Effect of Trace Rare Earth Element Cerium (Ce) on Microstructure and Mechanical Properties of High Strength Marine Engineering Steel
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Experimental Materials
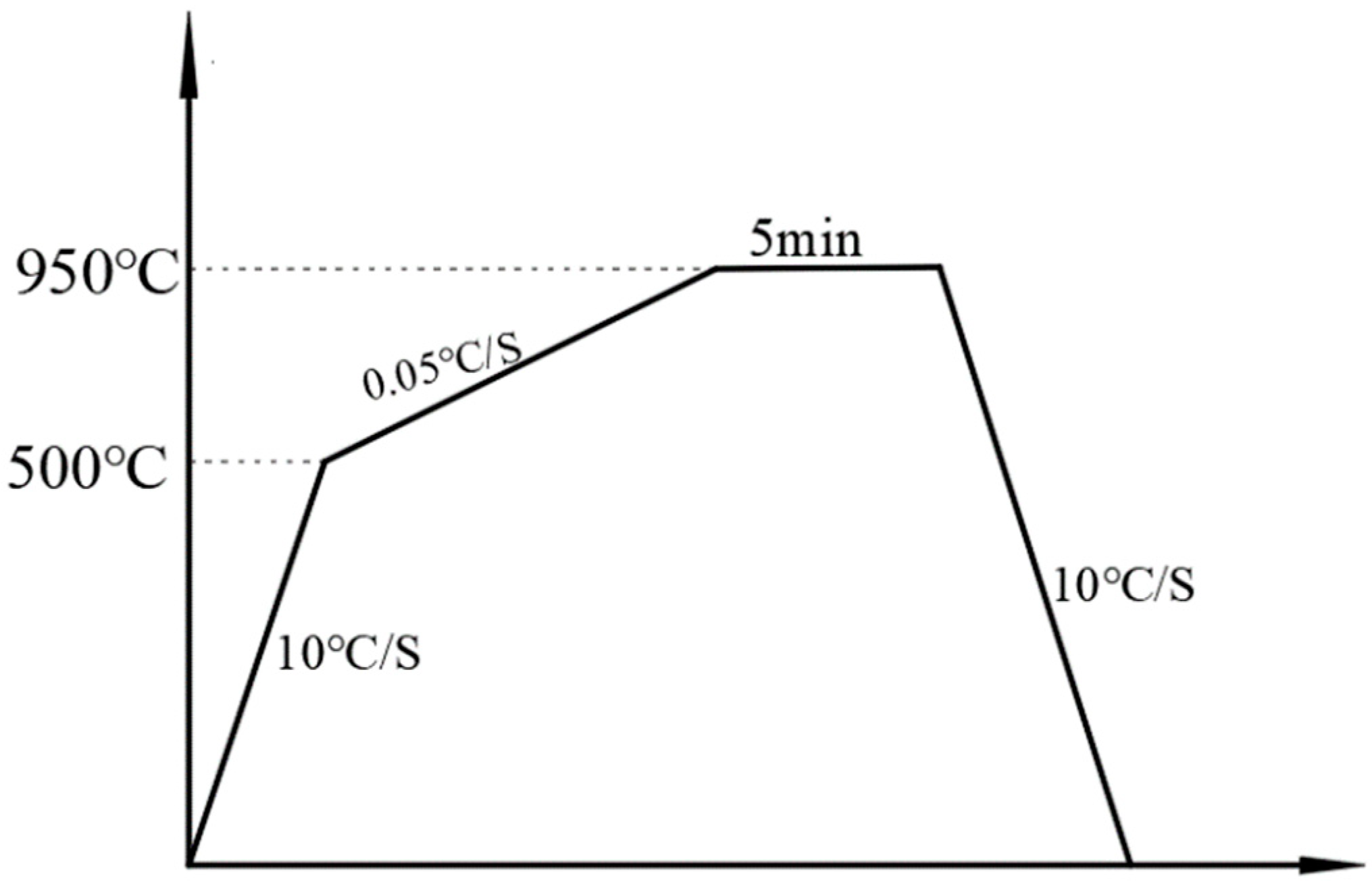
2.2. The Effect of Rare Earth Elements on the Phase Transition Point of the Test Steel
2.3. Morphology Observation and Composition Analysis of Inclusions
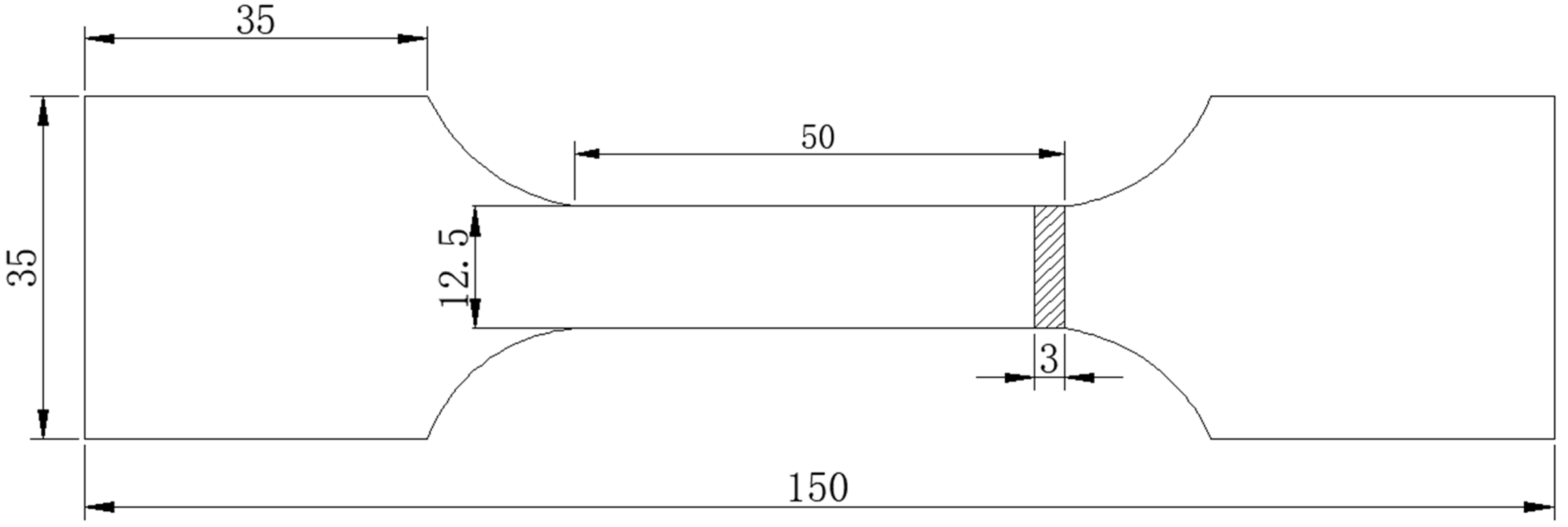
2.4. Mechanical Properties Test
3. Results Discussion
3.1. Original Microstructure Analysis and Phase Transformation Point Determination of Test Steel
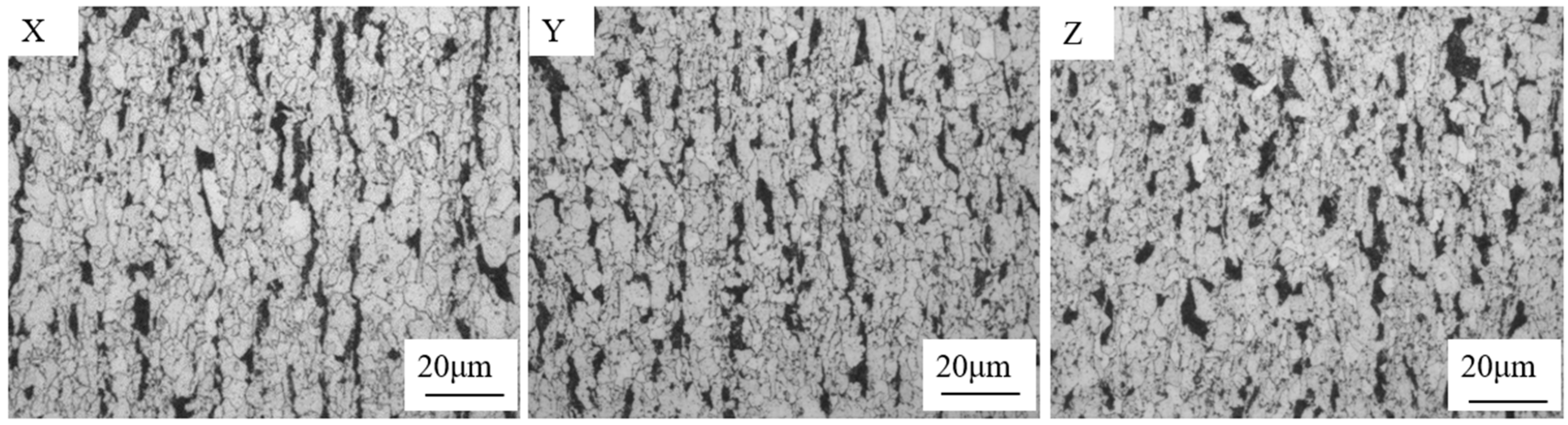
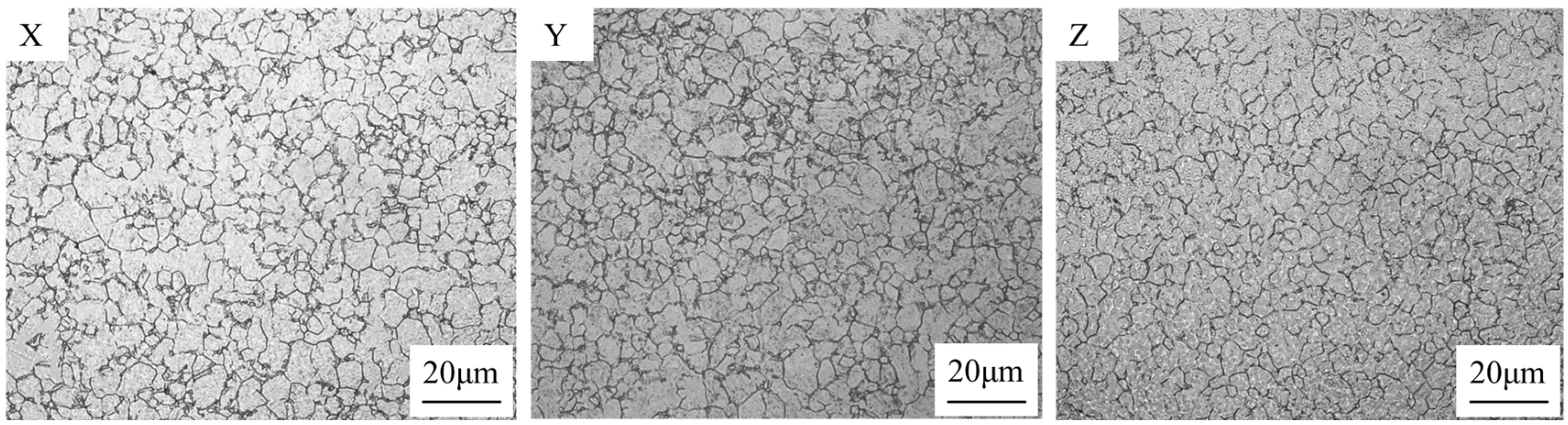
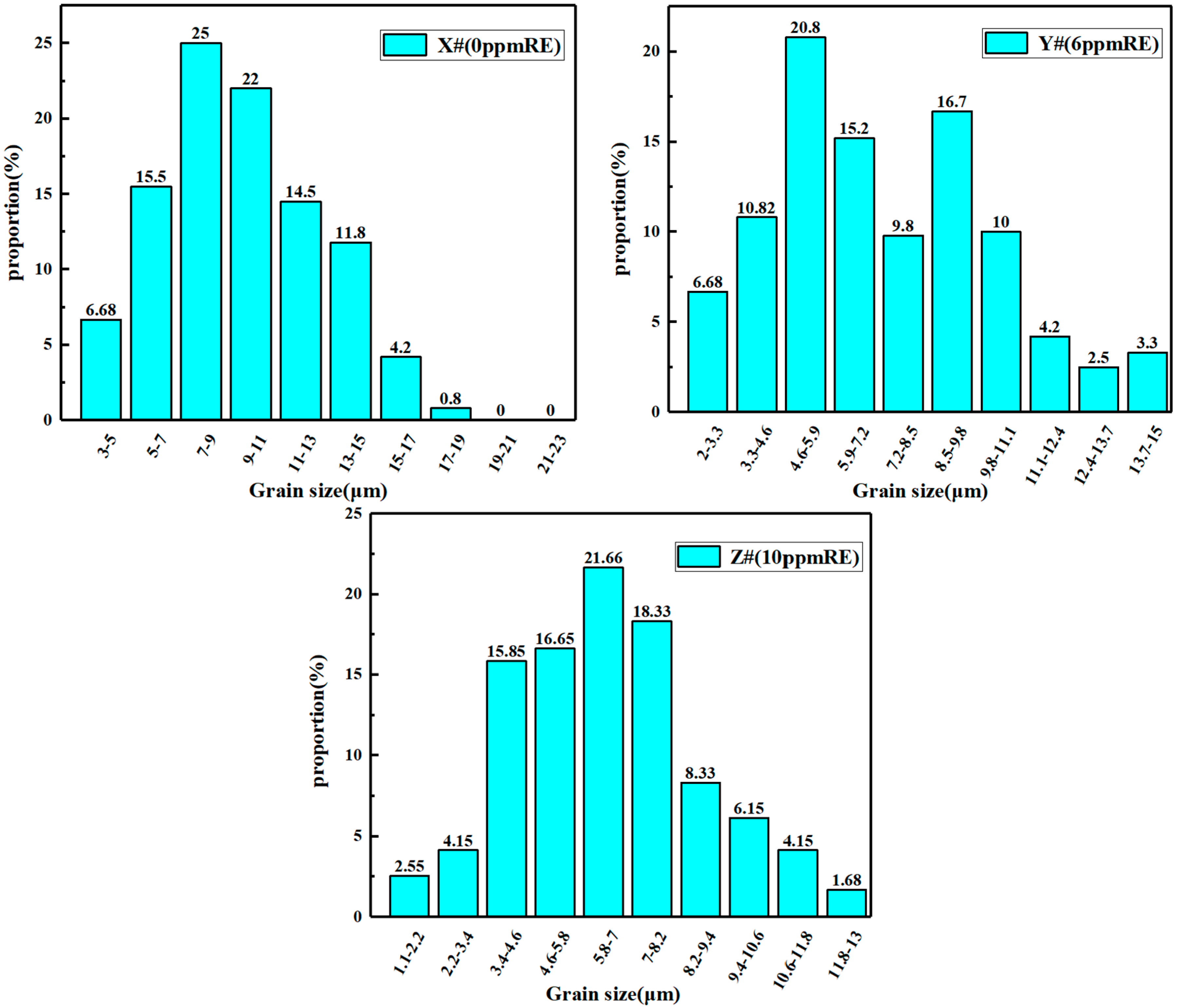
3.2. Effect of Trace Ce on the Original Austenite Grain Size
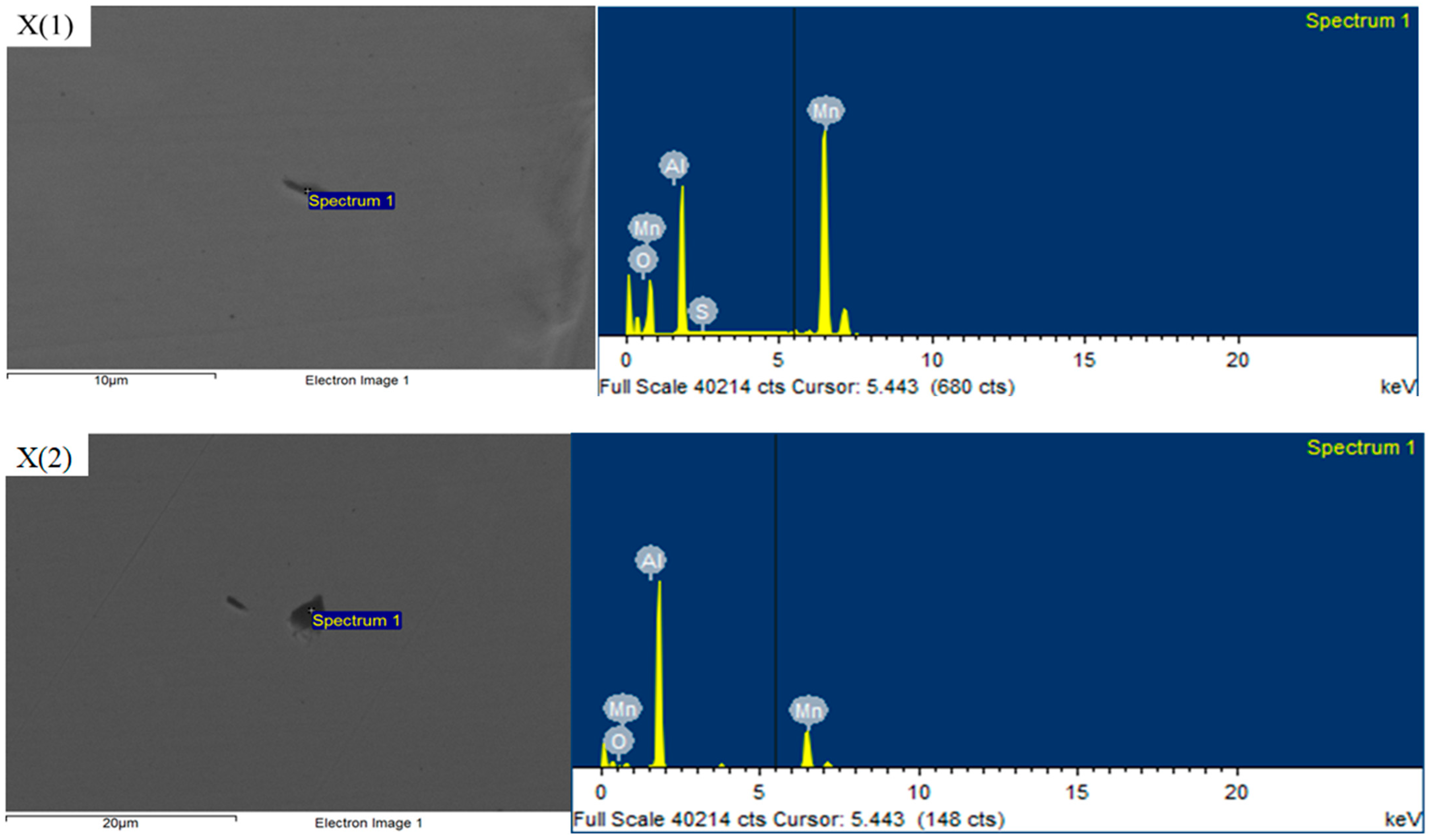
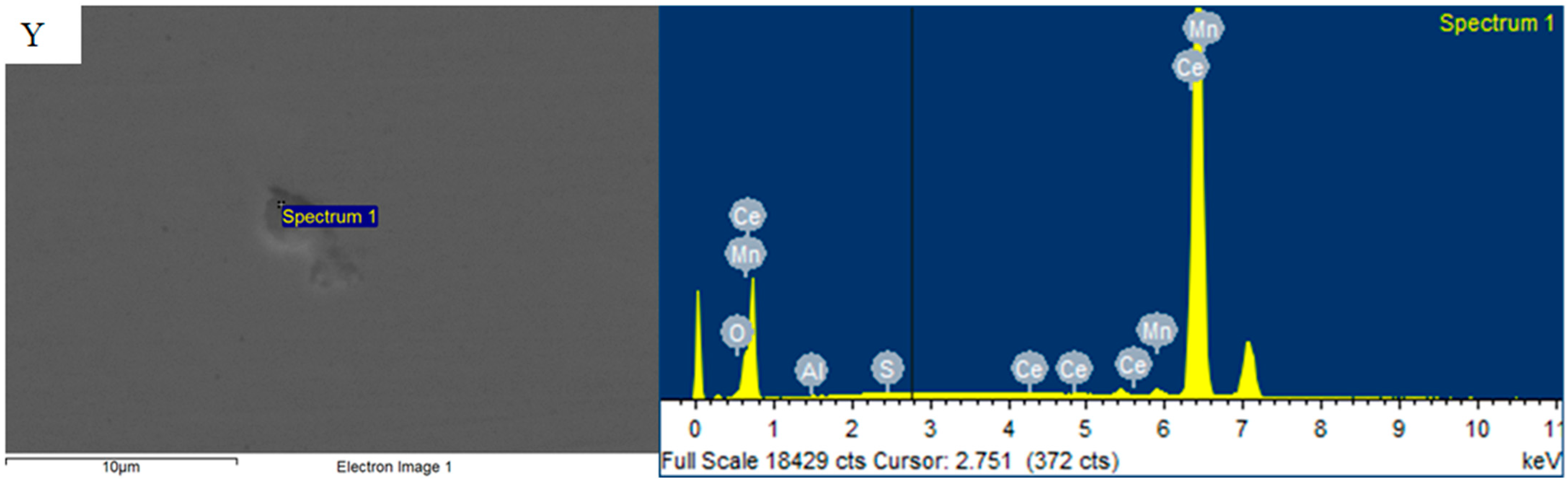
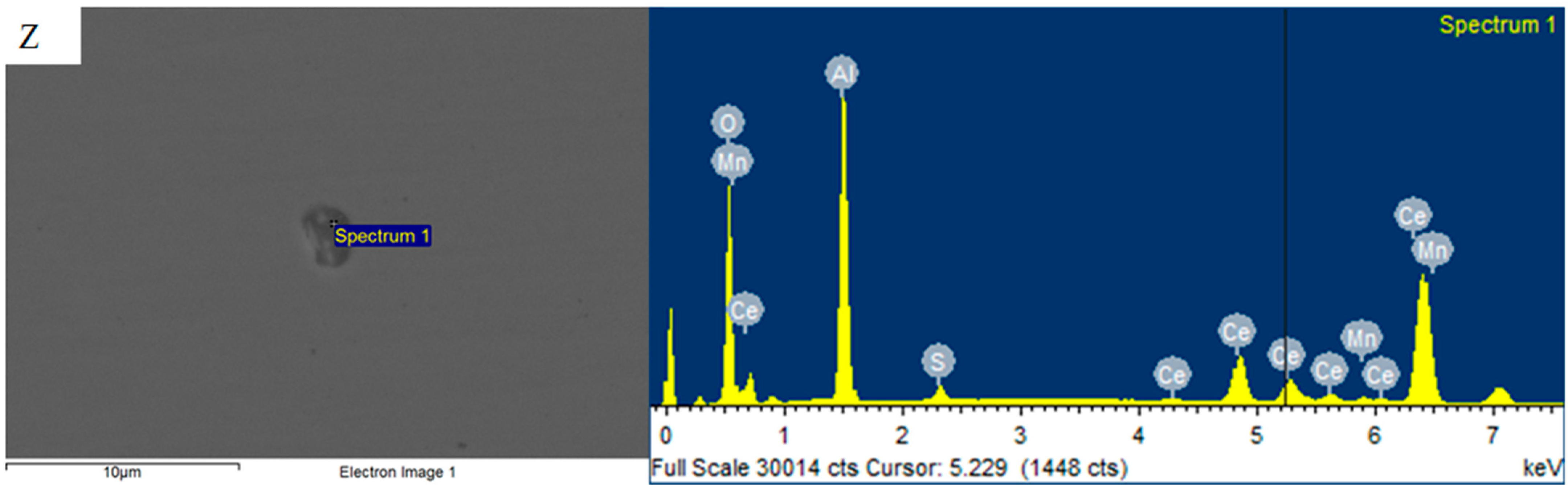
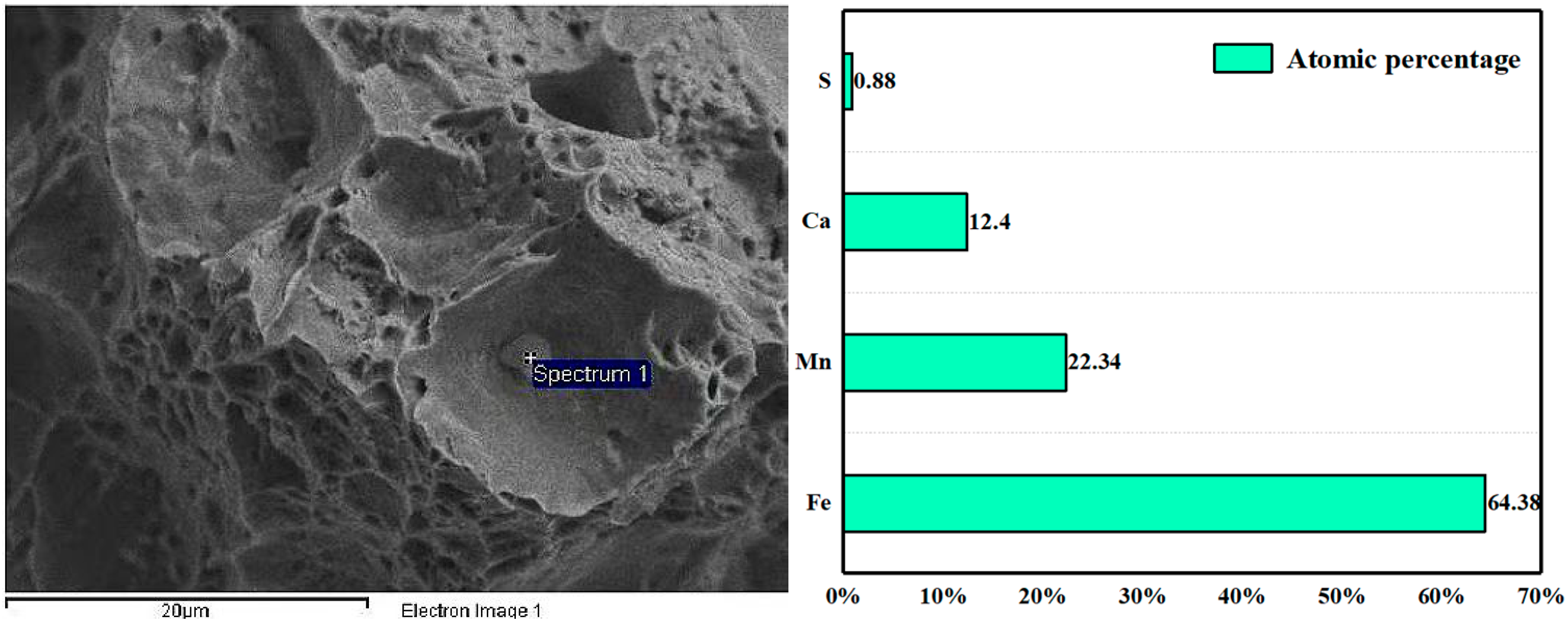
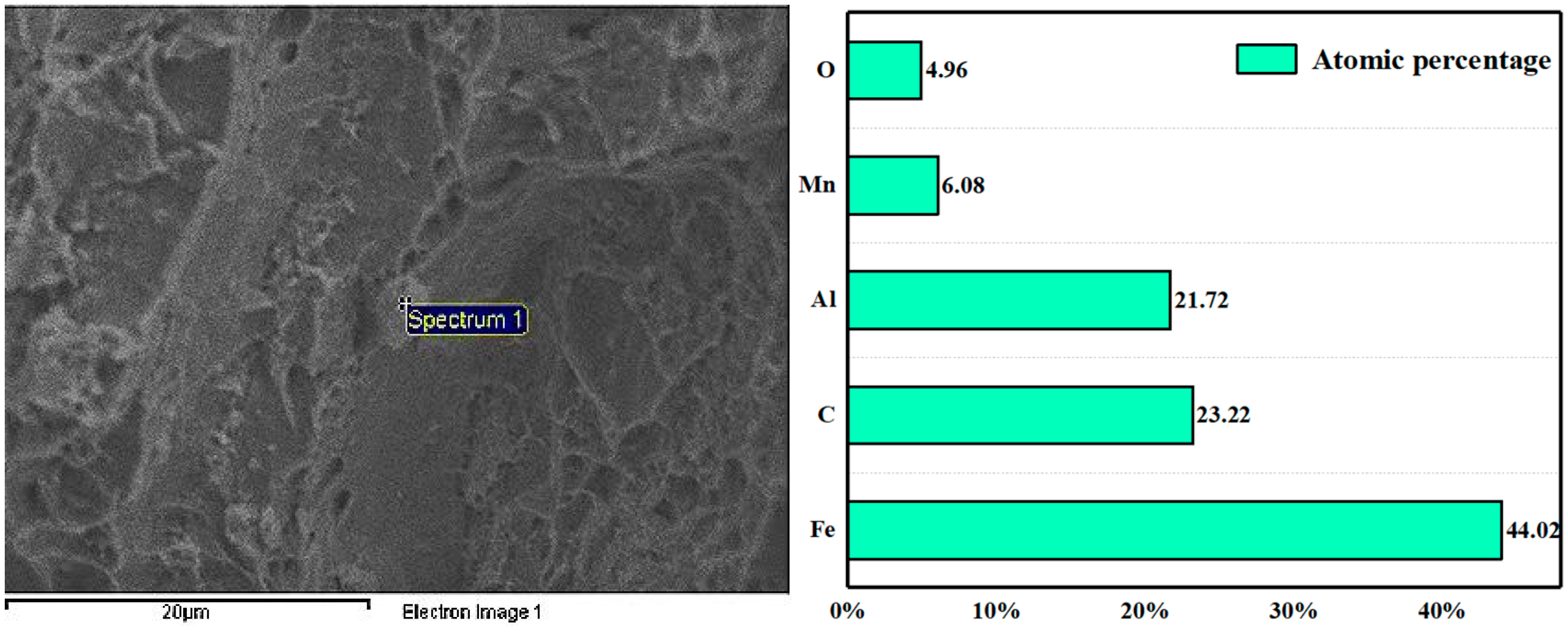
3.3. Mechanism of Rare Earth Element Ce on Inclusions
3.4. Effect of Trace Rare Earth Element Ce on Mechanical Properties of Test Steel
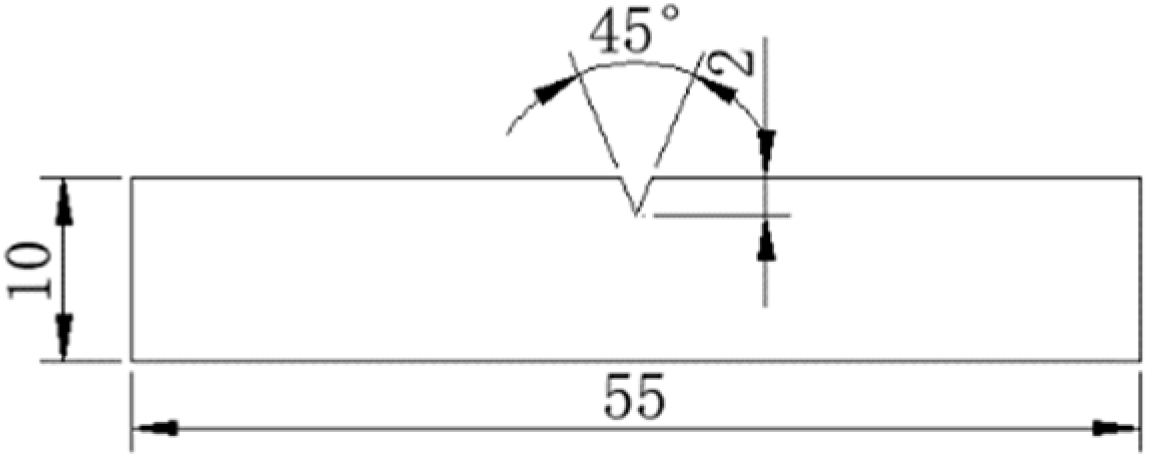
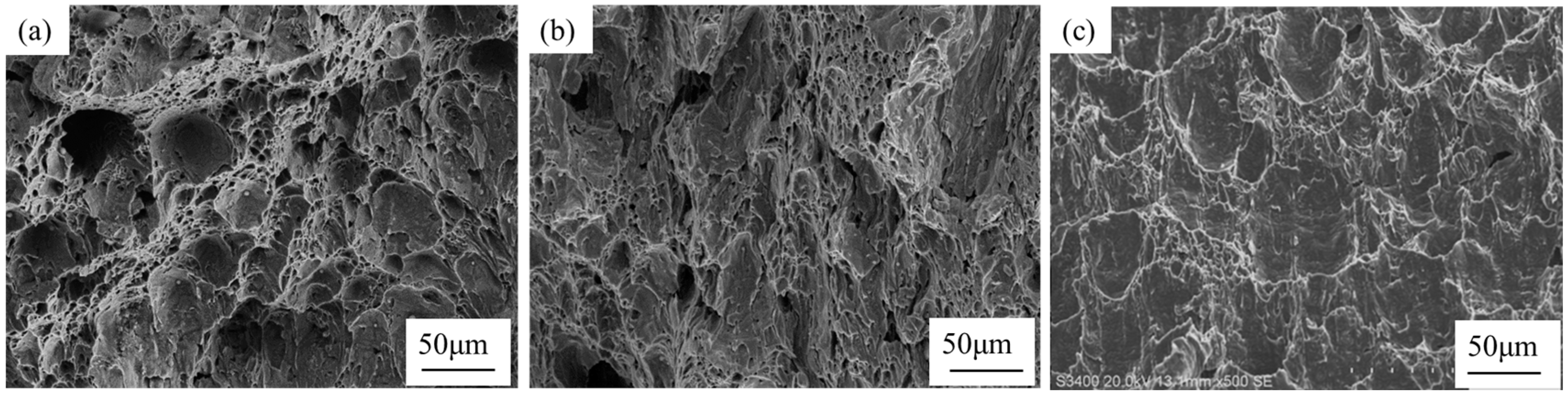
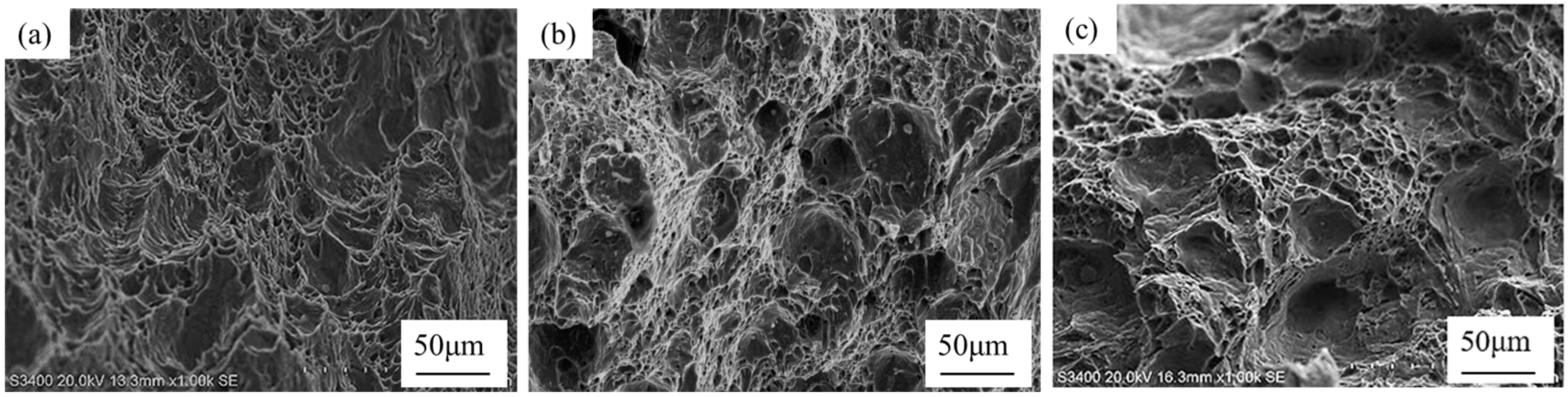
3.5. Test Steel Low Temperature Impact Fracture Analysis
4. Conclusions
- Compared with the blank test steel, the Ce element increases the Ac3 phase transformation point and decreases the Ac1 phase transformation point of the test steel, and expands the austenite phase region. This makes the test steel containing Ce element obtain a finer original austenite structure, and the distribution is more uniform, which plays the role of fine grain strengthening and can effectively improve the comprehensive mechanical properties of the test steel.
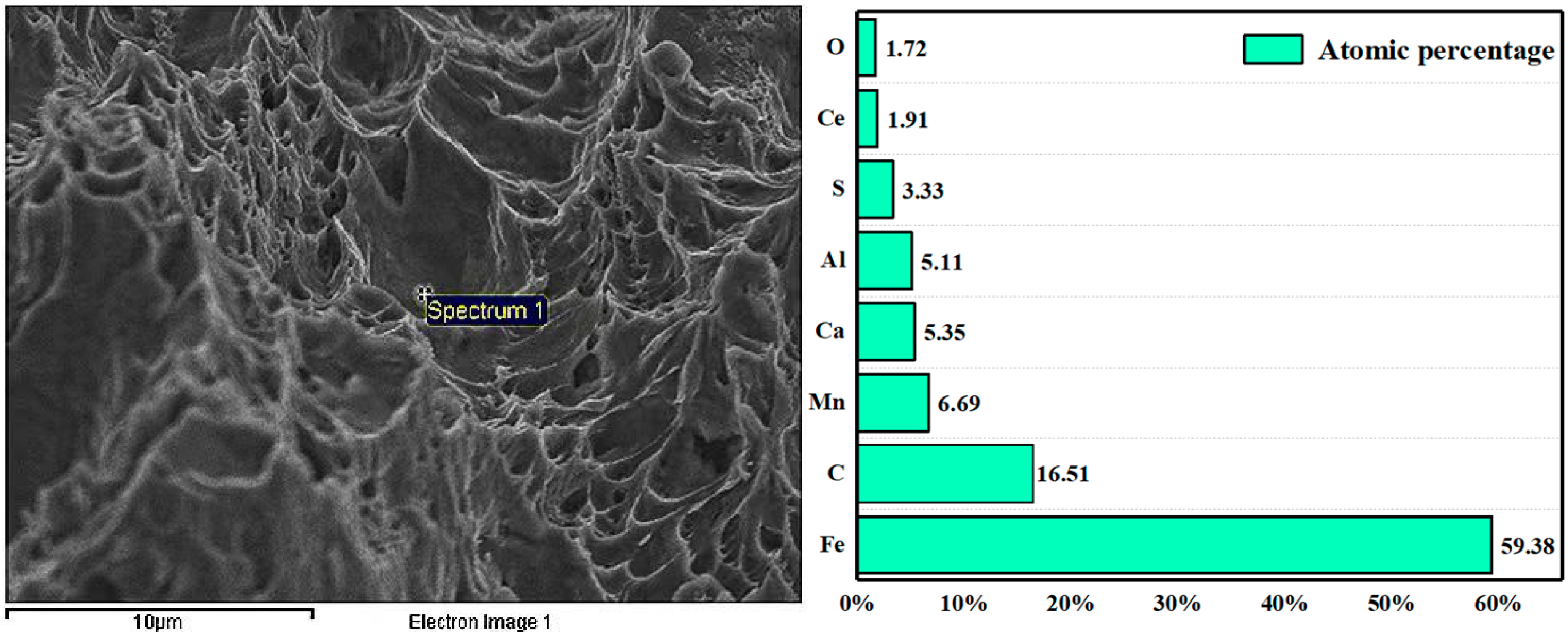
- After the addition of the Ce element, the long strip MnS and irregular Al2O3 can be modified into elliptical rare earth composite inclusions. In addition, the addition of Ce reduces the segregation of O and S at the grain boundary, plays a role in purifying the grain boundary, improves the strength of the grain boundary, and reduces the initiation of cracks at the grain boundary.
- The inclusions modified by the Ce element are only a few microns in size, which play a powerful role in dispersion strengthening and grain refinement. The continuity of the steel matrix is strengthened and the stress concentration of the steel matrix is reduced. With the increase in rare earth content at each temperature, the impact absorption energy is improved. The addition of the Ce element improves the low temperature impact toughness of steel.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, J. Research Status and Development of Shipbuilding Steel. Angang Technol. 2013, 382, 183–185. [Google Scholar]
- Hao, W.; Liu, Z.; Wang, X. Status and development trend of strength and corrosion resistance of high strength steel for offshore platform. Equip. Environ. Eng. 2014, 11, 50–56. [Google Scholar]
- Song, R.; Zhao, W.; Bao, X.; Chen, L.; Wang, X. Effect of rare earth element Ce and heat treatment on microstructure and mechanical properties of hypereutectoid rail steel. Heat Treat. Met. 2022, 47, 162–167. [Google Scholar]
- Chen, R.; Wang, Z.; He, J.; Zhu, F.; Li, C. Effects of Rare Earth Elements on Microstructure and Mechanical Properties of H13 Die Steel. Metals 2020, 10, 918. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, F.; Xi, X.; Yang, S.; Li, J. Effect of Y on inclusions characteristics and tensile properties in EH36 shipbuilding steel. Iron Steel 2019, 54, 61–67. [Google Scholar]
- Lian, X.-T.; Chen, L.; Fan, Z.-W.; Liu, T.S.; Xu, D.X.; Dong, H. Effects of Modified Inclusions and Precipitates Alloyed by Rare Earth Element on Corrosion and Impact Properties in Low Alloy Steel. Acta Metall. Sin. Engl. Lett. 2022, 35, 1719–1730. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Cai, C.; Li, A.; Lei, X.; Yang, C. Effect of Microalloyed Elements M (M = Ce, Ti, V, and Nb) on Mechanical Properties and Electronic Structures of gamma-Fe: Insights from a First-Principles Study. Steel Res. Int. 2021, 92, 2100053. [Google Scholar] [CrossRef]
- Meng, X.; Yu, Y.; Kang, J.; Su, J.; Wang, S. Effect of rare earth on inclusions and low temperature impact properties of A572. Gr65 steel. Heat Treat. Met. 2020, 45, 15–18. [Google Scholar]
- Yu, Z. Rare Earths in Steel; Metallurgical Industry Press: Beijing, China, 1982. [Google Scholar]
- GB/T 13298-91; Metallic Microstructure Test Method. China National Standardization Administration: Beijing, China, 1991.
- YB/T 5128-2018; Determination of Continuous Cooling Transformation Curve of Steel-Expansion Method. National Metallurgical Industry Bureau: Beijing, China, 2018.
- GB/T 712-2022; Structural Steel for Ship and Ocean Engineering. China National Standardization Administration: Beijing, China, 2022.
- GB/T 228.1-2010; Tensile Test of Metal Materials-Part 1: Room Temperature Test Method. National Standardization Administration of China: Beijing, China, 2010.
- GB/T 229-2007; Charpy Pendulum Impact Test Method for Metal Materials. China National Standardization Administration: Beijing, China, 2007.
- Fei, G.; Zheng, C.-W.; Wang, P.; Li, D.-Z.; Li, Y.-Y. Effects of Rare Earth on Austenite-Ferrite Phase Transformation in a Low-Carbon Fe-C Alloy. Acta Metall. Sin. Engl. Lett. 2022, 36, 141–146. [Google Scholar]
- Ji, Y.; Liu, Z.; Wang, H.; Ren, H. Effect of Solid-solution Rare Earth in Steel on Transformation of Super-cooled Austenite. Hot Work. Technol. 2015, 44, 113–118. [Google Scholar]
- Zhang, X. Effect of trace rare earth elements on microstructure and corrosion properties of offshore platform steel. Coll. Mater. Sci. Eng. 2022, 17–18. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, L.; Xia, M.; Qing, C.; Zhao, L. Effect of rare earth Ce on CCT curves of H13 steel. Heat Treat. Met. 2022, 47, 197–202. [Google Scholar]
- Mao, N.; Yang, W.; Chen, D.; Lu, W.; Zhang, X.; Chen, S.; Xu, M.; Pan, B.; Han, L.; Zhang, X.; et al. Effect of Lanthanum Addition on Formation Behaviors of Inclusions in Q355B Weathering Steel. Materials 2022, 15, 7952. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, L.; Zhang, Z. Effects of Hot Processing on Sulfide and Oxide Inclusions. Mater. Rev. 2021, 35, 13168–13176. [Google Scholar]
- Summers, J.M.; Chakraborty, S.; Bartlett, L.N.; O’Malley, R.J.; Buchely, M.F.; Pilon, R. On the Effect of Hot Rolling on Inclusion Size and Distribution in a Cast AISI 1070 Steel Railroad Wheel. Int. J. Met. 2022, 17, 1277–1295. [Google Scholar] [CrossRef]
- Cao, Y.; Li, G.; Hou, Y.; Moelans, N.; Guo, M. DFT study on the mechanism of inclusion-induced initial pitting corrosion of Al-Ti-Ca complex deoxidized steel with Ce treatment. Phys. B Condens. Matter 2019, 558, 10–19. [Google Scholar] [CrossRef]
- Xi, Z.; Sun, T.; Xiao, H.; Fan, Z.; Liu, T.; Lian, X.; Li, J.; Dong, H. Effects of Rare Earth Elements on Inclusions and Impact Toughness in a Weathering Steel. Trans. Indian Inst. Met. 2023, 77, 831–841. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Li, B.; Chen, W.; Zhang, J.; Mao, J. Inclusions modification by rare earth in steel and the resulting properties: A review. J. Rare Earths 2024, 42, 431–445. [Google Scholar] [CrossRef]
- Kusche, C.F.; Gibson, J.S.K.-L.; Wollenweber, M.A.; Korte-Kerzel, S. On the mechanical properties and deformation mechanisms of manganese sulphide inclusions. Mater. Des. 2020, 193, 108801. [Google Scholar] [CrossRef]
- Liu, C.; Revilla, R.I.; Zhang, D.; Liu, Z.; Lutz, A.; Zhang, F.; Zhao, T.; Ma, H.; Li, X.; Terryn, H. Role of Al2O3 inclusions on the localized corrosion of Q460NH weathering steel in marine environment. Corros. Sci. 2018, 138, 96–104. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Zhang, F.; Fu, X.; Li, H.; Yang, C. Experimental and DFT study on cerium inclusions in clean steels. J. Rare Earths 2021, 39, 477–486. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, W.; Zhang, L. Deformation of Non-metallic Inclusions in Steel during Rolling Process: A Review. ISIJ Int. 2022, 62, 2159–2171. [Google Scholar] [CrossRef]
- Yang, C.; Luan, Y.; Li, D.; Li, Y. Effects of rare earth elements on inclusions and impact toughness of high-carbon chromium bearing steel. J. Mater. Sci. Technol. 2019, 35, 1298–1308. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Wang, H.; Wan, X.; Hu, M.; Cao, Y. Achieving superior cryogenic impact toughness and sufficient tensile properties in a novel high-Mn austenitic steel weld metal via cerium addition. J. Mater. Res. Technol. 2023, 23, 5016–5030. [Google Scholar] [CrossRef]




| Simple | C | Mn | P | S | Si | Ca | Ni | Cr | Cu | Ti | Nb | Ce |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X# | 0.08 | 1.20 | <0.012 | <0.002 | 0.24 | 0.0012 | 0.378 | 0.536 | 0.405 | 0.015 | 0.045 | - |
| Y# | 0.08 | 1.21 | <0.012 | <0.002 | 0.24 | 0.0012 | 0.343 | 0.584 | 0.456 | 0.016 | 0.042 | 0.0006 |
| Z# | 0.07 | 1.19 | <0.012 | <0.002 | 0.25 | 0.0012 | 0.379 | 0.548 | 0.415 | 0.016 | 0.042 | 0.0010 |
| Sample | Ac1/°C | Ac3/°C |
|---|---|---|
| X# | 700 | 843 |
| Y# | 681 | 854 |
| Z# | 686 | 857 |
| Samples | σy/MPa | σt/MPa | ε/% |
|---|---|---|---|
| X (0 ppmRE) | 476 ± 3 | 572 ± 6 | 35 ± 2 |
| Y (6 ppmRE) | 462 ± 4 | 560 ± 3 | 34 ± 1 |
| Z (10 ppmRE) | 475 ± 3 | 590 ± 7 | 34 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, R.; Zhou, P.; Chen, J.; Lei, Y.; Yang, X.; Wu, Y.; Guo, J.; Cui, Q. Effect of Trace Rare Earth Element Cerium (Ce) on Microstructure and Mechanical Properties of High Strength Marine Engineering Steel. Crystals 2024, 14, 863. https://doi.org/10.3390/cryst14100863
Dong R, Zhou P, Chen J, Lei Y, Yang X, Wu Y, Guo J, Cui Q. Effect of Trace Rare Earth Element Cerium (Ce) on Microstructure and Mechanical Properties of High Strength Marine Engineering Steel. Crystals. 2024; 14(10):863. https://doi.org/10.3390/cryst14100863
Chicago/Turabian StyleDong, Ruifeng, Peiying Zhou, Jiquan Chen, Yuansu Lei, Xiong Yang, Yanjie Wu, Jing Guo, and Qingling Cui. 2024. "Effect of Trace Rare Earth Element Cerium (Ce) on Microstructure and Mechanical Properties of High Strength Marine Engineering Steel" Crystals 14, no. 10: 863. https://doi.org/10.3390/cryst14100863






