Abstract
Metal oxide nanoparticles (MONs) are particles with at least one dimension in the nanoscale range (1–100 nm). Their unique properties, significantly different from their bulk counterparts, make them promising materials for a wide range of applications in fields such as medicine, electronics, catalysis, environmental remediation, and energy storage. The precise control of MONs’ properties, including size, shape, composition, crystallinity, and surface chemistry, is significant for optimizing their performance. This study aims to investigate the characteristics of synthesis methods of MONs. Correlation between synthesis parameters and properties highlights that creating nanomaterials with defined and controlled dimensions is a complex task that requires a deep understanding of various factors. Also, this study presents a model with adaptive parameters for synthesis conditions to acquire desired nanometric scale for particles size, which represents an essential task.
1. Introduction
Nanotechnology has been acclaimed for its applications in various areas, including food production, environmental protection, cosmetics, treatment of diseases, pharmaceutics, electronics, automotive, etc. The classification of nanomaterials is performed by considering their morphological structure, size, and other properties. In recent years, the focus on metal oxide nanomaterials is evident due to their potential relevance. Metal oxide nanoparticles (MONs) have gathered significant attention across various scientific and technological domains due to their unique physicochemical properties.
These properties, including high surface-to-volume ratios, tunable electronic structures, and catalytic activity, make MONs promising materials for a wide range of applications. However, to fully exploit their potential, it is imperative to control their synthesis to produce nanoparticles with desired properties [1,2,3,4,5,6]. This study aims to explore the importance of adapting MON synthesis methods to meet specific functional requirements. By understanding the relationship between synthesis methods and nanoparticle characteristics, researchers and engineers can optimize the production of these materials for specific needs.
Metal oxide nanoparticles exhibit distinct optical phenomena such as localized surface plasmon resonance, which can be tuned by varying their size, shape, and composition. This property finds applications in biosensing, imaging, and optical devices. The vast surface area of MONs provides more active sites for chemical reactions, accelerating catalytic processes. Metal oxide nanoparticles can be tailored to exhibit high selectivity for specific reactions, minimizing unwanted products. Also, they can exhibit enhanced electrical conductivity compared to their bulk counterparts, making them valuable for electronic and sensor applications. Certain metal oxide nanoparticles possess magnetic properties that can be exploited in magnetic data storage, sensors, and biomedical applications.
Essentially, to identify research trends, a bibliometric study on MONs was carried out. An overview of the impact and relevance of the study is presented below. Analysis of the number of bibliographic references on the topic of metallic oxide nanoparticles in the databases Web of Science and Scopus highlights a consistent upward trend in the number of articles from 2014 to 2023, depicted in Figure 1.
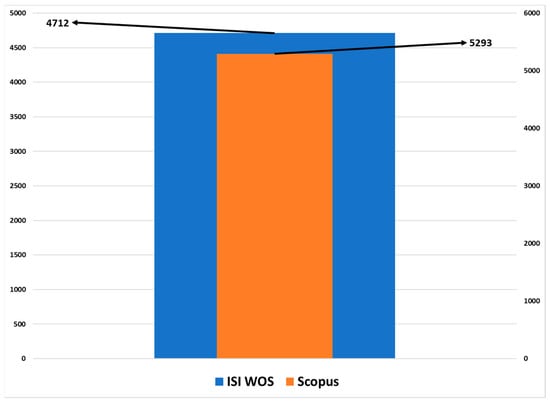
Figure 1.
Quantitative evolution of research articles in Web of Science and Scopus databases regarding MONs.
The percentage increase in the number of appearances of the topic “metal oxide nanoparticles”, compared to the previous year, is shown in Figure 2. This indicates a growing interest in understanding and utilizing these materials.
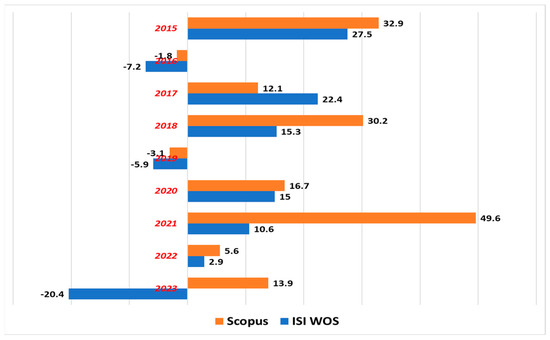
Figure 2.
Percentage evolution of number of appearances, compared to the previous year, of the topic of metal oxide nanoparticles.
Only in 2019 is there a decrease in the number of publications in both databases, Scopus and Web of Science, while in 2023, the number of articles published and indexed in the Web of Science decreases. Furthermore, the amount of research is increasing from year to year, leading to a better understanding of the factors influencing innovation in this field and identifying potential future directions.
In increased dynamics, the use of new types of adaptive models on the synthesis of metal oxide nanoparticles represents a new field, thanks to the integration of automatic analysis techniques in the synthesis processes. These innovative approaches allow precise and adaptive optimization of reaction parameters in real time, which is not possible with conventional methods. In addition, adaptive models offer superior control over nanoparticle properties, such as size or morphology, opening new opportunities in scientific research.
At the same time, the adaptive models used in the synthesis of metallic oxide nanoparticles allow the adjustment of reaction parameters such as temperature, pH, and precursor concentration.
The paper is structured as follows: in Section 2, the SWOT analysis of MONs synthesis methods is presented, followed, in Section 3, by the correlation between synthesis parameters and MON properties and continued, in Section 4, by exploitation of MONs. Section 5 is dedicated to the use of adaptive modeling in the synthesis of MONs.
2. SWOT Analysis of Metal Oxide Nanoparticle Synthesis
There are a few methods to fabricate nanostructures, which are divided into two main classes, bottom-up and top-down methods. The choice of synthesis method also plays a decisive role in determining MONs’ properties [6,7]. Top-down approach methods involve breaking down larger materials into metal oxide nanoparticles [7]. Some common top-down approaches are presented in what follows.
Mechanical milling (MM) concerns grinding a metal oxide bulk material into nanometric particles using high-energy collisions, considering the material properties, particularly when a dry product is desired. The reason for this is that drying usually causes a diminution in fine particles when the interface between particles is high. In recent times, additives have been used to prevent particle agglomeration during mechanical milling. Alcohols, amines, esters, carboxylic acids, ketones, electrolytes, surfactants, and neutral or changed polymers are used during the grinding of metal oxide bulk materials [8].
Laser ablation (LA) involves vaporizing a metal oxide target using a high-power laser, creating nanoparticles from the resulting plasma [9].
Sputtering (Sp) involves bombarding a target material with high-energy ions to discharge atoms, which deposit on a substrate to form a thin film [10,11].
Nanolithography (NL) involves constructing nanoscale patterns on a substrate using electron beam lithography or photolithography [12,13].
Arc discharge (AD) involves generating an electric arc between two electrodes, resulting in the vaporization of the electrode material and the formation of metal oxide nanoparticles [1,11,14,15].
A comparison of the presented methods is shown in Table 1.

Table 1.
Comparison of top-down methods.
Top-down synthesis methods offer a promising approach for producing metal oxide nanoparticles. However, careful consideration of the strengths, weaknesses, opportunities, and threats associated with these methods is essential for selecting the most appropriate approach for a given application, as presented in Figure 3.
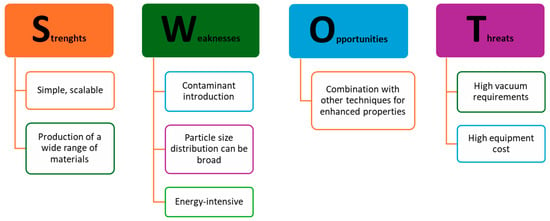
Figure 3.
SWOT analysis of top-down synthesis approaches of metal oxide nanoparticles [1,2,3,4,6,7,8,9,10,11,12,13].
Bottom-up approaches involve building nanoparticles from atoms or molecules.
Chemical reduction (CR) involves the reduction of a metal precursor to its metallic form, followed by oxidation to form the desired metal oxide. While it is primarily used for metal nanoparticle synthesis, it can be adapted for metal oxides, especially when coupled with other techniques or post-treatment processes [10,14].
Precipitation (Pre) and co-precipitation (Co-Pre) are widely used methods for synthesizing metal oxide nanoparticles due to their simplicity, low cost, and scalability [2,16]. The methods concern the simultaneous precipitation of metal ions from their aqueous solutions by adding a precipitating agent.
The process consists of five stages:
- Preparation of precursor solutions: Soluble metal salts (e.g., nitrates, chlorides) of the desired metal ions are dissolved in water to form precursor solutions.
- Precipitation: A precipitating agent (e.g., alkali hydroxide, ammonia) is added dropwise to the precursor solution, causing the formation of a metal hydroxide precipitate.
- Aging: The precipitate is aged at a specific temperature for a certain time to allow particle growth and crystallization.
- Washing and drying: The precipitate is washed with water or ethanol to remove impurities and then dried to obtain the metal oxide nanoparticles.
- Calcination: In many cases, the dried precipitate is calcined at high temperatures to convert the hydroxide into the desired metal oxide.
The conventional sol gel (SG) method is a chemical method in which metal alkoxide sol undergoes hydrolysis followed by condensation to a rigid gelatinous mass. Heat treatment of the obtained gels results in the formation of metal oxide nanoparticles. SG involves two main stages [17]:
- Sol formation: Metal alkoxides or inorganic salts are dissolved in a suitable solvent (often alcohol) to form a sol, which is a stable colloidal suspension of nanoparticles.
- Gelation: The sol undergoes a process called gelation, where the particles aggregate to form a three-dimensional complex, resulting in a gel. This can be induced by hydrolysis and condensation reactions.
After gelation, the gel is dried to remove the liquid phase, leading to the formation of a xerogel. In most cases, the xerogel is calcined at high temperatures to remove organic components and convert the metal hydroxide or oxyhydroxide into the desired metal oxide.
Hydrothermal (Hy) and solvothermal (SolHy) methods are powerful techniques for synthesizing metal oxide nanoparticles with precise control over size, shape, and crystallinity [18]. These methods involve carrying out chemical reactions in a closed system under high temperature and pressure conditions. Hydrothermal uses water as the solvent, while solvothermal employs organic solvents instead of water [19]. The method can be combined with microwave heating for faster reaction rates.
Micelle/microemulsion is a technique used to create nanoparticles or nanostructures by forming a stable dispersion of two immiscible liquids (typically oil and water) using surfactants. Such dynamic colloidal templates are known to produce particles that are smaller than those obtained by regular precipitation in aqueous systems. In fact, water-in-oil microemulsions have successfully been used to produce a variety of nanoparticle shapes and sizes [20,21]. Micelles are aggregates of surfactant molecules formed in aqueous solutions above a certain concentration (critical micelle concentration). They have a hydrophilic outer layer and a hydrophobic core. Microemulsions are thermodynamically stable dispersions of two immiscible liquids (e.g., oil and water) stabilized by surfactants. They are transparent and isotropic. Metal precursors (salts or organometallics) are introduced into the aqueous phase of the microemulsion. Metal ions are confined within the water droplets of the microemulsion, where they undergo hydrolysis and condensation to form metal oxide nanoparticles. The size of the nanoparticles is controlled by the size of the water droplets in the microemulsion. The nanoparticles are separated from the microemulsion phase and purified.
Template-based synthesis is an emergent technique for producing metal oxide nanoparticles with precise control over shape, size, and orientation. It involves using a pre-existing structure (template) to guide the formation of the desired material [22,23]. Nanoparticles are grown directly on or within the template.
Hard templates are rigid structures with defined pores or channels, such as: nanoporous alumina, zeolites, or colloidal crystals [23]. Also, solid structures such as microspheres, nanowires, and thin films can be used as templates to shape nanoparticles.
Soft templates are flexible structures, often polymers or surfactants, that can be removed after nanoparticle formation: micelles, block copolymers, or surfactant assemblies.
The synthesis process is based on four steps:
- The chosen template is prepared with the desired structure and pore size.
- Metal precursors are introduced into the template’s pores or channels.
- The metal precursors react and nucleate within the confined space of the template, forming nanoparticles.
- The template is removed, leaving behind the metal oxide nanoparticles with the desired shape.
Biological synthesis is a rapidly growing field due to its eco-friendly and sustainable approach. This method utilizes biological entities such as bacteria, fungi, plants, and algae to produce nanoparticles with specific properties [24,25,26]. Under specific conditions, the reduced metal species can undergo oxidation to form metal oxide nanoparticles. Biomolecules such as proteins, enzymes, or polysaccharides can act as capping agents, stabilizing the nanoparticles and preventing agglomeration [24,27,28,29]. The photocatalytic efficiency of ZnO nanoparticles from different sources (green synthesis vs. co-precipitation) varies significantly; one method is more environmentally friendly but potentially less efficient [30]. A brief comparison of these methods is presented in Table 2.

Table 2.
Comparison of bottom-up methods.
To summarize, bottom-up methods have the potential to enable the development of new and exciting applications for metal oxide nanoparticles, particularly in fields like electronics, medicine, and energy, but the economic viability strongly affect the scalability. A SWOT analysis of these approaches is presented in Figure 4.
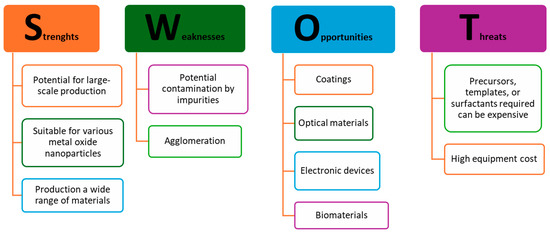
Figure 4.
SWOT analysis of bottom-up synthesis approaches of metal oxide nanoparticles [2,10,11,12,13,14,16,17,18,19,21,22,23,24,27,28,29,30].
3. Correlation between Synthesis and Properties of Metal-Oxide Nanoparticles
The synthesis of metal oxide nanoparticles involves a complex relationship of various factors that influence their properties, including size, shape, morphology, and crystallinity. The type of metal oxide nanoparticle is crucial in determining its properties and applications. Different metal oxides exhibit unique characteristics that make them suitable for specific purposes. MONs such as TiO2, Fe3O4, Fe2O3, Al2O3, CrO3, CuO, NiO, Ag2O, Sb2O3, and ZnO have been investigated in a variety of applications, including adsorption, photocatalytic activities, wastewater treatment, antifungal and antibacterial activities, cell viability, DNA damage, and oxidative stress. The specific metal and oxygen ratio in the nanoparticle determines its electronic structure, bandgap, and reactivity. For example, TiO2 is known for its photocatalytic properties, while ZnO is commonly used for its antimicrobial and UV-blocking abilities [31,32]. Also, CuO nanoparticles elaborated by sol-gel, with a size of particles between 36 and 52 nm, exhibit photocatalytic properties associated with optical ability [33]. Controlling particle size, morphology, and surface chemistry can optimize catalytic performance.
The choice of metal precursor significantly impacts the final composition of the MONs. The oxidation state of the metal ion determines the type of metal oxide formed. Doping is achieved by the introduction of foreign ions to modify the electronic properties and structure of the MONs. Liu et al. reported that the average crystal size of the Sb-doped SnO2 nanoparticles decreased from 16 to 7 nm by increasing the Sb doping concentration by chemical coprecipitation [34,35]. Also, Popov et al. demonstrated that the unit cell expansion of Sn-doped hematite nanoparticles is due to the replacement of Fe3+ by Sn4+, causing an increase in thickness of the particles. Impurities introduce energy levels within the bandgap, affecting the optical absorption and emission properties of ZnO36. Impurities act as donors or acceptors, modifying the electrical conductivity and carrier concentration of ZnO. Common donor impurities include elements from group III of the periodic table, such as Al, Ga, and In. When these elements replace Zn atoms in the lattice, they contribute an extra electron to the conduction band, increasing the electron concentration and, hence, the conductivity. Acceptor impurities are elements from group I, such as Cu and Ag, or Group V, like N. When these elements substitute for Zn or O atoms, they accept electrons from the valence band, leaving behind holes. This increases the hole concentration and can lead to p-type conductivity [36]. Another modification of catalytic activity consists in the degradation of tartrazine yellow azo dye by Cu-modified ZnO nanocomposite materials obtained by the hydrothermal method [18].
The size and shape of nanoparticles significantly impact their surface area, reactivity, and optical properties. Smaller particles often exhibit higher surface area, leading to increased catalytic activity or adsorption capacity. The balance between nucleation and growth kinetics determines particle size. Higher temperatures often lead to larger particles due to increased atomic mobility. Higher concentrations can result in faster nucleation and smaller particles. Different phases of a metal oxide can form at varying temperatures. A gradient can facilitate the formation of multiple phases within a single sample. Temperature gradients can influence particle shape, leading to anisotropic structures such as TiO2. Previous studies have shown that increasing the thermal annealing temperature increases the particle size and change the amorphous TiO2 to anatase and rutile at 450 °C and 900 °C, respectively [32].
Some metal oxides, especially when doped with metallic nanoparticles (e.g., Ag or Au), exhibit surface plasmon resonance (SPR), where free electrons in the metal resonate with light at specific wavelengths. This interaction leads to vivid color changes, which are dependent on the size, shape, and composition of the nanoparticles. For example, doping ZnO with Co (0.01%, 0.02%, 0.03% and 0.04%) can make the material appear green due to changes in its absorption spectrum [37]. Li et al. prepared nano-iron red oxide pigment via an ammonia process, with urea as the precipitant, started from cyanided tailings [38].
Different crystal structures (e.g., anatase, rutile for TiO2) influence properties like bandgap, reactivity, and stability. Surfactants and capping agents can control particle growth and shape by adsorbing onto specific crystal facets, affecting the crystal’s reactivity and stability. Higher temperatures promote crystal growth and improve crystallinity. Slow cooling allows for better crystal formation.
The solution pH influences the surface charge of the MONs. Precise pH regulation can influence the solubility of precursors, nucleation rates, and particle growth mechanisms [39]. At different pH levels, the solubility of metal ions changes, affecting nucleation and growth processes. A lower pH typically promotes the formation of smaller particles due to faster nucleation, while a higher pH can lead to larger, more stable particles through controlled growth [40]. Additionally, pH impacts the surface charge and zeta potential of nanoparticles, influencing their dispersion and aggregation in solution [16,41,42]. Also, elevated pressures can enhance reaction kinetics, promote phase transformations, and influence particle morphology. Kumar S. demonstrated the capacity of ZnO to capture CO2 due to particle size and pore volume [43].
Surfactant gel [44] or microwave [45] assisted synthesis can be attractive alternatives to the conventional sol gel method for the preparation of metal oxide nanoparticles.
Metal oxide nanoparticles synthesized using the surfactant gel method can be useful for a variety of applications [44]. For example, nanostructured ZnO [30], TiO2 [31], CeO2 [46], MgO [47], CuO [33], and ZrO2 [48] can be utilized as photocatalysts, for optoelectronic applications, or as adsorbents for the removal of waterborne pollutants.
Microwave radiation can induce non-thermal effects, such as the generation of reactive species, which can influence nanoparticle properties. Microwaves offer rapid and uniform heating, leading to accelerated reaction rates and improved product quality. Microwave energy can be selectively absorbed by specific components in the reaction mixture, enabling precise control over nanoparticle formation [45].
Ultrasonic waves generate cavitation bubbles, which collapse violently, creating localized high temperatures and pressures [49,50]. Cavitation improves mass transfer, leading to increased reaction rates and homogeneous nucleation. Ultrasonic waves can fragment larger particles, resulting in smaller and more uniform nanoparticles [49].
4. Functionalization of Metal Oxide Nanoparticles for Emerging Applications
Metal oxide nanoparticles (MONs) are particularly attractive for functionalization due to their unique properties such as high surface area, tunable electronic properties, and diverse chemical reactivity. Functionalization mechanisms can be performed through physical adsorption, chemical process, or layer-by-layer assembly.
In the physical adsorption process, the Van der Waals forces are responsible for molecule adsorption on the nanoparticle surface, but at the same time, hydrogen bonding can occur. Covalent, ionic, or coordination bonding are the bases of the formation of chemical bonds between the surface of the nanoparticles and the functional groups in chemical processes.
The surface chemistry of nanoparticles, including functional groups and charge, affects their interactions with other materials and biological systems. Metal oxide nanoparticles like TiO₂ and ZnO have surface hydroxyl groups that can generate reactive oxygen species (ROS), such as hydroxyl radicals (OH), under UV or visible light irradiation. These ROS are highly reactive and can degrade organic pollutants in water [19,46]. The presence of functional groups that stabilize ROS on the surface of nanoparticles enhances their catalytic activity [49,50,51]. Furthermore, the surface charge of the nanoparticles affects their interaction with pollutants; for example, negatively charged surfaces are more likely to attract and degrade positively charged pollutants. In some cases, the adsorbed pollutant can undergo degradation on the nanoparticle’s surface. This can be due to catalytic activity or other chemical reactions facilitated by the nanoparticle. MONs, such as zinc oxide nanoparticles, can effectively remove pollutants like heavy metals and organic contaminants from water through adsorption and catalytic processes [52].
Metal oxide nanoparticles are excellent candidates for gas sensors due to their high surface area, which enhances interaction with gas molecules. Their electronic properties are also sensitive to changes in the surrounding environment, making them suitable for detecting a variety of gases. ZnO-based gas sensors are used in various applications, including alcohol breathalyzers, environmental monitoring, and industrial process control [53]. SnO2 nanostructured sensors are used in applications such as indoor air quality monitoring, automotive exhaust gas sensing, and personal safety devices [54]. WO3-based sensors are used in industrial process control, environmental monitoring, and personal safety devices [54].
MONs, particularly those of transition metals like zinc oxide (ZnO) and titanium dioxide (TiO2), are being explored as electrode materials for rechargeable batteries, enhancing energy density and charging efficiency [55]. Also, they can be used as efficient charge carriers in solar cells, increasing light absorption and improving overall energy conversion efficiency [56].
The surface charge of metal oxide nanoparticles determines their interaction with cell membranes. For instance, cell membranes are typically negatively charged due to the presence of phospholipids. Nanoparticles with a positive surface charge (e.g., functionalized with amino groups -NH₂) can strongly interact with the cell membrane, promoting cellular uptake. These interactions can lead to increased cytotoxicity due to membrane disruption, production of ROS, or initiation of cellular stress responses [57]. ZnO NPs are known for their antibacterial properties, but they can also induce cytotoxicity in mammalian cells [58]. Also, currently, the use of TiO2 for sunscreens and pigments is becoming a concern [59]. On the other hand, nanoparticles with a negative surface charge may experience repulsion from the cell membrane, leading to lower cellular uptake and reduced cytotoxicity [57,60].
Metal oxide nanoparticles can be functionalized with specific ligands (e.g., antibodies, peptides) to target cells or tissues. These ligands are typically attached via covalent bonds to surface functional groups (e.g., carboxyl (−COOH) or amine (−NH₂) groups). So, the functional groups on the nanoparticle surface can determine the stability of the drug-nanoparticle conjugate in biological environments and the efficiency of drug release at the target site. For example, pH-sensitive linkages can be used for drug release in acidic tumor microenvironments [61].
Metal oxide nanoparticles can interact with enzymes through their surface functional groups, either promoting or inhibiting enzymatic activity [62]. For example, ZnO nanoparticles may inhibit enzymes by binding them to active sites via their surface hydroxyl groups or metal ions. This can lead to oxidative stress, DNA damage, or apoptosis in cells. Nanoparticles with specific functional groups may also interact with DNA, potentially causing genotoxic effects if they disrupt DNA replication or transcription processes.
MONs can be integrated into biosensors for early detection of diseases and monitoring of biological parameters [63]. Fluorescent MONs can be used as contrast agents in medical imaging techniques like MRI and fluorescence microscopy to visualize biological processes [64].
5. Adaptive Modeling of Synthesis Conditions for Particle Size Control
Creating nanomaterials with precise and controlled dimensions is a complex task that requires a deep understanding of various factors. Currently the Design of Experiment (DOE) is used to model the effect of synthesis conditions on nanoparticle properties. DOE matrix is a quantitative or qualitative combination of factor levels. This consists of designing an experiment to vary synthesis conditions (e.g., temperature, concentration, pH) and measure the resulting nanoparticle properties. Quantitative factors include time, temperature, stirring speed, homogenization pressure, etc. Qualitative factors take on a discrete number of values and include operation mode, supplier, type of surfactant, etc. The response can be nanoparticle size, nanoparticle shape or nanoparticle distribution, as presented in Figure 5.
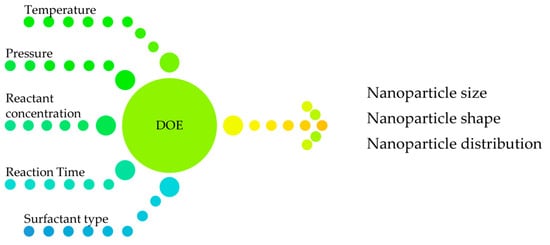
Figure 5.
DOE chart.
Using Regression Analysis is important to model the relationship between synthesis conditions and nanoparticle properties [65]. Also, Response Surface Methodology (RSM) is useful if multiple factors are involved. RSM can be used to optimize nanoparticle properties by finding the optimal combination of synthesis conditions.
For example, in an investigation of how reactant concentration and microwave power can affect the size of MgO nanoparticles, a 22 factorial design can be used with four experiments, taking into account the experiments conducted by Modan et al. [16]. The various input sizes were concentration (CC), in mol/l, microwave power (MP), in W, and the output size is the average crystallite size determined by XRD, in nm. As a model/equation of dependence of output quantities as a function of input quantities, for each material researched, the linear model was adopted, a model widely used in process modeling. Considering that the input variables are independent, the particle size equations are of the form:
where C, a, b, c, d, e, g are constants to be determined based on experimental data.
D = Cx + ax⋅A + bx⋅B + cx⋅C + dx⋅A⋅B + ex⋅A⋅C + gx⋅B⋅C
The processing of the obtained data will be carried out using the ANOVA method also known as dispersion analysis or analysis of variance.
The analysis was performed using the Minitab 16 program. The Taguchi method allows for the calculation of the S/N ratio, the choice of the performance characteristic between “smaller is better”, “large is better”, or “normal is better”. The signal-to-noise ratio (S/N ratio) is the ratio of the desired value to the undesirable value as output characteristics.
The values of the process parameters in their natural values corresponding to the two levels (+1, −1) are presented in Table 3.

Table 3.
Values of the parameters of the process of elaboration of MgO nanoparticles.
An experimental research plan for determining the particle size in natural and normed variables, respectively, is presented inTable 4.

Table 4.
Experimental parameters of the process of elaboration of MgO nanoparticles.
To determine the influence of solution concentration, experiments 1 and 3 or experiments 2 and 4 can be compared. The decrease in concentration (from 1 M to 0.5 M) does not significantly affect the crystallite size under the same microwave power. Comparing experiments 1 and 2, an increase in microwave power from 850 W to 1000 W (for concentration 1M), leads to a decrease in crystallite size of 13.37%, from 7.33 to 6.35 nm.
Comparing experiments 3 and 4, an increase in microwave power from 850 W to 1000 W (for concentration 0.5 M) leads to a decrease in crystallite size of 4.88%, from 7.18 to 6.83 nm.
To gain a comprehensive understanding of how concentration and microwave power influence the crystallite dimension, the ANOVA method was used. The analysis was performed in the Minitab 16 program, as presented in Table 5.

Table 5.
Coded coefficients.
To find the statistical significance of the process variables and their interactions on the particle size, the ANOVA analysis was performed. The crystallite size model based on the linear regression of ANOVA analysis is:
DC = 6.922 − 0.08250 CC − 0.3325 MP − 0.1575 CC × MP (nm)
Pareto can be used to graphic representation of the relative importance of parameters in the synthesis process. The Pareto chart of the effects is generated in Figure 6.
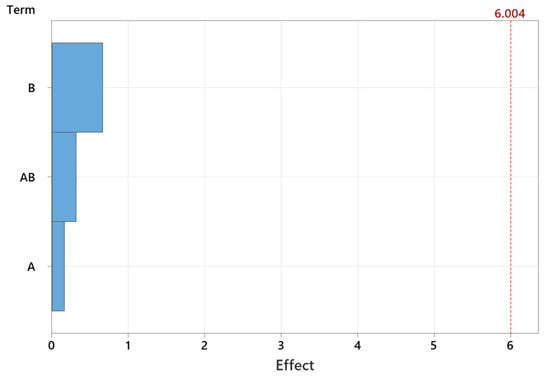
Figure 6.
Pareto chart of the effects (A—concentration, B—microwave power, AB—concentration or microwave power)).
So, in conclusion, using statistical analysis, we can assume that the size of MgO nanoparticles is influenced by both reactant concentration (CC) and microwave power (MP). The interaction term in the model suggests that the relationship between particle size and CC or MP is not strictly linear.
6. Conclusions
Metal oxide nanoparticles have emerged as a class of materials with immense potential across various fields. The analysis of the number of publications presenting the results of research in the field of metallic oxide nanoparticles shows an increase in the last 10 years.
The synthesis of MONs is a crucial step in determining their final properties and performance. By carefully controlling the synthesis process, researchers can tailor the size, shape, morphology, and composition of MONs to optimize their suitability for specific purposes. The development of efficient and versatile synthesis strategies is essential for realizing their full potential.
This study has provided an overview of the most common synthesis techniques of MONs and challenges in the field. The top-down and bottom-up syntheses present both advantages and disadvantages, according to the SWOT analysis carried out. Also, the study highlights the correlation between synthesis parameters and MON properties. It is concluded that the functionalization of this class of nanoparticles proves to be a tool for specific properties.
In this paper, it is demonstrated that, by using adaptive models, the control over the size of the nanoparticles is improved, obtaining uniform products with improved functional properties. By combining DOE and ANOVA, a comprehensive understanding of how synthesis parameters influence the response variable and identify the optimal conditions for achieving desired properties can be aquired. Using these methods, the consumption of energy and raw materials can be significantly reduced, facilitating more sustainable and efficient processes.
The influence of other factors, such as temperature, stirring rate, or microwave frequency, might also be investigated. The integration of experimental and theoretical approaches will continue to drive advancements in this field, enabling the development of novel materials with tailored functionalities for a wide range of applications.
Author Contributions
Conceptualization, A.-G.S. and M.O.; Methodology, D.M.I.; Software, D.M.I.; Validation, M.O.; Formal analysis, M.O.; Investigation, A.-G.S.; Resources, A.-M.I.; Data curation, A.-G.S.; Writing—original draft preparation, A.-G.S.; Writing—review and editing, L.M.C. and M.O.; Visualization, M.O.; Supervision, D.M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Subramani, K.; Ahmed, W. Nanotechnology and the Future of Dentistry. In Emerging Nanotechnologies in Dentistry: Processes, Materials and Applications; William Andrew: Norwich, NY, USA, 2011; pp. 1–14. [Google Scholar] [CrossRef]
- Yang, L. Fundamentals of Nanotechnology and Orthopedic Materials. In Nanotechnology-Enhanced Orthopedic Materials; Woodhead Publishing: Sawston, UK, 2015; pp. 1–25. [Google Scholar] [CrossRef]
- Hári, J.; Pukánszky, B. Nanocomposites: Preparation, Structure, and Properties. In Applied Plastics Engineering Handbook: Processing and Materials; Elsevier: Amsterdam, The Netherlands, 2011; pp. 109–142. [Google Scholar] [CrossRef]
- Ibrahim, I.D.; Jamiru, T.; Sadiku, E.R.; Hamam, Y.; Alayli, Y.; Eze, A.A. Application of Nanoparticles and Composite Materials for Energy Generation and Storage. IET Nanodielectrics 2019, 2, 115–122. [Google Scholar] [CrossRef]
- Shurygina, I.A.; Shurygin, M.G.; Sukhov, B.G. Nanobiocomposites of Metals as Antimicrobial Agents. In Antibiotic Resistance: Mechanisms and New Antimicrobial Approaches; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 167–186. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An Overview of Synthesis, Classification, Characterization, and Applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Chen, F.; Yan, T.H.; Bashir, S.; Liu, J.L. Synthesis of Nanomaterials Using Top-down Methods. In Advanced Nanomaterials and Their Applications in Renewable Energy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 37–60. [Google Scholar] [CrossRef]
- Arbain, R.; Othman, M.; Palaniandy, S. Preparation of Iron Oxide Nanoparticles by Mechanical Milling. Miner. Eng. 2011, 24, 1–9. [Google Scholar] [CrossRef]
- Balachandran, A.; Sreenilayam, S.P.; Madanan, K.; Thomas, S.; Brabazon, D. Nanoparticle Production via Laser Ablation Synthesis in Solution Method and Printed Electronic Application—A Brief Review. Results Eng. 2022, 16, 100646. [Google Scholar] [CrossRef]
- Bosso, P.; Del Sole, R.; Milella, A.; Mengucci, P.; Barucca, G.; Armenise, V.; Bianco, G.V.; Fracassi, F.; Palumbo, F. Nanostructured Iron Oxide Thin Films Deposited by RF Sputtering as Catalysts for the Heterogeneous Solar Photo-Fenton Reaction. Vacuum 2023, 207, 111646. [Google Scholar] [CrossRef]
- Shaji, K.; Haviar, S.; Zeman, P.; Kos, Š.; Čerstvý, R.; Čapek, J. Controlled Sputter Deposition of Oxide Nanoparticles-Based Composite Thin Films. Surf. Coat. Technol. 2024, 477, 130325. [Google Scholar] [CrossRef]
- Nanolithography—An overview. ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/chemistry/nanolithography (accessed on 30 August 2024).
- Novembre, A.; Liu, S. Chemistry and Processing of Resists for Nanolithography. In Nanolithography: The Art of Fabricating Nanoelectronic and Nanophotonic Devices and Systems; Woodhead Publishing: Sawston, UK, 2013; pp. 194–286. [Google Scholar] [CrossRef]
- Elwakil, B.H.; Toderas, M.; El-Khatib, M. Arc Discharge Rapid Synthesis of Engineered Copper Oxides Nano Shapes with Potent Antibacterial Activity against Multi-Drug Resistant Bacteria. Sci. Rep. 2022, 12, 20209. [Google Scholar] [CrossRef]
- Hemben, A.; Chianella, I.; Leighton, G.J.T. Surface Engineered Iron Oxide Nanoparticles Generated by Inert Gas Condensation for Biomedical Applications. Bioengineering 2021, 8, 38. [Google Scholar] [CrossRef]
- Modan, M.; Schiopu, A.-G.; Marian Ducu, C.; Oproescu, M.; Magdalena Modan, E.; Schiopu, A.; Georgian Moga, S.; Aurelian Negrea, D.; Oproescu, M.; Gabriel Iana, V.; et al. The influence of precursors on the morpho-structure of zinc oxide. J. Sci. Arts 2024, 24, 399–408. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, Z.U.H.; Sabahat, S.; Sun, J.; Shah, N.S.; Khan, Z.U.; Muhammad, N.; Mir, S.; Rahim, A.; Nadeem, M.; et al. Innovations in Metal Oxides-Biochar Nanoparticles for Dye Removal. Nano-Struct. Nano-Objects 2024, 39, 101269. [Google Scholar] [CrossRef]
- Rehman, F.U.; Zada, Z.; Qayyum, I. Hydrothermal Synthesis of Copper Doped Zinc Oxide Nano Composites to Achieve Optimum Removal of Organic Pollutant Dye from Waste Water Following the Photo Catalytic Degradation. J. Nanomater. Mol. Nanotechnol. 2022, 11, 2. [Google Scholar]
- Han, Z.; Adeleye, A.S.; Keller, A.A. Engineered Nanomaterials for Water Treatment. Encycl. Nanomater. 2023, 1, 418–455. [Google Scholar] [CrossRef]
- Bumajdad, A.; Eastoe, J.; Zaki, M.I.; Heenan, R.K.; Pasupulety, L. Generation of Metal Oxide Nanoparticles in Optimised Microemulsions. J. Colloid. Interface Sci. 2007, 312, 68–75. [Google Scholar] [CrossRef]
- Alameen, A.S.; Undre, S.B.; Undre, P.B. Synthesis, Dispersion, Functionalization, Biological and Antioxidant Activity of Metal Oxide Nanoparticles: Review. Nano-Struct. Nano-Objects 2024, 39, 101298. [Google Scholar] [CrossRef]
- Mohandesi, M.; Tavakolian, M.; Rahimpour, M.R. Eggplant as an Appreciable Bio-Template for Green Synthesis of NiO Nanoparticles: Study of Physical and Photocatalytic Properties. Ceram. Int. 2022, 48, 22820–22826. [Google Scholar] [CrossRef]
- Kaur, A.; Bajaj, B.; Kaushik, A.; Saini, A.; Sud, D. A Review on Template Assisted Synthesis of Multi-Functional Metal Oxide Nanostructures: Status and Prospects. Mater. Sci. Eng. B 2022, 286, 116005. [Google Scholar] [CrossRef]
- Kousar, R.; Khan, Z.U.H.; Sabahat, S.; Sun, J.; Muhammad, N.; Shah, N.S.; Iqbal, J.; Khasim, S.; Salam, M.A. Synergetic Comparative Study: Photocatalytic and Biological Investigations of Green-Synthesized Metal Oxide Nanoparticles. Nano-Struct. Nano-Objects 2024, 38, 101184. [Google Scholar] [CrossRef]
- Purkayastha, D.D. Biogenic Synthesis of Silver Nanoparticles Using Lichens. In Green Synthesis of Silver Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 513–523. [Google Scholar] [CrossRef]
- Aisida, S.O.; Onwujiobi, C.; Ahmad, I.; Zhao, T.-k.; Maaza, M.; Ezema, F.I. Biogenic Synthesis of Zinc Oxide Nanorods for Biomedical Applications and Photodegradation of Rhodamine B. Mater. Today Commun. 2022, 33, 104660. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Y.; Yang, X.; Yu, P.; Ban, D. Green Synthesis of Gold Nanoparticles Mediated by Extract of Curcuma Longa under Ultrasonic Condition: Investigation of Its Application for Reduction of Dye Pollutants and Repairing the Articular Cartilage in an Animal Model of Osteoarthritis of the Knee. Inorg. Chem. Commun. 2024, 162, 112169. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Osibote, O.A. Green Synthesis of Metal Oxide Nanoparticles, and Their Various Applications. J. Hazard. Mater. Adv. 2024, 13, 100401. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Chaudhry, S.A.; Ikram, S. A Review on Biogenic Synthesis of ZnO Nanoparticles Using Plant Extracts and Microbes: A Prospect towards Green Chemistry. J. Photochem. Photobiol. B 2017, 166, 272–284. [Google Scholar] [CrossRef]
- Aalami, Z.; Hoseinzadeh, M.; Hosseini Manesh, P.; Aalami, A.H.; Es’haghi, Z.; Darroudi, M.; Sahebkar, A.; Hosseini, H.A. Synthesis, Characterization, and Photocatalytic Activities of Green Sol-Gel ZnO Nanoparticles Using Abelmoschus Esculentus and Salvia Officinalis: A Comparative Study versus Co-Precipitation-Synthesized Nanoparticles. Heliyon 2024, 10, e24212. [Google Scholar] [CrossRef]
- Marycleopha, M.; Yaou Balarabe, B.; Adjama, I.; Moussa, H.; Anandaram, H.; Abdoul Razak, M.W. Anhydrous Sol-Gel Synthesis of TiO2 Nanoparticles: Evaluating Their Impact on Protein Interactions in Biological Systems. J. Trace Elem. Miner. 2024, 7, 100114. [Google Scholar] [CrossRef]
- Sugapriya, S.; Sriram, R.; Lakshmi, S. Effect of Annealing on TiO2 Nanoparticles. Optik 2013, 124, 4971–4975. [Google Scholar] [CrossRef]
- Sivayogam, D.; Kartharinal Punithavathy, I.; Johnson Jayakumar, S.; Mahendran, N. Study on Structural, Electro-Optical and Optoelectronics Properties of CuO Nanoparticles Synthesis via Sol Gel Method. Mater. Today Proc. 2022, 48, 508–513. [Google Scholar] [CrossRef]
- Liu, S.M.; Ding, W.Y.; Chai, W.P. Influence of Sb Doping on Crystal Structure and Electrical Property of SnO2 Nanoparticles Prepared by Chemical Coprecipitation. Phys. B Condens. Matter 2011, 406, 2303–2307. [Google Scholar] [CrossRef]
- Popov, N.; Ristić, M.; Bošković, M.; Perović, M.; Musić, S.; Stanković, D.; Krehula, S. Influence of Sn Doping on the Structural, Magnetic, Optical and Photocatalytic Properties of Hematite (α-Fe2O3) Nanoparticles. J. Phys. Chem. Solids 2022, 161, 110372. [Google Scholar] [CrossRef]
- Rahman, M.; Kamruzzaman, M.; Zapien, J.A.; Afrose, R.; Anam, T.K.; Liton, M.N.H.; Helal, M.A.; Khan, M.K.R. Conversion of N-Type to p-Type Conductivity in ZnO by Incorporation of Ag and Ag-Li. Mater. Today Commun. 2022, 33, 104278. [Google Scholar] [CrossRef]
- Zhou, N.; Zhang, Y.; Nian, S.; Li, W.; Li, J.; Cao, W.; Wu, Z. Synthesis and Characterization of Zn1-XCoxO Green Pigments with Low Content Cobalt Oxide. J. Alloys Compd. 2017, 711, 406–413. [Google Scholar] [CrossRef]
- Dengxin, L.; Guolong, G.; Fanling, M.; Chong, J. Preparation of Nano-Iron Oxide Red Pigment Powders by Use of Cyanided Tailings. J. Hazard. Mater. 2008, 155, 369–377. [Google Scholar] [CrossRef]
- Stepan, T.; Tété, L.; Laundry-Mottiar, L.; Romanovskaia, E.; Hedberg, Y.S.; Danninger, H.; Auinger, M. Effect of Nanoparticle Size on the Near-Surface PH-Distribution in Aqueous and Carbonate Buffered Solutions. Electrochim. Acta 2022, 409, 139923. [Google Scholar] [CrossRef]
- Modan, E.M.; Plăiașu, A.G. Advantages and Disadvantages of Chemical Methods in the Elaboration of Nanomaterials. In The Annals of “Dunarea de Jos” University of Galati. Fascicle IX, Metallurgy and Materials Science; Galati University Press: Galati, Romania, 2020; Volume 43, pp. 53–60. [Google Scholar] [CrossRef]
- Walton, K.S.; Snurr, R.Q. Applicability of the BET Method for Determining Surface Areas of Microporous Metal-Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar] [CrossRef]
- Shaji, A.; Zachariah, A.K. Surface Area Analysis of Nanomaterials. In Thermal and Rheological Measurement Techniques for Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; Volume 3, pp. 197–231. [Google Scholar] [CrossRef]
- Kumar, S. The Effect of Elevated Pressure, Temperature and Particles Morphology on the Carbon Dioxide Capture Using Zinc Oxide. J. CO2 Util. 2014, 8, 60–66. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Pan, S.; Barman, A.; McLaughlin, J.A.; Roy, S.S. Oil Swollen Surfactant Gel Based Synthesis of Metal Oxides Nanoparticles: An Attractive Alternative for the Conventional Sol Gel Synthesis. Ceram. Int. 2016, 42, 12119–12128. [Google Scholar] [CrossRef]
- Veronesi, P.; Colombini, E.; Canarslan, Ö.S.; Baldi, G.; Leonelli, C. Procedure to Generate a Selection Chart for Microwave Sol-Gel Synthesis of Nanoparticles. Chem. Eng. Process.-Process Intensif. 2023, 189, 109383. [Google Scholar] [CrossRef]
- Alsolami, E.S.; Mkhalid, I.A.; Shawky, A.; Hussein, M.A. Metal Oxide-Combined Sol-Gel Synthesized Ceria Nanoparticles: An Operative Photocatalyst for Visible-Light-Driven Mineralization of Ciprofloxacin Antibiotic in Water. J. Phys. Chem. Solids 2024, 195, 112289. [Google Scholar] [CrossRef]
- Mohammed Salman, K.; Renuka, C.G. Modified Sol-Gel Technique for the Synthesis of Pure MgO and ZnO Nanoparticles to Study Structural and Optical Properties for Optoelectronic Applications. Mater. Today Proc. 2023, 89, 84–89. [Google Scholar] [CrossRef]
- Gutierrez-Sanchez, C.D.; Téllez-Jurado, L.; Dorantes-Rosales, H.J. Synthesis of Zirconia Nanoparticles by Sol-Gel. Influence of Acidity-Basicity on the Stability Transformation, Particle, and Crystallite Size. Ceram. Int. 2024, 50, 20547–20560. [Google Scholar] [CrossRef]
- Banitaba, S.H. Collaboration of Ultrasonic Irradiation and Silica Nanoparticles in the Diastereoselective Synthesis of Trans-2,3-Dihydrofuran Derivatives: An Exceptional Catalytic Activity of Sound Cavities and SiO2 Nanoparticles. Polycycl. Aromat. Compd. 2023, 43, 2321–2334. [Google Scholar] [CrossRef]
- Arularasu, M.V.; Vinitha, P.; Muthukrishnaraj, A. Ultrasonic Assisted Synthesis of Nanoporous Carbon/CeVO4 Nanocomposite for Supercapacitor and Photocatalytic Applications. Nano-Struct. Nano-Objects 2024, 39, 101305. [Google Scholar] [CrossRef]
- Kainat, S.; Gull, N.; Khan, S.M.; Zia, S.; Munir, S. Physicochemical Attributes, Structural Characterization, and Catalytic Properties of Nanomaterials. In Nanomaterials in Biomass Conversion: Advances and Applications for Bioenergy, Biofuels, and Bio-based Products; Woodhead Publishing: Sawston, UK, 2024; pp. 143–167. [Google Scholar] [CrossRef]
- Badri, A.; Slimi, S.; Guergueb, M.; Kahri, H.; Mateos, X. Green Synthesis of Copper Oxide Nanoparticles Using Prickly Pear Peel Fruit Extract: Characterization and Catalytic Activity. Inorg. Chem. Commun. 2021, 134, 109027. [Google Scholar] [CrossRef]
- Sha, R.; Basak, A.; Maity, P.C.; Badhulika, S. ZnO Nano-Structured Based Devices for Chemical and Optical Sensing Applications. Sens. Actuators Rep. 2022, 4, 100098. [Google Scholar] [CrossRef]
- Yang, B.; Tran, T.T.; Milam-Guerrero, J.A.; To, D.T.; Stahovich, T.; Myung, N.V. Enhancing Gas Sensing Performance of Tungsten Trioxide (WO3) Nanofibers through Diameter and Crystallinity Control. Sens. Actuators Rep. 2024, 7, 100182. [Google Scholar] [CrossRef]
- Das, A.; Peu, S.D.; Hossain, M.S.; Akanda, M.A.M.; Salah, M.M.; Akanda, M.M.H.; Rahman, M.; Das, B.K. Metal Oxide Nanosheet: Synthesis Approaches and Applications in Energy Storage Devices (Batteries, Fuel Cells, and Supercapacitors). Nanomaterials 2023, 13, 1066. [Google Scholar] [CrossRef]
- Oproescu, M.; Schiopu, A.G.; Calinescu, V.M.; Iana, V.G.; Bizon, N.; Sallah, M. Influence of Supplementary Oxide Layer on Solar Cell Performance. Eng. Technol. Appl. Sci. Res. 2024, 14, 13274–13282. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, B.; Kumar, R.; Chhabra, D.; Ghosh, M.; Manuja, M.; Brar, B.; Pal, Y.; Tripathi, B.N.; Prasad, M. Metal/Metal Oxide Nanoparticles: Toxicity Concerns Associated with Their Physical State and Remediation for Biomedical Applications. Toxicol. Rep. 2021, 8, 1970–1978. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Touzani, A.; Ramos-Pan, L.; Alba-González, A.; Folgueira, M.; Moreda-Piñeiro, J.; Méndez, J.; Pásaro, E.; Fernández-Bertólez, N.; Laffon, B. Cytotoxic Effects of Zinc Oxide Nanoparticles on Human Glial Cells. Mater. Proc. 2023, 14, 23. [Google Scholar] [CrossRef]
- Miquel-Jeanjean, C.; Déric Cré Pel, F.; Ronique Raufast, V.; Payre, B.; Datas, L.; Bessou-Touya, S.; Lène Duplan, H. Penetration Study of Formulated Nanosized Titanium Dioxide in Models of Damaged and Sun-Irradiated Skins. Photochem. Photobiol. 2012, 88, 1513–1521. [Google Scholar] [CrossRef]
- Miu, D.-M.; Sha’at, F.; Neagu, G.; Albulescu, A.; Pavaloiu, R.-D.; Eremia, M.-C.; Jinga, S.-I. Biopolymer nanoparticles loaded with curcumin for biomedical applications. UPB Bull. Series B 2024, 86, 175–188. [Google Scholar]
- Tavares Luiz, M.; Santos Rosa Viegas, J.; Palma Abriata, J.; Viegas, F.; Testa Moura de Carvalho Vicentini, F.; Lopes Badra Bentley, M.V.; Chorilli, M.; Maldonado Marchetti, J.; Tapia-Blácido, D.R. Design of Experiments (DoE) to Develop and to Optimize Nanoparticles as Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2021, 165, 127–148. [Google Scholar] [CrossRef]
- Khan, S.; Babadaei, M.M.N.; Hasan, A.; Edis, Z.; Attar, F.; Siddique, R.; Bai, Q.; Sharifi, M.; Falahati, M. Enzyme–Polymeric/Inorganic Metal Oxide/Hybrid Nanoparticle Bio-Conjugates in the Development of Therapeutic and Biosensing Platforms. J. Adv. Res. 2021, 33, 227–239. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Sensors and Biosensors Based on Metal Oxide Nanomaterials. TrAC Trends Anal. Chem. 2019, 121, 115690. [Google Scholar] [CrossRef]
- Xu, H.; Yu, P.; Bandari, R.P.; Smith, C.J.; Aro, M.R.; Singh, A.; Ma, L. Bimodal MRI/Fluorescence Nanoparticle Imaging Contrast Agent Targeting Prostate Cancer. Nanomaterials 2024, 14, 1177. [Google Scholar] [CrossRef]
- Mathew, D.; Bhat, S.G. Statistical Design for Biogenesis of Melanin Nanoparticles from Producer Strain Pseudomonas Stutzeri BTCZ 109 through Taguchi DOE. Biocatal. Agric. Biotechnol. 2022, 42, 102366. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).