–1382. [Google Scholar] [CrossRef]
- Fei, Y. Thermal expansion. In Mineral Physics & Crystallography; AGU Reference Shelf; Wiley: Hoboken, NJ, USA, 1995; pp. 29–44. [Google Scholar]
- Maier, C.G.; Kelley, K.K. An equation for the representation of high-temperature heat content data. J. Am. Chem. Soc. 2002, 54, 3243–3246. [Google Scholar] [CrossRef]
- Haas, J.L.; Fisher, J.R. Simultaneous evaluation and correlation of thermodynamic data. Am. J. Sci. 1976, 276, 525–545. [Google Scholar] [CrossRef]
- Berman, R.G.; Brown, T.H. Heat capacity of minerals in the system Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-SiO2-TiO2-H2O-CO2: Representation, estimation, and high temperature extrapolation. Contrib. Mineral. Petrol. 1985, 89, 168–183. [Google Scholar] [CrossRef]
- Richet, P.; Fiquet, G. High-temperature heat capacity and premelting of minerals in the system MgO-CaO-Al2O3-SiO2. J. Geophys. Res. 1991, 96, 445–456. [Google Scholar] [CrossRef]
- Hofmeister, A.M.; Ito, E. Thermodynamic properties of MgSiO3 ilmenite from vibrational spectra. Phys. Chem. Miner. 1992, 18, 423–432. [Google Scholar] [CrossRef]
- Saxena, S.K.; Chatterjee, N.; Fei, Y.; Shen, G. Thermodynamic Data on Oxides and Silicates, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Chopelas, A. Thermal expansivity of mantle relevant magnesium silicates derived from vibrational spectroscopy at high pressure. Am. Mineral. 2000, 85, 270–278. [Google Scholar] [CrossRef]
- Watanabe, H. Thermochemical Properties of Synthetic High-Pressure Compounds Relevant to the Earth’s Mantle; Springer: Dordrecht, The Netherlands, 1982; pp. 441–464. [Google Scholar]
- Benisek, A.; Dachs, E. The accuracy of standard enthalpies and entropies for phases of petrological interest derived from density-functional calculations. Contrib. Mineral. Petrol. 2018, 173, 90. [Google Scholar] [CrossRef] [PubMed]
- Vočadlo, L.; Poirer, J.P.; Price, G.D. Grüneisen parameters and isothermal equations of state. Am. Mineral. 2000, 85, 390–395. [Google Scholar] [CrossRef]
- Yusa, H.; Akaogi, M.; Ito, E. Calorimetric study of MgSiO3 garnet and pyroxene: Heat capacities, transition enthalpies, and equilibrium phase relations in MgSiO3 at high pressures and temperatures. J. Geophys. Res. Solid Earth 1993, 98, 6453–6460. [Google Scholar] [CrossRef]
- Yu, Y.G.; Wentzcovitch, R.M.; Vinograd, V.L.; Angel, R.J. Thermodynamic properties of MgSiO3 majorite and phase transitions near 660 km depth in MgSiO3 and Mg2SiO4: A first principles study. J. Geophys. Res. 2011, 116, B02208. [Google Scholar] [CrossRef]
- Wentzcovitch, R.M.; Stixrude, L.; Karki, B.B.; Kiefer, B. Akimotoite to perovskite phase transition in MgSiO3. Geophys. Res. Lett. 2004, 31, L10611. [Google Scholar] [CrossRef]











| Data type | Temperature Range | Pressure Range | Method | References |
|---|---|---|---|---|
| K | GPa | |||
| Sound velocity | ~1500 | ~25.7 | Ultrasonic interferometry | Zhou et al. (2014) [3] |
| Unit-cell volume | 298–876 | Ambient | X-ray diffraction | Ashida et al. (1988) [16] |
| 298–773 | Ambient | X-ray diffraction | Kojitani et al. (2022) [17] | |
| Heat capacity | 170–700 | Ambient | Calorimetry | Ashida et al. (1988) [16] |
| 1.90–302.43 | Ambient | Thermal relaxation method | Akaogi et al. (2008) [18] | |
| 300–820 | Ambient | Calorimetry | Kojitani et al. (2022) [17] | |
| Standard entropy | 298.15 | Ambient | Calorimetry | Akaogi et al. (2008) [18] |
| Fitting Equations | Reduced χ2 | R2 | References of the Empirical Equations |
|---|---|---|---|
| 1.19 | 0.996 | Kojitani et al. (2022) [17] | |
| 1.21 | 0.996 | Hass and Fisher (1976) [27] | |
| 1.18 | 0.996 | Berman and Brown (1985) [28] | |
| 1.19 | 0.996 | Richet and Fiquet (1991) [29] |
| Temperature | KT | KS | G | References |
|---|---|---|---|---|
| K | GPa | GPa | GPa | |
| M0 (GPa) | ||||
| 300 | -- | 212 | 132 | Weidner and Ito (1985) [8] |
| 298 | 212 | -- | -- | Reynard et al. (1996) [9] |
| 300 | 201 | -- | -- | Karki & Wentzcovitch (2002) [11] |
| 298 | 210 | -- | -- | Wang et al. (2004) [10] |
| 300 | 221 | 226 | 136 | Zhang et al. (2005) [12] |
| 2000 | 158.1 (6) | -- | 85.7 | Li et al. (2009) [13] |
| 300 | 207 (3) | 218.9 (6) | 131.8 (3) | Zhou et al. (2014) [3] |
| 300 | 202 | 204 | 127 | Hao et al. (2019) [14] |
| 300 | 205 (1) | 209 (2) | -- | Siersch et al. (2021) [4] |
| 300 | 221.5 (11) | 222.4 (13) | 130.8 (5) | This study |
| ∂M/∂P | ||||
| 300 | 4.64 | -- | -- | Karki & Wentzcovitch (2002) [11] |
| 298 | 5.6 (8) | -- | -- | Wang et al. (2004) [10] |
| 300 | 3.94 | 3.85 | 1.04 | Zhang et al. (2005) [12] |
| 2000 | 3.7 (2) | -- | 4.5 (3) | Li et al. (2009) [13] |
| 300 | 4.6 | 4.62 (3) | 1.64 (1) | Zhou et al. (2014) [3] |
| 300 | 4.40 | 4.39 | 1.64 | Hao et al. (2019) [14] |
| 300 | -- | 4.4 | -- | Siersch et al. (2021) [4] |
| 300 | 4.49 (1) | 4.39 (1) | 1.54 (1) | This study |
| ∂M/∂T (×10−2 GPa/K) | ||||
| 300 | −2.5 | -- | -- | Karki & Wentzcovitch (2002) [11] |
| 298 | −4.0 (1) | -- | -- | Wang et al. (2004) [10] |
| 300 | −3.0 | −2.16 | −1.79 | Zhang et al. (2005) [12] |
| 300 | -- | −1.99(9) | −1.58(4) | Zhou et al. (2014) [3] |
| 300 | −2.3 | −1.719 | −1.242 | Hao et al. (2019) [14] |
| 300 | −2.943 (1) | −1.937 (1) | −1.645 (1) | This study |
| ∂2M/∂T2 (×10−6 GPa/K2) | ||||
| 300 | -- | −2.9 (8) | −6.7 (4) | Zhou et al. (2014) [3] |
| 300 | -- | −1.26 | −1.94 | Hao et al. (2019) [14] |
| 300 | −0.847 (6) | −2.060 (8) | −5.351 (4) | This study |
| T K | α 10−5/K | CP J/mol∙K | S J/mol∙K | γ |
|---|---|---|---|---|
| N0 | ||||
| 10−5/K | J/mol∙K | J/mol∙K | ||
| 300 | 1.35 (4) | 77 (17) | 54(1) | 1.03 (25) |
| 700 | 1.44 (3) | 116 (9) | 138 (10) | 1.35 (2) |
| 1000 | 3.03 (20) | 124 (8) | 181 (14) | 1.37 (2) |
| 1500 | 3.37 (27) | 130 (7) | 233 (16) | 1.41 (7) |
| 2000 | 3.66 (35) | 133 (6) | 271 (18) | 1.44 (12) |
| ∂N/∂P | ||||
| 10−7/K∙GPa | J/mol∙K∙GPa | J/mol∙K∙GPa | 10−2/GPa | |
| 300 | −5.84 (2) | −0.8066 (6) | −0.315 (2) | −2.200 (7) |
| 700 | −5.79 (2) | −0.2356 (6) | −0.681 (2) | −0.981 (3) |
| 1000 | −6.64 (3) | −0.2078 (11) | −0.758 (2) | −1.096 (4) |
| 1500 | −8.01 (4) | −0.2472 (20) | −0.848 (3) | −1.377 (5) |
| 2000 | −9.42 (5) | −0.3178 (28) | −0.929 (4) | −1.643 (6) |
| ∂2N/∂P2 | ||||
| 10−9/K∙GPa2 | 10−3J/mol∙K∙GPa2 | 10−3J/mol∙K∙GPa2 | 10−4/GPa2 | |
| 300 | 6.40 (6) | 0.53 (2) | 5.14 (6) | −1.210 (2) |
| 700 | 6.29 (6) | 1.60 (2) | 5.99 (6) | 0.762 (10) |
| 1000 | 7.13 (10) | 2.36 (3) | 6.69 (7) | 0.945 (13) |
| 1500 | 9.12 (14) | 3.72 (6) | 7.90 (9) | 1.243 (18) |
| 2000 | 11.19 (18) | 5.20 (9) | 9.17 (11) | 1.482 (21) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Song, W.; Yang, G.; Liu, Y.; Li, Q. Pressure Effects on the Thermodynamic Properties of MgSiO3 Akimotoite. Crystals 2024, 14, 837. https://doi.org/10.3390/cryst14100837
Su C, Song W, Yang G, Liu Y, Li Q. Pressure Effects on the Thermodynamic Properties of MgSiO3 Akimotoite. Crystals. 2024; 14(10):837. https://doi.org/10.3390/cryst14100837
Chicago/Turabian StyleSu, Chang, Wei Song, Guang Yang, Yonggang Liu, and Qingyi Li. 2024. "Pressure Effects on the Thermodynamic Properties of MgSiO3 Akimotoite" Crystals 14, no. 10: 837. https://doi.org/10.3390/cryst14100837
APA StyleSu, C., Song, W., Yang, G., Liu, Y., & Li, Q. (2024). Pressure Effects on the Thermodynamic Properties of MgSiO3 Akimotoite. Crystals, 14(10), 837. https://doi.org/10.3390/cryst14100837






