Study on Synthesis of CSH Gel and Its Immobilization of Heavy Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis Methods
2.2. Analytical Methods
3. Results and Discussion
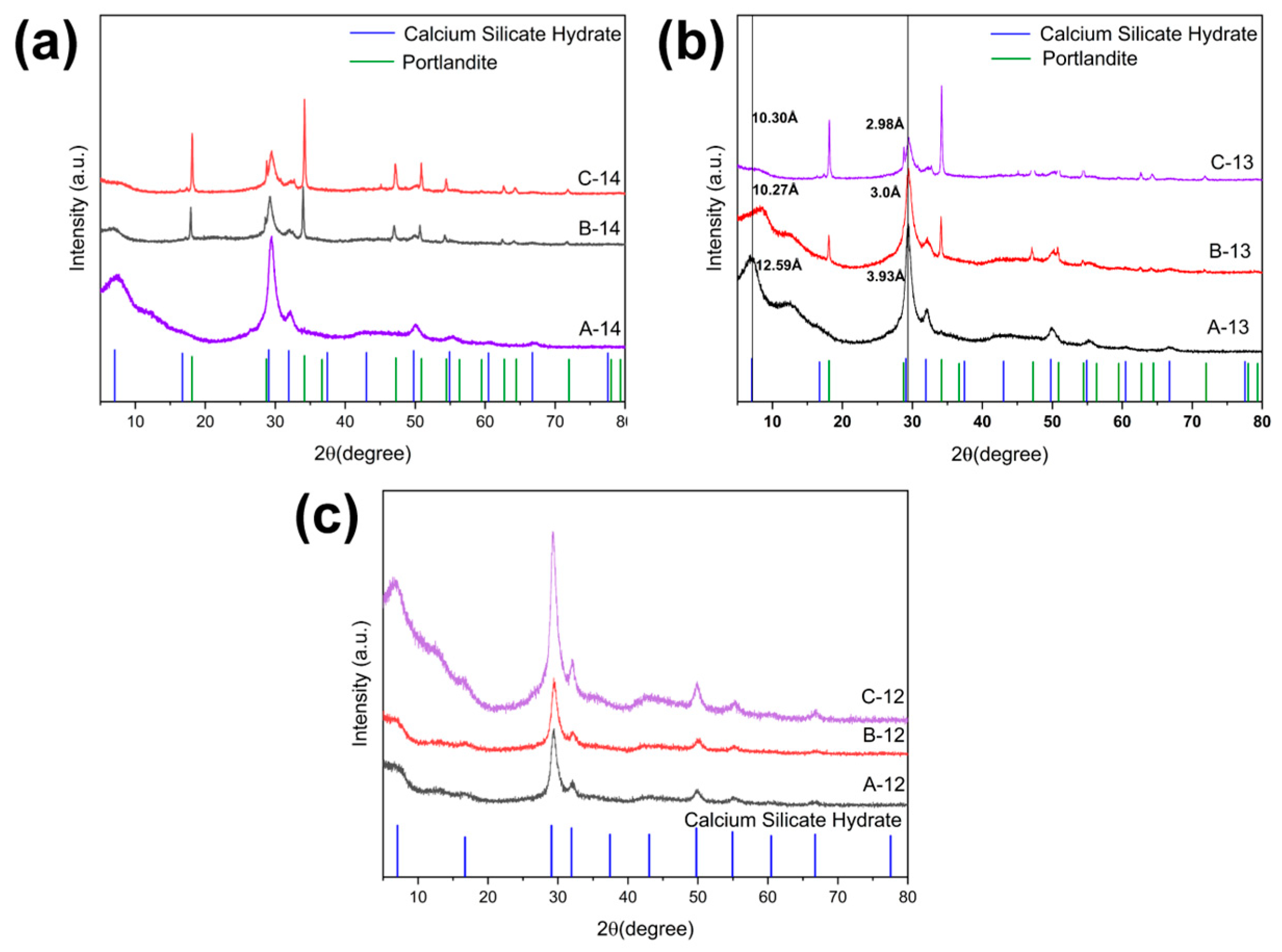
3.1. Study on CSH Gel Formation at Different pH Values
3.2. Effect of Ca/Si on the Composition and Structure of CSH Gel
3.2.1. ICP Analyses of CSH Gel
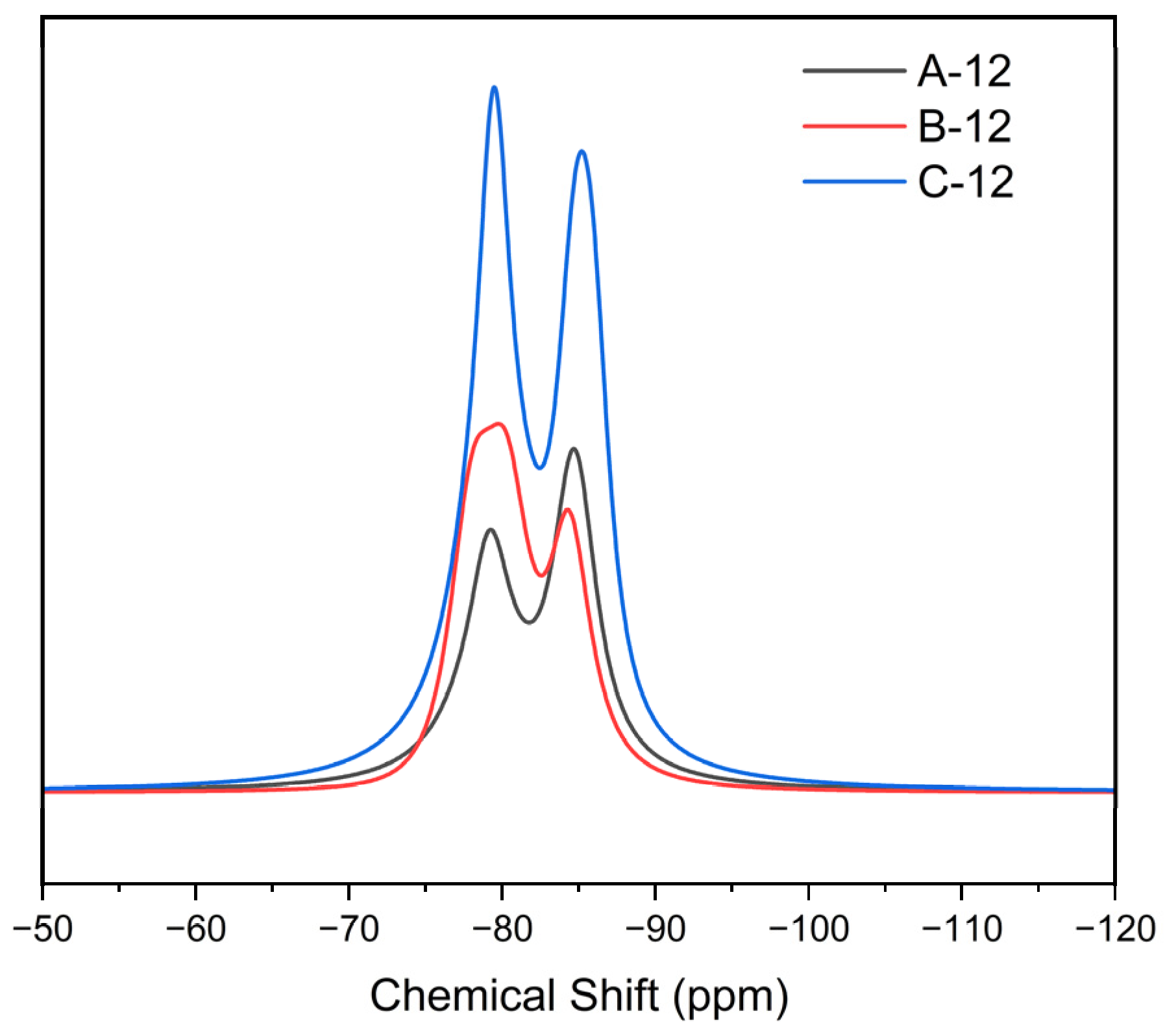
3.2.2. SSNMR
3.2.3. Raman
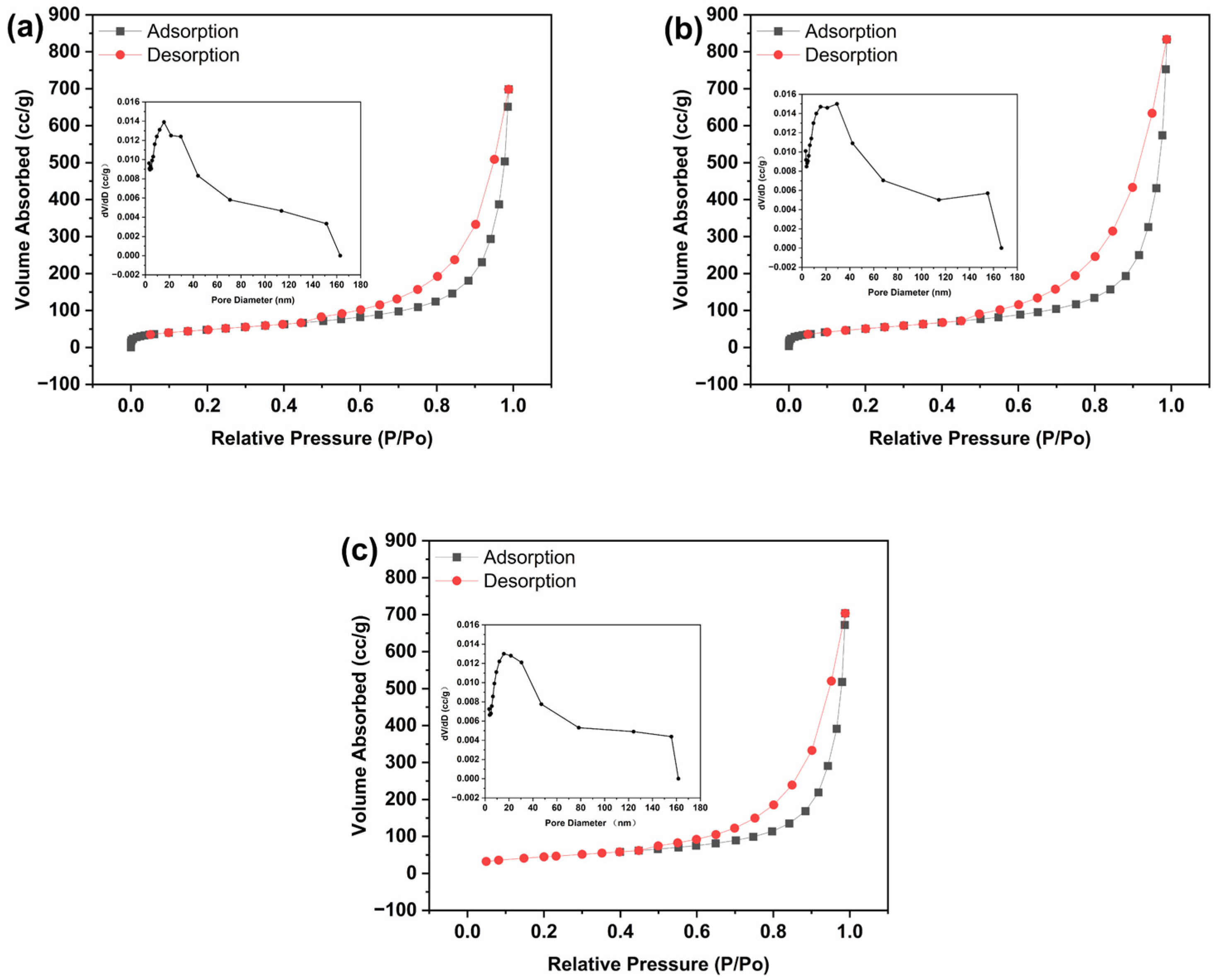
3.2.4. BET
3.2.5. FT-IR Analyses
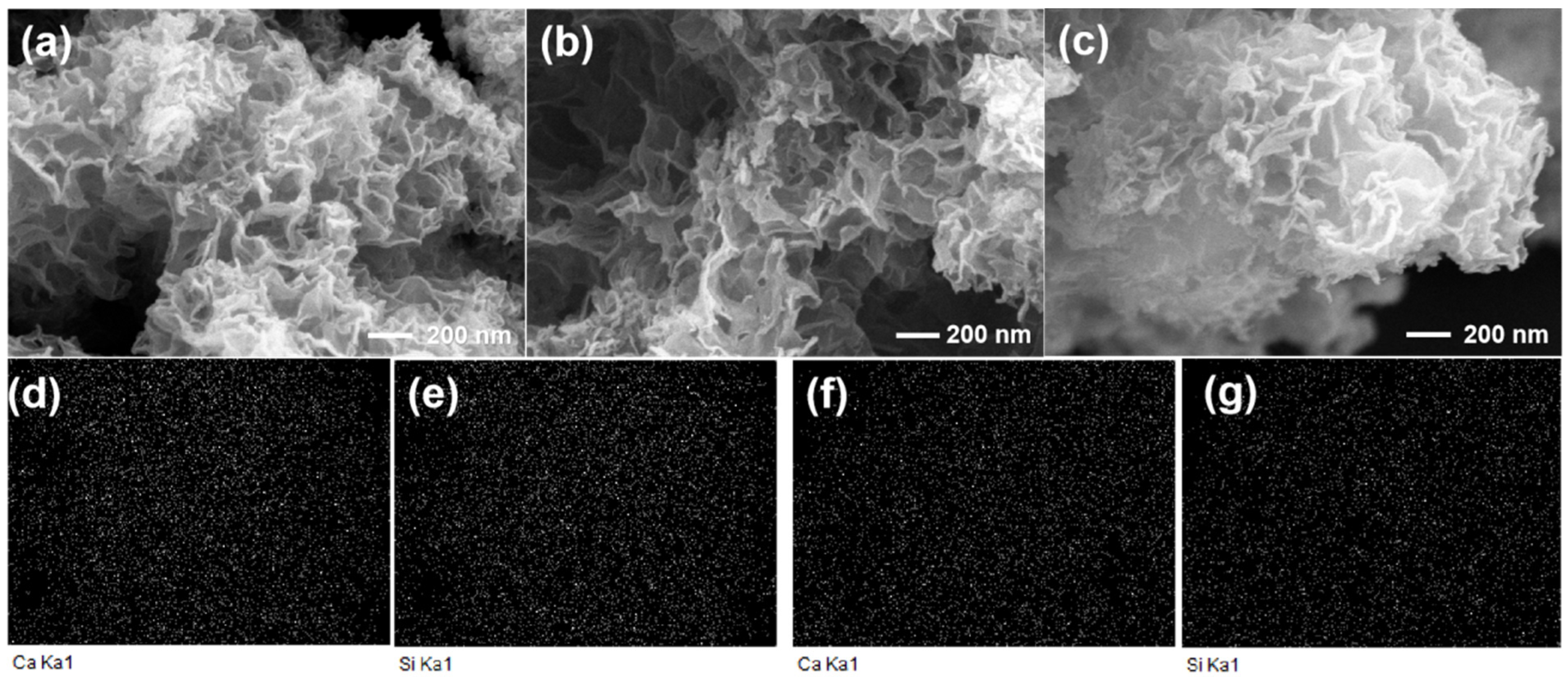
3.2.6. SEM Observations
3.3. Immobilization of Heavy Metal Ions in CSH Gel Reaction Systems
3.3.1. Immobilization of Heavy Metal Ions in CSH Gel Reaction System at Different pH Values
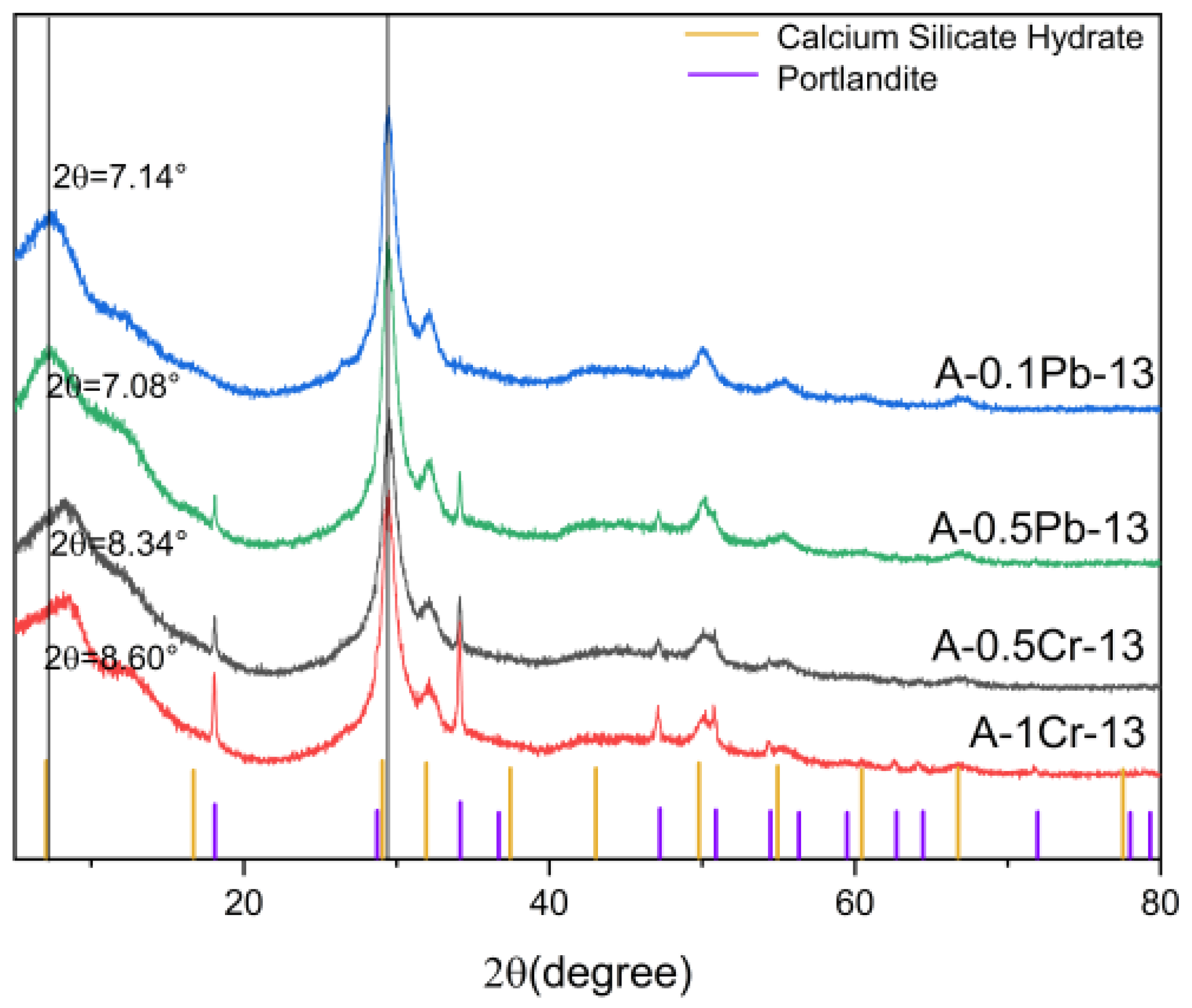
3.3.2. Immobilization of Heavy Metal Ions in CSH Gel Reaction Systems with Different Ca/Si
4. Conclusions
- (1)
- Synthetic experiments indicate that the structure of CSH gel synthesized at pH = 14 changes by varying Ca/Si, accompanied by a decreasing trend in interplanar spacing and the concurrent formation of calcium hydroxide impurities. At pH = 12, the CSH gel exhibited an initial increase in silicon chain length, followed by a decrease as the Ca/Si ratio increased, without the formation of any impurity phases. As the Ca/Si increases, the interplanar spacing of the CSH gel tends to decrease, accompanied by the extension of silicon chains and the removal of Ca2+ ions. The released Ca2+ ions react with the NaOH present in the solution, forming calcium hydroxide.
- (2)
- The study delved into the immobilization mechanism of heavy metal ions within the CSH gel reaction system. Characterization results from XRD, FT-IR, and Raman spectroscopy indicate that both Cr3+ and Pb2+ can substitute for Ca2+ in the CSH gel and generate calcium hydroxide, with Cr3+ having the additional capability of substituting for Si4+ within the CSH structure. The findings of this study reveal that the immobilization mechanism of lead and chromium within the CSH gel reaction system involves ion substitution.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuesta, A.; Zea-Garcia, J.D.; Londono-Zuluaga, D.; De La Torre, A.G.; Santacruz, I.; Vallcorba, O.; Dapiaggi, M.; Sanfélix, S.G.; Aranda, M.A.G. Multiscale Understanding of Tricalcium Silicate Hydration Reactions. Sci. Rep. 2018, 8, 8544. [Google Scholar] [CrossRef] [PubMed]
- Land, G.; Stephan, D. The Effect of Synthesis Conditions on the Efficiency of C-S-H Seeds to Accelerate Cement Hydration. Cem. Concr. Compos. 2018, 87, 73–78. [Google Scholar] [CrossRef]
- Kumar, A.; Walder, B.J.; Kunhi Mohamed, A.; Hofstetter, A.; Srinivasan, B.; Rossini, A.J.; Scrivener, K.; Emsley, L.; Bowen, P. The Atomic-Level Structure of Cementitious Calcium Silicate Hydrate. J. Phys. Chem. C 2017, 121, 17188–17196. [Google Scholar] [CrossRef]
- Goracci, G.; Monasterio, M.; Jansson, H.; Cerveny, S. Dynamics of Nano-Confined Water in Portland Cement—Comparison with Synthetic C-S-H Gel and Other Silicate Materials. Sci. Rep. 2017, 7, 8258. [Google Scholar] [CrossRef]
- Yan, Y.; Bernard, E.; Miron, G.D.; Rentsch, D.; Ma, B.; Scrivener, K.; Lothenbach, B. Kinetics of Al Uptake in Synthetic Calcium Silicate Hydrate (C-S-H). Cem. Concr. Res. 2023, 172, 107250. [Google Scholar] [CrossRef]
- Xiang-peng, F.; Li-ping, G.; Jian-dong, W.; Bang-cheng, L.; Ying-jie, C.; Xu-yan, S. Impact of Temperature, pH Value and Multiple Ions on the Physisorption of Chloride Ion on C-S-H Gel Surface. Constr. Build. Mater. 2023, 392, 131967. [Google Scholar] [CrossRef]
- Maddalena, R.; Li, K.; Chater, P.A.; Michalik, S.; Hamilton, A. Direct Synthesis of a Solid Calcium-Silicate-Hydrate (C-S-H). Constr. Build. Mater. 2019, 223, 554–565. [Google Scholar] [CrossRef]
- Nithurshan, M.; Elakneswaran, Y.; Yoda, Y.; Yano, K.; Kitagaki, R.; Hiroyoshi, N. Exploring the Reinforcing Mechanism of Graphene Oxide in Cementitious Materials through Microstructural Analysis of Synthesised Calcium Silicate Hydrate. Cem. Concr. Compos. 2024, 153, 105717. [Google Scholar] [CrossRef]
- Yoshida, S.; Elakneswaran, Y.; Nawa, T. Electrostatic Properties of C–S–H and C-A-S-H for Predicting Calcium and Chloride Adsorption. Cem. Concr. Compos. 2021, 121, 104109. [Google Scholar] [CrossRef]
- Labbez, C.; Jönsson, B.; Pochard, I.; Nonat, A.; Cabane, B. Surface Charge Density and Electrokinetic Potential of Highly Charged Minerals: Experiments and Monte Carlo Simulations on Calcium Silicate Hydrate. J. Phys. Chem. B 2006, 110, 9219–9230. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, Y.; Xu, L.; Zhu, Z.; Zhou, Y.; Pan, F.; Wu, K. Insight into the Local C-S-H Structure and Its Evolution Mechanism Controlled by Curing Regime and Ca/Si Ratio. Constr. Build. Mater. 2022, 333, 127388. [Google Scholar] [CrossRef]
- Bernard, E.; Yan, Y.; Lothenbach, B. Effective Cation Exchange Capacity of Calcium Silicate Hydrates (C-S-H). Cem. Concr. Res. 2021, 143, 106393. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, S.-Y.; Miron, G.D.; Collings, I.E.; L’Hôpital, E.; Skibsted, J.; Winnefeld, F.; Scrivener, K.; Lothenbach, B. Effect of Alkali Hydroxide on Calcium Silicate Hydrate (C-S-H). Cem. Concr. Res. 2022, 151, 106636. [Google Scholar] [CrossRef]
- Yang, Z.; Kang, D.; Zhang, D.; Yan, C.; Zhang, J. Crystal Transformation of Calcium Silicate Minerals Synthesized by Calcium Silicate Slag and Silica Fume with Increase of C/S Molar Ratio. J. Mater. Res. Technol. 2021, 15, 4185–4192. [Google Scholar] [CrossRef]
- John, E.; Epping, J.D.; Stephan, D. The Influence of the Chemical and Physical Properties of C-S-H Seeds on Their Potential to Accelerate Cement Hydration. Constr. Build. Mater. 2019, 228, 116723. [Google Scholar] [CrossRef]
- Sun, G.K.; Young, J.F.; Kirkpatrick, R.J. The Role of Al in C–S–H: NMR, XRD, and Compositional Results for Precipitated Samples. Cem. Concr. Res. 2006, 36, 18–29. [Google Scholar] [CrossRef]
- Shen, X.; Feng, P.; Zhang, Q.; Lu, J.; Liu, X.; Ma, Y.; Jin, P.; Wang, W.; Ran, Q.; Hong, J. Toward the Formation Mechanism of Synthetic Calcium Silicate Hydrate (C-S-H)—pH and Kinetic Considerations. Cem. Concr. Res. 2023, 172, 107248. [Google Scholar] [CrossRef]
- Harris, M.; Simpson, G.; Scrivener, K.; Bowen, P. A Method for the Reliable and Reproducible Precipitation of Phase Pure High Ca/Si Ratio (>1.5) Synthetic Calcium Silicate Hydrates (C S H). Cem. Concr. Res. 2022, 151, 106623. [Google Scholar] [CrossRef]
- Chen, J.J.; Thomas, J.J.; Taylor, H.F.W.; Jennings, H.M. Solubility and Structure of Calcium Silicate Hydrate. Cem. Concr. Res. 2004, 34, 1499–1519. [Google Scholar] [CrossRef]
- Lothenbach, B.; Nonat, A. Calcium Silicate Hydrates: Solid and Liquid Phase Composition. Cem. Concr. Res. 2015, 78, 57–70. [Google Scholar] [CrossRef]
- Richardson, I.G. Model Structures for C-(A)-S-H(I). Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 903–923. [Google Scholar] [CrossRef] [PubMed]
- García Lodeiro, I.; Fernández-Jimenez, A.; Palomo, A.; Macphee, D.E. Effect on Fresh C-S-H Gels of the Simultaneous Addition of Alkali and Aluminium. Cem. Concr. Res. 2010, 40, 27–32. [Google Scholar] [CrossRef]
- Pantuzzo, F.L.; Santos, L.R.G.; Ciminelli, V.S.T. Solubility-Product Constant of an Amorphous Aluminum-Arsenate Phase (AlAsO4·3.5H2O) AT 25 °C. Hydrometallurgy 2014, 144–145, 63–68. [Google Scholar] [CrossRef]
- Teramoto, H.; Koie, S. Early Hydration of a Superhigh-Early-Strength Portland Cement Containing Chromium. J. Am. Ceram. Soc. 1976, 59, 522–525. [Google Scholar] [CrossRef]
- Serclérat, I.; Moszkowicz, P.; Pollet, B. Retention Mechanisms in Mortars of the Trace Metals Contained in Portland Cement Clinkers. Waste Manag. 2000, 20, 259–264. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Cui, Y.; Pang, L. Assessing Heavy-Metal Leaching Risk in Cement-Industrial Slag Composite Pastes Using a Mechanism-Based Thermodynamic Approach. Cem. Concr. Compos. 2023, 140, 105074. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Huang, Z. Reuse of Copper Slag as a Supplementary Cementitious Material: Reactivity and Safety. Resour. Conserv. Recycl. 2020, 162, 105037. [Google Scholar] [CrossRef]
- Wang, H.; Ju, C.; Zhou, M.; Chen, J.; Dong, Y.; Hou, H. Sustainable and Efficient Stabilization/Solidification of Pb, Cr, and Cd in Lead-Zinc Tailings by Using Highly Reactive Pozzolanic Solid Waste. J. Environ. Manage. 2022, 306, 114473. [Google Scholar] [CrossRef]
- Hekal, E.E.; Kishar, E.A.; Mohamed, M.R.; Mohamed, B.A. Inertisation of Cd2+ Ions by Using Blended Cement Pastes. Adv. Cem. Res. 2013, 25, 80–85. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Dai, J.-G.; Wang, L.; Tsang, D.C.W.; Poon, C.S. Influence of Lead on Stabilization/Solidification by Ordinary Portland Cement and Magnesium Phosphate Cement. Chemosphere 2018, 190, 90–96. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, L.; Wang, H.; Liu, S.; Ren, J. Stabilization and Solidification Mechanism of Pb in Phosphogypsum Slag-Based Cementitious Materials. Constr. Build. Mater. 2023, 368, 130427. [Google Scholar] [CrossRef]
- Wang, F.; Shen, Z.; Liu, R.; Zhang, Y.; Xu, J.; Al-Tabbaa, A. GMCs Stabilized/Solidified Pb/Zn Contaminated Soil under Different Curing Temperature: Physical and Microstructural Properties. Chemosphere 2020, 239, 124738. [Google Scholar] [CrossRef] [PubMed]
- Tajuelo Rodriguez, E.; Garbev, K.; Merz, D.; Black, L.; Richardson, I.G. Thermal Stability of C-S-H Phases and Applicability of Richardson and Groves’ and Richardson C-(A)-S-H(I) Models to Synthetic C-S-H. Cem. Concr. Res. 2017, 93, 45–56. [Google Scholar] [CrossRef]
- Cuesta, A.; Santacruz, I.; De La Torre, A.G.; Dapiaggi, M.; Zea-Garcia, J.D.; Aranda, M.A.G. Local Structure and Ca/Si Ratio in C-S-H Gels from Hydration of Blends of Tricalcium Silicate and Silica Fume. Cem. Concr. Res. 2021, 143, 106405. [Google Scholar] [CrossRef]
- Foley, E.M.; Kim, J.J.; Reda Taha, M.M. Synthesis and Nano-Mechanical Characterization of Calcium-Silicate-Hydrate (C-S-H) Made with 1.5 CaO/SiO2 Mixture. Cem. Concr. Res. 2012, 42, 1225–1232. [Google Scholar] [CrossRef]
- Leukel, S.; Panthöfer, M.; Mondeshki, M.; Kieslich, G.; Wu, Y.; Krautwurst, N.; Tremel, W. Mechanochemical Access to Defect-Stabilized Amorphous Calcium Carbonate. Chem. Mater. 2018, 30, 6040–6052. [Google Scholar] [CrossRef]
- Gasharova, B.; Garbev, K.; Bornefeld, M.; Black, L.; Stemmermann, P. Raman and IR Spectroscopic Study of Nano-Crystalline Calcium Silicate Hydrates of Type C-S-H(I). Acta Crystallogr. A 2006, 62, s208. [Google Scholar] [CrossRef]
- Vetter, M.; Gonzalez-Rodriguez, J.; Nauha, E.; Kerr, T. The Use of Raman Spectroscopy to Monitor Phase Changes in Concrete Following High Temperature Exposure. Constr. Build. Mater. 2019, 204, 450–457. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Chen, Y.; Zhuang, Z.; Chen, F.-F.; Zhu, Y.-J.; Yu, Y. Recycling Heavy Metals from Wastewater for Photocatalytic CO2 Reduction. Chem. Eng. J. 2020, 402, 125922. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, Y.; Chen, F. Ultrathin Calcium Silicate Hydrate Nanosheets with Large Specific Surface Areas: Synthesis, Crystallization, Layered Self-Assembly and Applications as Excellent Adsorbents for Drug, Protein, and Metal Ions. Small 2013, 9, 2911–2925. [Google Scholar] [CrossRef]
- Dambrauskas, T.; Davidoviciene, D.; Baltakys, K.; Eisinas, A.; Jaskunas, A.; Siler, P.; Rudelis, V.; Svedaite, E. Effect of Intercalated Metal Ions on the Specific Surface Area and Porosity of Dibasic Calcium Silicate Hydrate. Surf. Interfaces 2022, 33, 102243. [Google Scholar] [CrossRef]
- Liu, X.; Feng, P.; Cai, Y.; Yu, X.; Yu, C.; Ran, Q. Carbonation Behavior of Calcium Silicate Hydrate (C-S-H): Its Potential for CO2 Capture. Chem. Eng. J. 2022, 431, 134243. [Google Scholar] [CrossRef]
- Wang, D.; Xiong, C.; Li, W.; Chang, J. Growth of Calcium Carbonate Induced by Accelerated Carbonation of Tricalcium Silicate. ACS Sustain. Chem. Eng. 2020, 8, 14718–14731. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, C.; Jiang, Y.; Wang, Y.; Yang, W.; Cheng, T.; Zhou, G. Effect of Sodium Dodecyl Benzene Sulfonate on Morphology and Structure of Calcium Silicate Hydrate Prepared via Precipitation Method. Colloids Surf. Physicochem. Eng. Asp. 2018, 540, 249–255. [Google Scholar] [CrossRef]
- Basquiroto De Souza, F.; Sagoe-Crentsil, K.; Duan, W. A Century of Research on Calcium Silicate Hydrate (C–S–H): Leaping from Structural Characterization to Nanoengineering. J. Am. Ceram. Soc. 2022, 105, 3081–3099. [Google Scholar] [CrossRef]
- Qiao, G.; Hou, D.; Li, W.; Yin, B.; Zhang, Y.; Wang, P. Molecular Insights into Migration of Heavy Metal Ion in Calcium Silicate Hydrate (CSH) Surface and Intra-CSH (Ca/Si = 1.3). Constr. Build. Mater. 2023, 365, 130097. [Google Scholar] [CrossRef]
- Chen, L.; Nakamura, K.; Hama, T. Review on Stabilization/Solidification Methods and Mechanism of Heavy Metals Based on OPC-Based Binders. J. Environ. Manage. 2023, 332, 117362. [Google Scholar] [CrossRef]
- Król, M.; Florek, P.; Marzec, M.; Wójcik, S.; Dziża, K.; Mozgawa, W. Structural Studies of Calcium Silicate Hydrate Modified with Heavy Metal Cations. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2024, 321, 124681. [Google Scholar] [CrossRef]
- Gedeike, I.; Baltakys, K.; Eisinas, A. Effect of Cr3+ Ions on the Crystallization of Calcium Silicate Hydrates (CaO/SiO2 = 1.5) under Hydrothermal Conditions and Their Thermal Stability. J. Therm. Anal. Calorim. 2023, 148, 1501–1510. [Google Scholar] [CrossRef]
- Li, G.; Li, M.; Zhang, X.; Cao, P.; Jiang, H.; Luo, J.; Jiang, T. Hydrothermal Synthesis of Zeolites-Calcium Silicate Hydrate Composite from Coal Fly Ash with Co-Activation of Ca(OH)2-NaOH for Aqueous Heavy Metals Removal. Int. J. Min. Sci. Technol. 2022, 32, 563–573. [Google Scholar] [CrossRef]
- Niuniavaite, D.; Baltakys, K.; Dambrauskas, T.; Eisinas, A.; Rubinaite, D.; Jaskunas, A. Microstructure, Thermal Stability, and Catalytic Activity of Compounds Formed in CaO-SiO2-Cr(NO3)3-H2O System. Nanomaterials 2020, 10, 1299. [Google Scholar] [CrossRef]






| Composition | Ca(NO3)2 Solution (mol/L) | Na2SiO3 Solution (mol/L) | Ca/Si* | pH | |
|---|---|---|---|---|---|
| Samples | |||||
| A-14 | 0.15 | 0.1 | 1.5 | 14 | |
| B-14 | 0.18 | 0.1 | 1.8 | 14 | |
| C-14 | 0.2 | 0.1 | 2.0 | 14 | |
| A-13 | 0.15 | 0.1 | 1.5 | 13 | |
| B-13 | 0.18 | 0.1 | 1.8 | 13 | |
| C-13 | 0.2 | 0.1 | 2.0 | 13 | |
| A-12 | 0.15 | 0.1 | 1.5 | 12 | |
| B-12 | 0.18 | 0.1 | 1.8 | 12 | |
| C-12 | 0.2 | 0.1 | 2.0 | 12 | |
| Composition | Ca(NO3)2 Solution (mol/L) | Na2SiO3 Solution (mol/L) | Cr(NO3)3 (mmol) | Pb(NO3)2 (mmol) | Ca/Si* | pH | |
|---|---|---|---|---|---|---|---|
| Samples | |||||||
| A-0.1Pb-13 | 0.15 | 0.1 | 0 | 0.1 | 1.5 | 13 | |
| A-0.5Pb-13 | 0.15 | 0.1 | 0 | 0.5 | 1.5 | 13 | |
| A-0.5Cr-13 | 0.15 | 0.1 | 0.5 | 0 | 1.5 | 13 | |
| A-1Cr-13 | 0.15 | 0.1 | 1 | 0 | 1.5 | 13 | |
| A-0.1Pb-12 | 0.15 | 0.1 | 0 | 0.1 | 1.5 | 12 | |
| A-0.5Pb-12 | 0.15 | 0.1 | 0 | 0.5 | 1.5 | 12 | |
| A-0.5Cr-12 | 0.15 | 0.1 | 0.5 | 0 | 1.5 | 12 | |
| A-1Cr-12 | 0.15 | 0.1 | 1 | 0 | 1.5 | 12 | |
| B-0.5Pb-12 | 0.18 | 0.1 | 0 | 0.5 | 1.8 | 12 | |
| B-1Cr-12 | 0.18 | 0.1 | 1 | 0 | 1.8 | 12 | |
| C-0.5Pb-12 | 0.2 | 0.1 | 0 | 0.5 | 2.0 | 12 | |
| C-1Cr-12 | 0.2 | 0.1 | 1 | 0 | 2.0 | 12 | |
| Ion Concentration | Ca (mg/L) | Si (mg/L) | Ca/Si | |
|---|---|---|---|---|
| Samples | ||||
| A-12 | 243.2 | 162.4 | 1.03 | |
| B-12 | 223.2 | 120.5 | 1.34 | |
| C-12 | 202.7 | 103.3 | 1.38 | |
| Samples | A-12 | B-12 | C-12 |
|---|---|---|---|
| MCL | 4 | 5 | 4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, K.; Wang, L.; Liao, L.; Bai, Y.; Hu, J. Study on Synthesis of CSH Gel and Its Immobilization of Heavy Metals. Crystals 2024, 14, 864. https://doi.org/10.3390/cryst14100864
Zhu K, Wang L, Liao L, Bai Y, Hu J. Study on Synthesis of CSH Gel and Its Immobilization of Heavy Metals. Crystals. 2024; 14(10):864. https://doi.org/10.3390/cryst14100864
Chicago/Turabian StyleZhu, Kunqian, Lijuan Wang, Libing Liao, Yunlong Bai, and Jing Hu. 2024. "Study on Synthesis of CSH Gel and Its Immobilization of Heavy Metals" Crystals 14, no. 10: 864. https://doi.org/10.3390/cryst14100864






