Growth and Faceting of Tungsten and Oxides in Scandate Cathode Particles during In Situ Heating in the Scanning Electron Microscope
Abstract
:1. Introduction
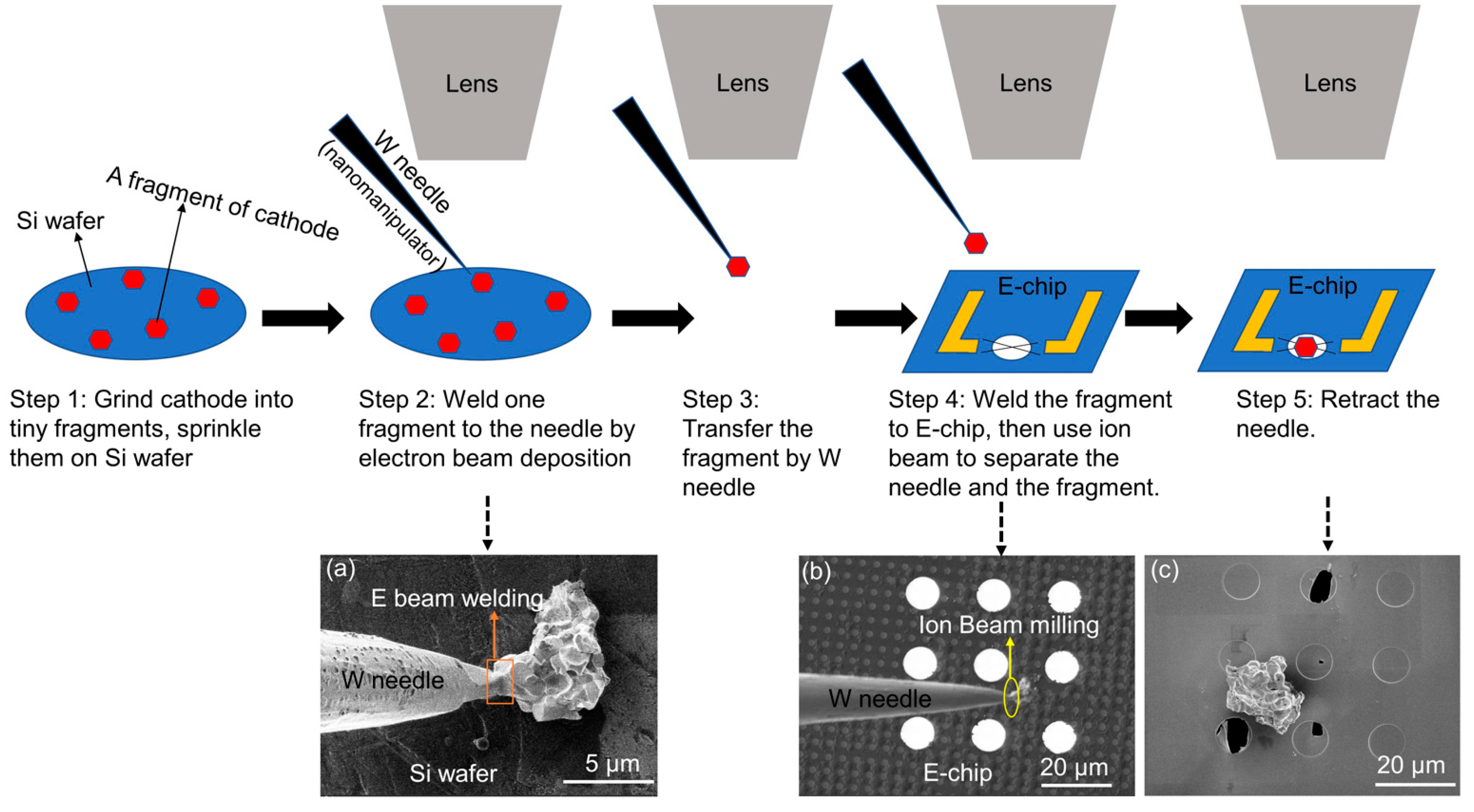
2. Materials and Methods
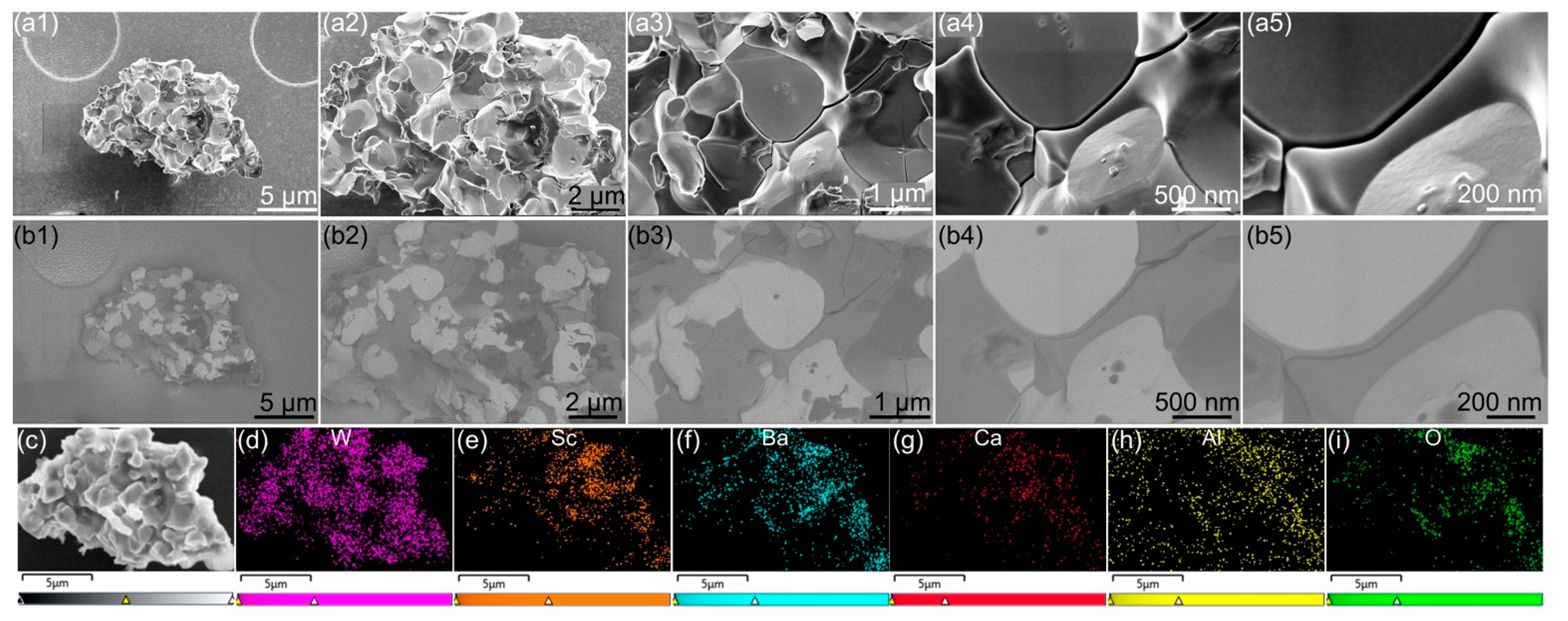
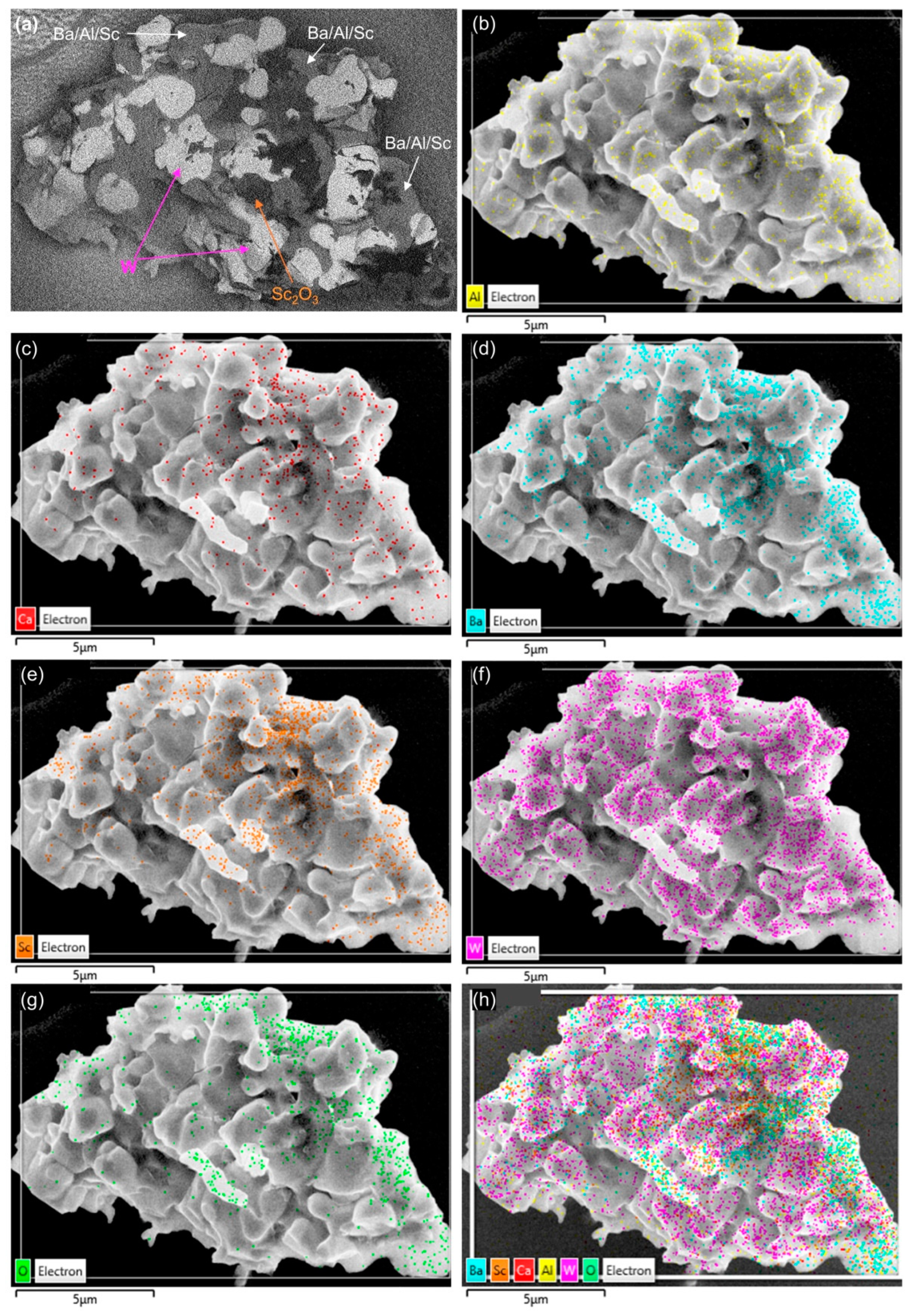
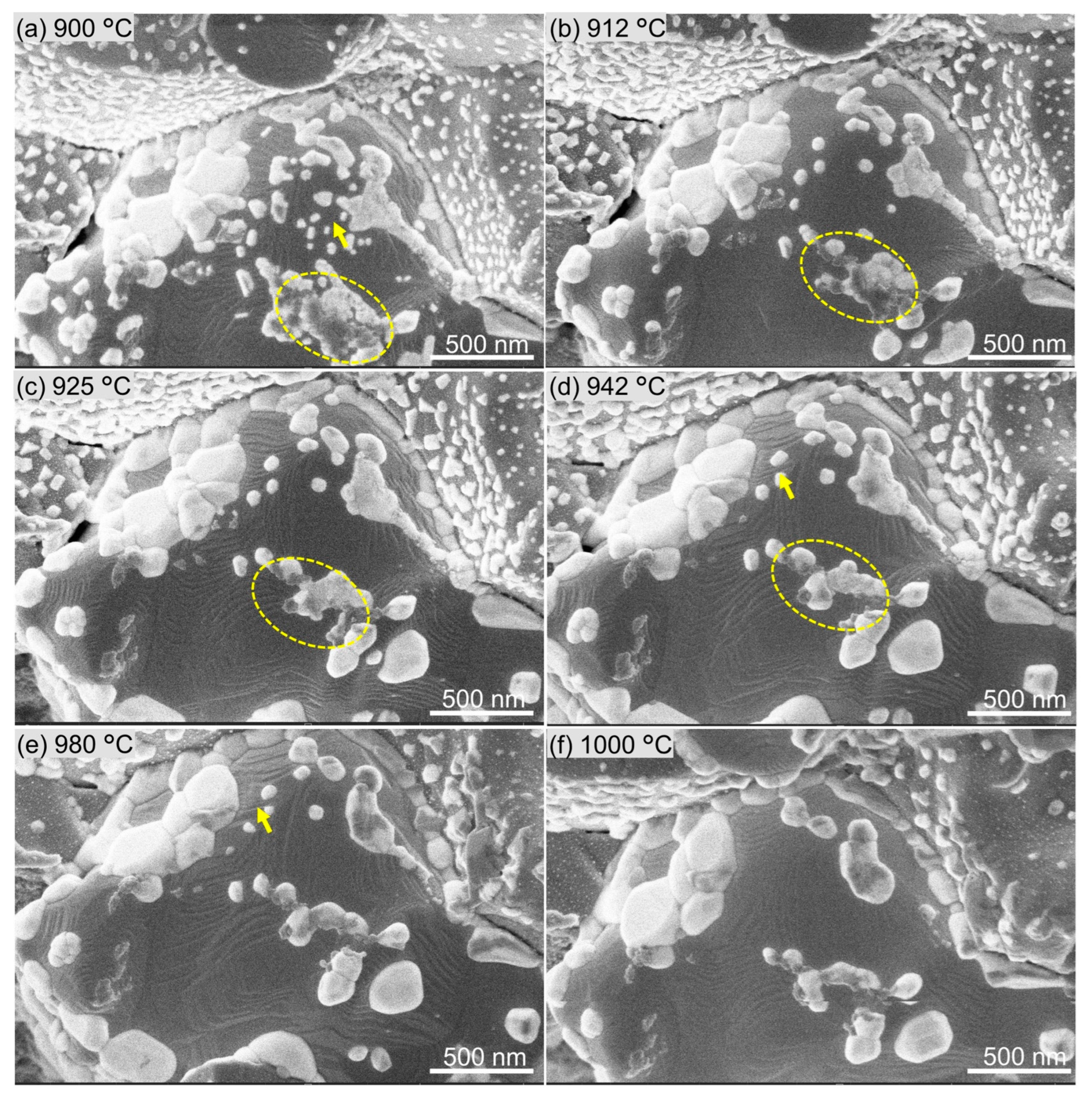
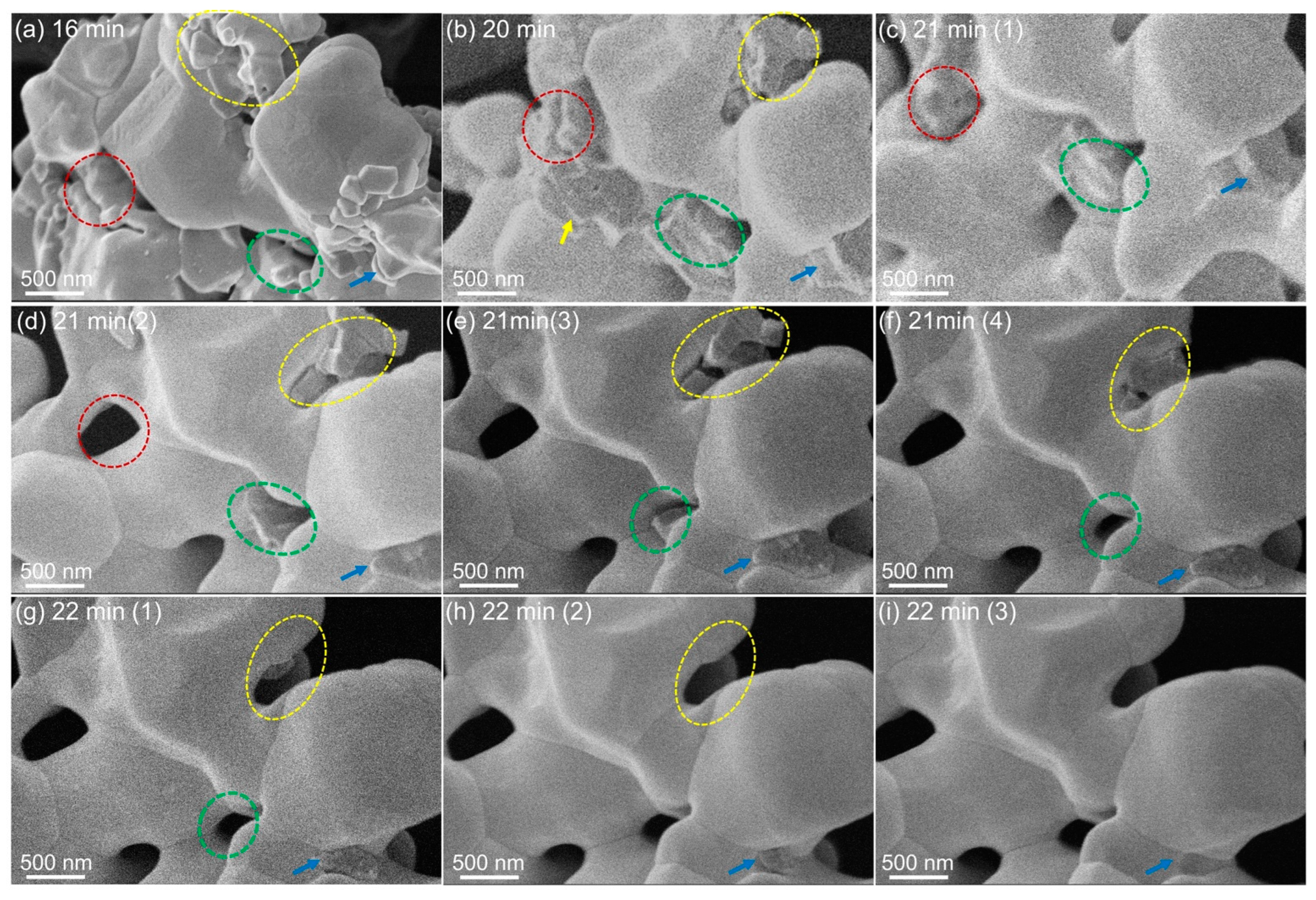
3. Results and Analysis
4. Discussion
4.1. Behavior of Impregnant Materials during Heating Experiments
4.2. Incipient Faceting and Formation of Nanoscale Steps on W Grains
4.3. Scandate Dispenser Cathode Activation
5. Conclusions
- (1)
- EDS analysis of faceted impregnant particles indicated they were predominantly composed of barium-containing oxides, with BaAl2O4 observed frequently.
- (2)
- As temperature was increased, impregnants exhibited phase separation and began to sublimate.
- (3)
- Concurrently, nanoscale steps emerged on tungsten surfaces, although the tungsten grains did not achieve a highly faceted morphology.
- (4)
- As samples approached a temperature of 1200 °C, impregnant materials underwent rapid dissipation, most likely via sublimation.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bugaris, D.E.; Goggin, C.; Zhang, X.; Balk, J.; Busbaher, D.; Tucek, J. High-Performance Scandate Cathode. In Proceedings of the 2019 International Vacuum Electronics Conference (IVEC), Busan, Republic of Korea, 28 April–1 May 2019; pp. 1–2. [Google Scholar]
- Gao, J.Y.; Yang, Y.F.; Zhang, X.K.; Li, S.L.; Hu, P.; Wang, J.S. A review on recent progress of thermionic cathode. Tungsten 2020, 2, 289–300. [Google Scholar] [CrossRef]
- Kirkwood, D.M.; Gross, S.J.; Balk, T.J.; Beck, M.J.; Booske, J.; Busbaher, D.; Jacobs, R.; Kordesch, M.E.; Mitsdarffer, B.; Morgan, D.; et al. Frontiers in Thermionic Cathode Research. IEEE Trans. Electron Devices 2018, 65, 2061–2071. [Google Scholar] [CrossRef]
- Liu, X.; Vancil, B.K.; Durham, D.B.; Ogletree, D.F.; Barnard, E.S.; Balk, T.J. Observation of Ultrathin Sc-Containing Surface Layer on Life-Tested Scandate Cathodes. IEEE Trans. Electron Devices 2023, 70, 2876–2882. [Google Scholar] [CrossRef]
- Gong, T.; Zhou, Y.; Zhang, Z.; Xu, L.; Zhou, X.; Fu, H. Environmental Stability of 612-aluminate with Different Phase Structures. In Proceedings of the 2023 24th International Vacuum Electronics Conference (IVEC), Chengdu, China, 25–28 April 2023; pp. 1–2. [Google Scholar]
- Sun, J.; Gao, J.; Yang, Y.; Zhang, R.; Liu, H.; Wang, J. Investigation on Scadium Thin Films on Dispenser Cathodes. In Proceedings of the 2023 24th International Vacuum Electronics Conference (IVEC), Chengdu, China, 25–28 April 2023; pp. 1–2. [Google Scholar]
- Cai, Y.; Yang, Y.; Liu, H.; Sun, J.; Li, Z.; Zhou, W.; Hu, Y.; Wang, J. Synthesis of impregnants for dispenser cathodes via homogeneous-lyophilization: Phase structure, optical response and emission property of four aluminates. Ceram. Int. 2024, 50, 11341–11350. [Google Scholar] [CrossRef]
- Lai, C.; Guo, G.; Dong, L.; Cai, Y.; Zhou, F.; Zheng, Z.; Yang, Y.; Zhou, W.; Wang, J. The effects of aluminate compounds on the free Ba generation and electron emission performance of dispenser cathode. Ceram. Int. 2024, in press. [CrossRef]
- Shang, Y.; Zheng, Q.; Cao, Y.; Hu, J.; Fu, H.; Luo, Y.; Yang, D. Effect of sintering atmospheres on the structure and properties of the 612 type barium-calcium-aluminate for the dispenser cathodes. Ceram. Int. 2019, 45, 14816–14820. [Google Scholar] [CrossRef]
- Ives, R.L.; Falce, L.R.; Miram, G.; Collins, G. Controlled-Porosity Cathodes for High-Current-Density Applications. IEEE Trans. Plasma Sci. 2010, 38, 1345–1353. [Google Scholar] [CrossRef]
- Swartzentruber, P.D. Microstructure and Work Function of Dispenser Cathode Coatings: Effects on Thermionic Emission. Ph.D. Dissertation, University of Kentucky, Lexington, KY, USA, 2014. [Google Scholar]
- Liu, X.; Zhou, Q.; Maxwell, T.L.; Vancil, B.K.; Beck, M.J.; Balk, T.J. Scandate cathode surface characterization: Emission testing, elemental analysis and morphological evaluation. Mater. Charact. 2019, 148, 188–200. [Google Scholar] [CrossRef]
- Liu, X. Characterization of Nanostructure, Materials, and Electron Emission Performance of Next-Generation Thermionic Scandate Cathodes. Ph.D. Dissertation, University of Kentucky, Lexington, KY, USA, 2019. [Google Scholar]
- Liu, X.; Vancil, B.K.; Beck, M.J.; Balk, T.J. Near-Surface Material Phases and Microstructure of Scandate Cathodes. Materials 2019, 12, 636. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, X.; Maxwell, T.; Vancil, B.; Balk, T.J.; Beck, M.J. BaxScyOz on W (0 0 1), (1 1 0), and (1 1 2) in scandate cathodes: Connecting to experiment via μO and equilibrium crystal shape. Appl. Surf. Sci. 2018, 458, 827–838. [Google Scholar] [CrossRef]
- Kapustin, V.; Li, I.; Shumanov, A.V.; Lebedinskii, Y.Y.; Zablotskii, A.V.E. Physical operating principles of scandate cathodes for microwave devices. Tech. Phys. 2017, 62, 116–126. [Google Scholar] [CrossRef]
- Shukla, S.; Singh, A.; Barik, R. Synthesis of Rhenium–Scandia doped tungsten nanoparticles for shrinkage investigation. J. Sol-Gel Sci. Technol. 2020, 95, 384–392. [Google Scholar] [CrossRef]
- Barik, R.K.; Sattorov, M.; Park, G.-S. Cathode Technologies for Terahertz Source. In Advances in Terahertz Source Technologies; Jenny Stanford Publishing: Singapore, 2024; pp. 665–700. [Google Scholar]
- Zheng, Q.; Huang, Z.; Shang, Y.; Liu, M.; Li, Y.; Luo, Y.; Fu, H. Single-phase (Ba,Ca)2ScAlO5 impregnant for dispenser cathodes with enhanced thermionic emission performance. Ceram. Int. 2021, 47, 1632–1639. [Google Scholar] [CrossRef]
- Sun, J.; Yang, Y.; Dong, L.; Liu, Z.; Liu, P.; Cai, Y.; Liu, H.; Wang, J. Vacuum evaporation synthesizing Sc film layer and elucidation of near-surface Ba-O-Sc electronic configuration for dispenser cathode. Appl. Surf. Sci. 2024, 679, 161241. [Google Scholar] [CrossRef]
- Li, N.; Zhang, K.; Feng, J. Effect of Sr-Doping on the Emission-Active Materials of Scandate Cathodes. IEEE Trans. Electron Devices 2023, 70, 5883–5889. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Liu, W.; Wang, Y.; Wang, Y.; Zhou, M. Sc2O3–W matrix impregnated cathode with spherical grains. J. Phys. Chem. Solids 2008, 69, 2103–2108. [Google Scholar] [CrossRef]
- Lai, C.; Wang, J.; Zhou, F.; Liu, W.; den Engelsen, D.; Miao, N. Emission and evaporation properties of 75 at.% Re-25 at.% W mixed matrix impregnated cathode. Appl. Surf. Sci. 2018, 427, 874–882. [Google Scholar] [CrossRef]
- Krachkovskaya, T.; Storublev, A.; Sakhadji, G.; Emelyanov, A. Performance Investigation for Nanocarbon Modified Dispenser Cathode. J. Russ. Univ. Radioelectron. 2018, 4, 57–63. [Google Scholar] [CrossRef]
- Ao, B.; Zheng, Q.; Yan, R.; Luo, Y. Effect of Surface Features on Emission Properties of Impregnated Scandate Dispenser Cathode. In Proceedings of the 2023 24th International Vacuum Electronics Conference (IVEC), Chengdu, China, 25–28 April 2023; pp. 1–2. [Google Scholar]
- Gaertner, G.; Vancil, B.K. Electron Emission Model of Ba Dispenser Scandate Cathodes Based on the Beamlet Effect. e-J. Surf. Sci. Nanotechnol. 2024, 22, 179–187. [Google Scholar] [CrossRef]
- Gharaati, A.; Mardani, D. Fabrication and enhancement of a new high-current density scandate electron emitter for a pulsed Pierce electron gun. Eur. Phys. J. Plus 2022, 137, 615. [Google Scholar] [CrossRef]
- Zheng, Q.; Gong, T.; Wu, F.; Ilyas, N.; Huang, Z.; Li, Y.; Luo, Y.; Fu, H. Effect of Sc2O3 addition on the phase composition and electron emission performance of Co-precipitated aluminates for impregnated cathodes. Ceram. Int. 2021, 47, 18831–18837. [Google Scholar] [CrossRef]
- Zhao, J.; Gamzina, D.; Li, N.; Li, J.; Spear, A.G.; Barnett, L.; Banducci, M.; Risbud, S.; Luhmann, N.C. Scandate dispenser cathode fabrication for a high-aspect-ratio high-current-density sheet beam electron gun. IEEE Trans. Electron Devices 2012, 59, 1792–1798. [Google Scholar] [CrossRef]
- Lu, Z.; Yin, S.; Zhang, Z.; Ren, F.; Lv, X. Study on Low-Temperature Emission Performance of Scandate Cathode with Micro-Blade-Type Arrays. Materials 2019, 13, 100. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, P.; Wang, J.; Zhang, R.; Yang, Y.; Zhou, W.; Liu, H.; Sun, J. The effect of Ir and Sc on the emission capacity of W-Ir matrix scandate cathodes prepared via a novel in situ method. Acta Mater. 2023, 261, 119400. [Google Scholar] [CrossRef]
- Zhou, Y.; Gong, T.; Wu, M.; Xu, L.; Fu, H. Thermionic Emission Capability of the Ba–Sc–Al–O Compound Synthesized by High-Temperature Solid-State Process. IEEE Trans. Electron Devices 2023, 70, 1294–1298. [Google Scholar] [CrossRef]
- Wang, M.; Hu, K.; Wu, R.; Khanlari, K.; Zhang, F.; Lin, B. Effect of tungsten powder particle shape on the emission properties of the barium tungsten cathode. Adv. Powder Technol. 2023, 34, 104133. [Google Scholar] [CrossRef]
- Mantica, A.M.; Detisch, M.J.; Balk, T.J. Thermionic Emission Characterization of M-Type Cathodes Using Kelvin Probe in an Ultra-High Vacuum Environment. IEEE Trans. Electron Devices 2023, 70, 2864–2871. [Google Scholar] [CrossRef]
- Mantica, A. Surface Properties, Work Function, and Thermionic Electron Emission Characterization of Materials for Next-Generation Dispenser Cathodes. Ph.D. Dissertation, University of Kentucky, Lexington, KY, USA, 2023. [Google Scholar]
- Podor, R.; Le Goff, X.; Lautru, J.; Brau, H.P.; Barreau, M.; Carrier, X.; Mendonca, J.; Nogues, D.; Candeias, A. Direct Observation of the Surface Topography at High Temperature with SEM. Microsc. Microanal. 2020, 26, 397–402. [Google Scholar] [CrossRef]
- Gregori, G.; Kleebe, H.-J.; Siegelin, F.; Ziegler, G. In situ SEM imaging at temperatures as high as 1450 °C. Microscopy 2002, 51, 347–352. [Google Scholar]
- Bai, H.; Briot, N.J.; Beck, M.; Balk, T.J. Crystallographic Faceting of Bulk Tungsten Surfaces Observed During In Situ Heating in an Environmental Scanning Electron Microscope. Mater. Charact. 2024, 212, 113925. [Google Scholar] [CrossRef]
- Bai, H.; Balk, T.J. Observations of Temperature-Induced Material Transformations in Impregnated Scandate Cathode Samples During in situ Heating in the SEM. In Proceedings of the 2022 23rd International Vacuum Electronics Conference (IVEC), Monterey, CA, USA, 25–29 April 2022; pp. 238–239. [Google Scholar]
- Howe, J.; Walker, L.; Allard, L.; Demers, H. Early Experiments with a New In Situ SEM Heating Technology. Microsc. Microanal. 2017, 17, 432–433. [Google Scholar] [CrossRef]
- Mujin, M.; Nakamura, M.; Matsumoto, M. In-situ FE-SEM observation of the growth behaviors of Fe particles at magmatic temperatures. J. Cryst. Growth 2021, 560–561, 126043. [Google Scholar] [CrossRef]
- Leng, Y. Materials Characterization: Introduction to Microscopic and Spectroscopic Methods; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Zhu, Y.; Liu, D.; Jing, H.; Zhang, F.; Zhang, X.; Hu, S.; Zhang, L.; Wang, J.; Zhang, L.; Zhang, W. Oxygen activation on Ba-containing perovskite materials. Sci. Adv. 2022, 8, eabn4072. [Google Scholar] [CrossRef] [PubMed]
- Vancil, B.; Brodie, I.; Lorr, J.; Schmidt, V. Scandate dispenser cathodes with sharp transition and their application in microwave tubes. IEEE Trans. Electron Devices 2014, 61, 1754–1759. [Google Scholar] [CrossRef]
- Vancil, B.; Jones, D.; Kleschuk, M.; Schmidt, V.; Vancil, A.; Ohlinger, W.; Green, M. Recent Progress on Scandate Cathodes. In Proceedings of the 2020 IEEE 21st International Conference on Vacuum Electronics (IVEC), Monterey, CA, USA, 19–22 October 2020; pp. 81–82. [Google Scholar]
- Bai, H.; Maxwell, T.L.; Kordesch, M.E.; Balk, T.J. Physical vapor deposition and thermally induced faceting of tungsten nanoparticles. Mater. Charact. 2023, 198, 112724. [Google Scholar] [CrossRef]
- Acsente, T.; Negrea, R.F.; Nistor, L.C.; Matei, E.; Grisolia, C.; Birjega, R.; Dinescu, G. Tungsten nanoparticles with controlled shape and crystallinity obtained by magnetron sputtering and gas aggregation. Mater. Lett. 2017, 200, 121–124. [Google Scholar] [CrossRef]
- Togaru, M.; Sainju, R.; Zhang, L.; Jiang, W.; Zhu, Y. Direct observation of tungsten oxidation studied by in situ environmental TEM. Mater. Charact. 2021, 174, 111016. [Google Scholar] [CrossRef]
- Yu, H.; Das, S.; Liu, J.; Hess, J.; Hofmann, F. Surface terraces in pure tungsten formed by high temperature oxidation. Scr. Mater. 2019, 173, 110–114. [Google Scholar] [CrossRef]
- Szczepkowicz, A.; Bryl, R. Observation of vertex-rounding transition for a crystal in equilibrium: Oxygen-covered tungsten. Phys. Rev. B 2005, 71, 113416. [Google Scholar] [CrossRef]
- Szczepkowicz, A.; Bryl, R. From hill-and-valley faceting to global faceting of a crystal: Oxygen-covered tungsten. Surf. Sci. 2004, 559, L169–L172. [Google Scholar] [CrossRef]
- Kresse, R.; Baudis, U.; Jäger, P.; Riechers, H.H.; Wagner, H.; Winkler, J.; Wolf, H.U. Barium and barium compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2000. [Google Scholar]
- Wang, Y.; Wang, J.; Liu, W.; Zhang, X.; Li, L. Emission mechanism of high current density scandia-doped dispenser cathodes. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2011, 29, 04E106. [Google Scholar] [CrossRef]
- Busbaher, D.; Zhao, J.; Gamzina, D.; Luhmann, N.C. Performance comparison between sintered tungsten dispenser cathodes and nano-composite scandate dispenser cathodes. In Proceedings of the 2013 IEEE 14th International Vacuum Electronics Conference (IVEC), Paris, France, 21–23 May 2013; pp. 1–2. [Google Scholar]
- Huang, Z.; Wu, F.; Zheng, Q.; Xiong, H.; Fang, J.; Liu, M.; Yan, R.; Luo, Y.; Ilyas, N.; Fu, H. Structure and emission properties of barium calcium aluminates synthesized by room-temperature solid-state reaction approach. Mater. Chem. Phys. 2021, 263, 124418. [Google Scholar] [CrossRef]
- Kordesch, M.; Vaughn, J.; Wan, C.; Jamison, K. Model scandate cathodes investigated by thermionic emission microscopy. In Proceedings of the 2010 8th International Vacuum Electron Sources Conference and Nanocarbon, Nanjing, China, 14–16 October 2010; pp. 41–42. [Google Scholar]
- Kordesch, M.E.; Wan, C. Barium de-wetting and thermionic emission from Ba on W. In Proceedings of the 2013 26th International Vacuum Nanoelectronics Conference (IVNC), Roanoke, VA, USA, 8–12 July 2013; pp. 1–2. [Google Scholar]
- Vaughn, J.M.; Wan, C.; Jamison, K.D.; Kordesch, M.E. Thermionic electron emission microscopy of metal-oxide multilayers on tungsten. IBM J. Res. Dev. 2011, 55, 14:11–14:16. [Google Scholar] [CrossRef]
- Worthing, A. The true temperature scale of tungsten and its emissive powers at incandescent temperatures. Phys. Rev. 1917, 10, 377. [Google Scholar] [CrossRef]
- Rutgers, G.A.W.; De Vos, J.C. Relation between brightness, temperature, true temperature and colour temperature of tungsten. Luminance of tungsten. Physica 1954, 20, 715–720. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Balk, T.J. Growth and Faceting of Tungsten and Oxides in Scandate Cathode Particles during In Situ Heating in the Scanning Electron Microscope. Crystals 2024, 14, 840. https://doi.org/10.3390/cryst14100840
Bai H, Balk TJ. Growth and Faceting of Tungsten and Oxides in Scandate Cathode Particles during In Situ Heating in the Scanning Electron Microscope. Crystals. 2024; 14(10):840. https://doi.org/10.3390/cryst14100840
Chicago/Turabian StyleBai, Huanhuan, and Thomas John Balk. 2024. "Growth and Faceting of Tungsten and Oxides in Scandate Cathode Particles during In Situ Heating in the Scanning Electron Microscope" Crystals 14, no. 10: 840. https://doi.org/10.3390/cryst14100840





