The Influence of Deposition Temperature on the Microscopic Process of Diamond-like Carbon (DLC) Film Deposition on a 2024 Aluminum Alloy Surface
Abstract
1. Introduction
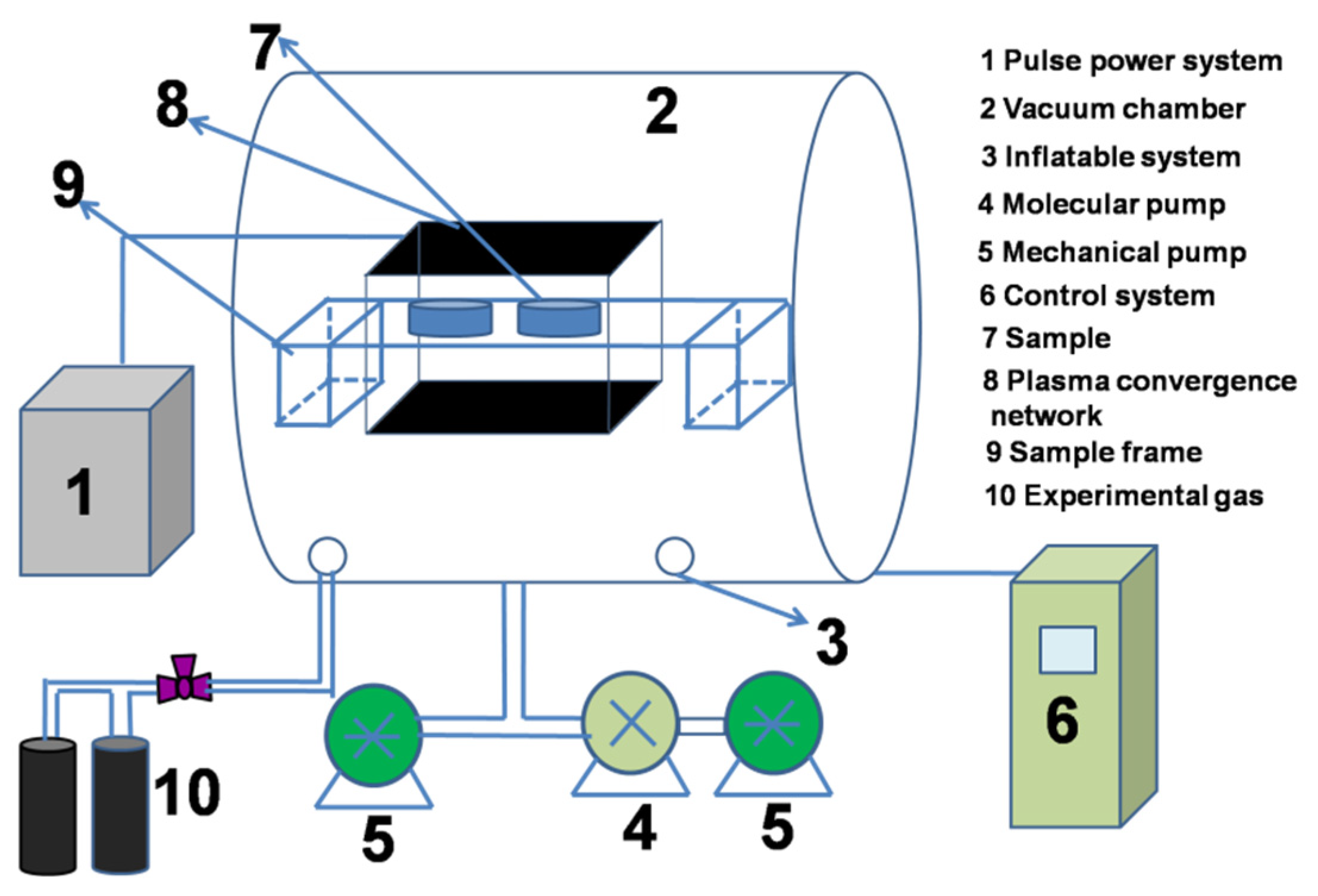
2. Experimental Process
2.1. Experimental Materials
2.2. Experimental Process and Parameters
3. Experimental Results
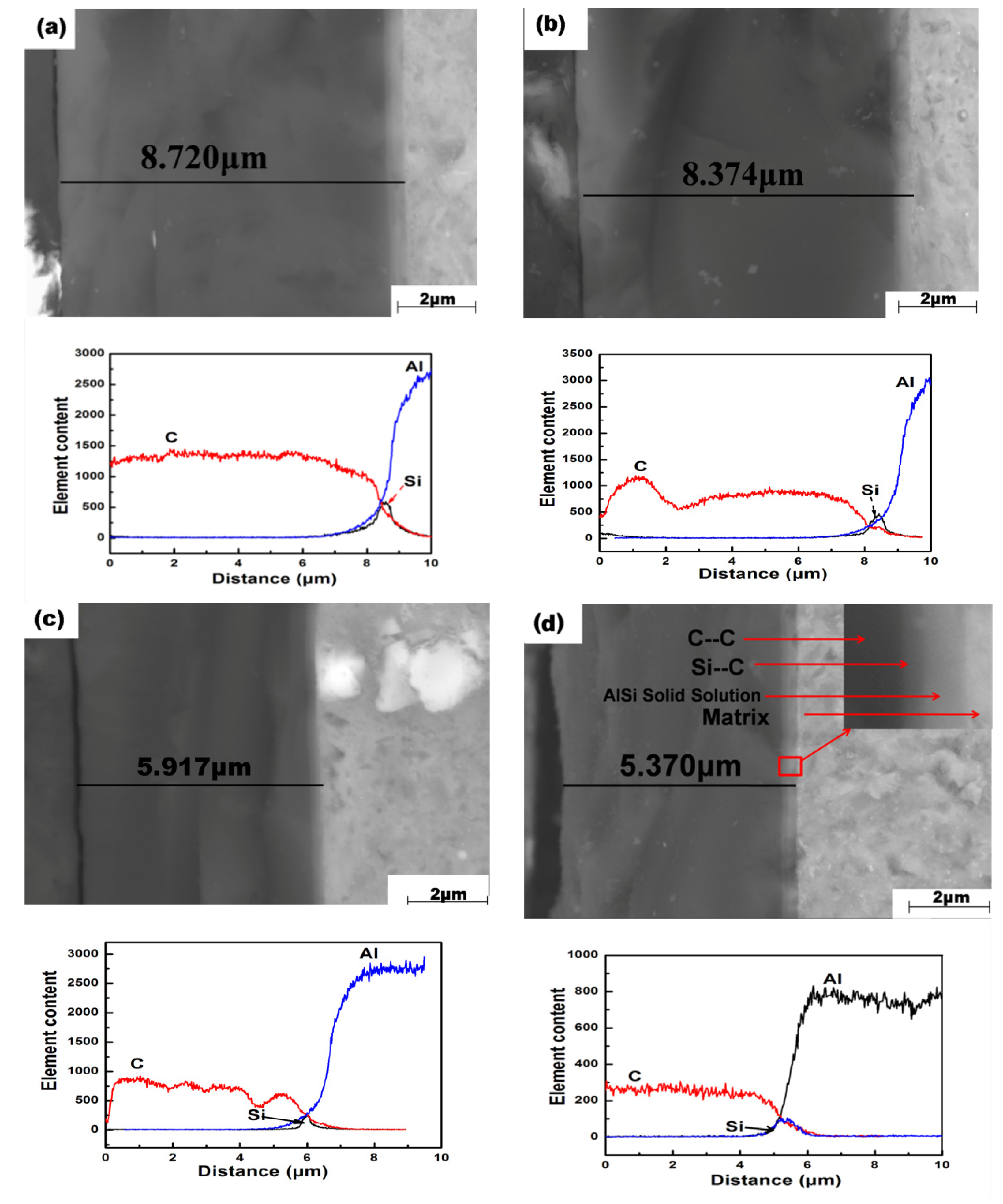
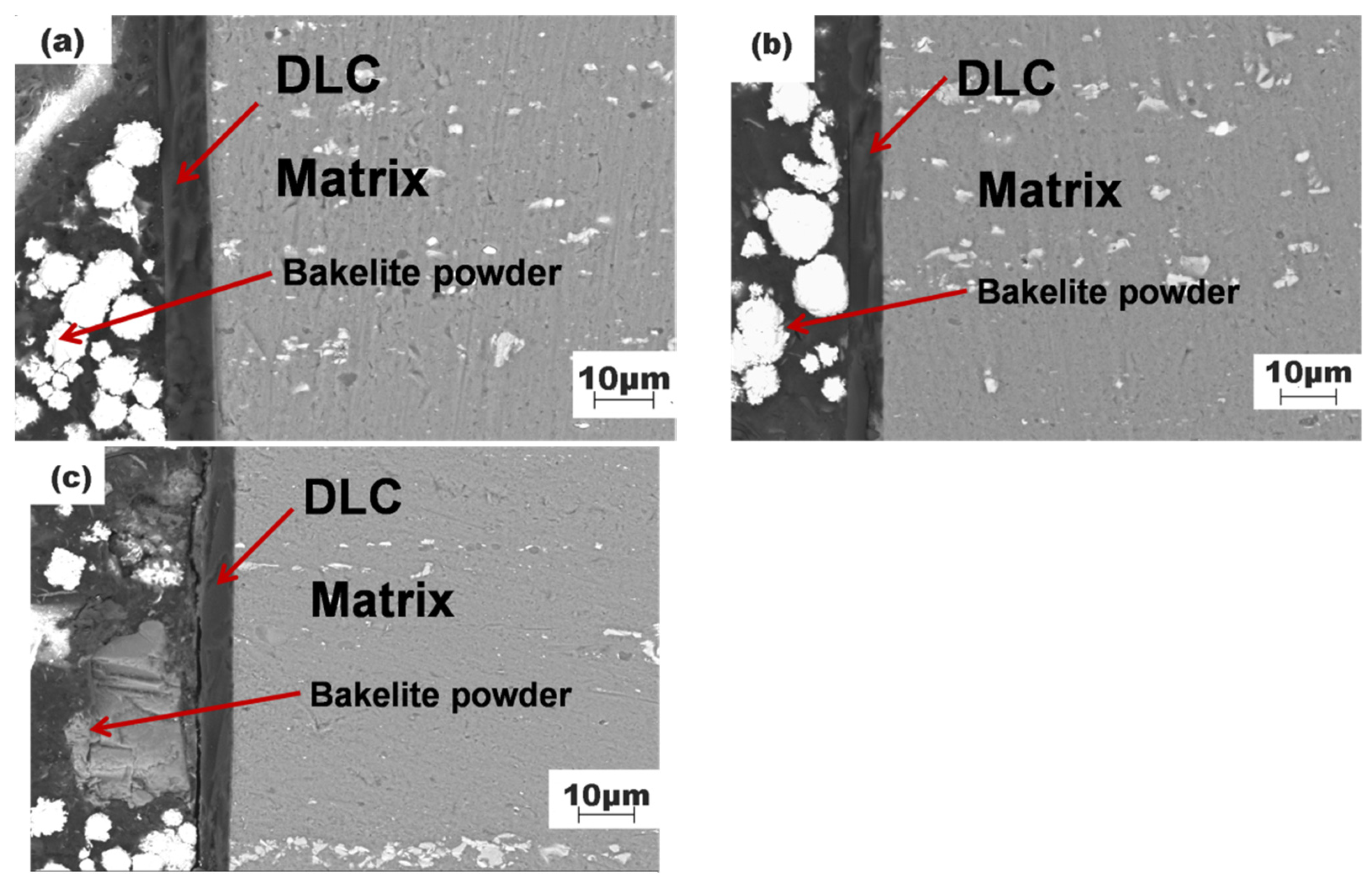
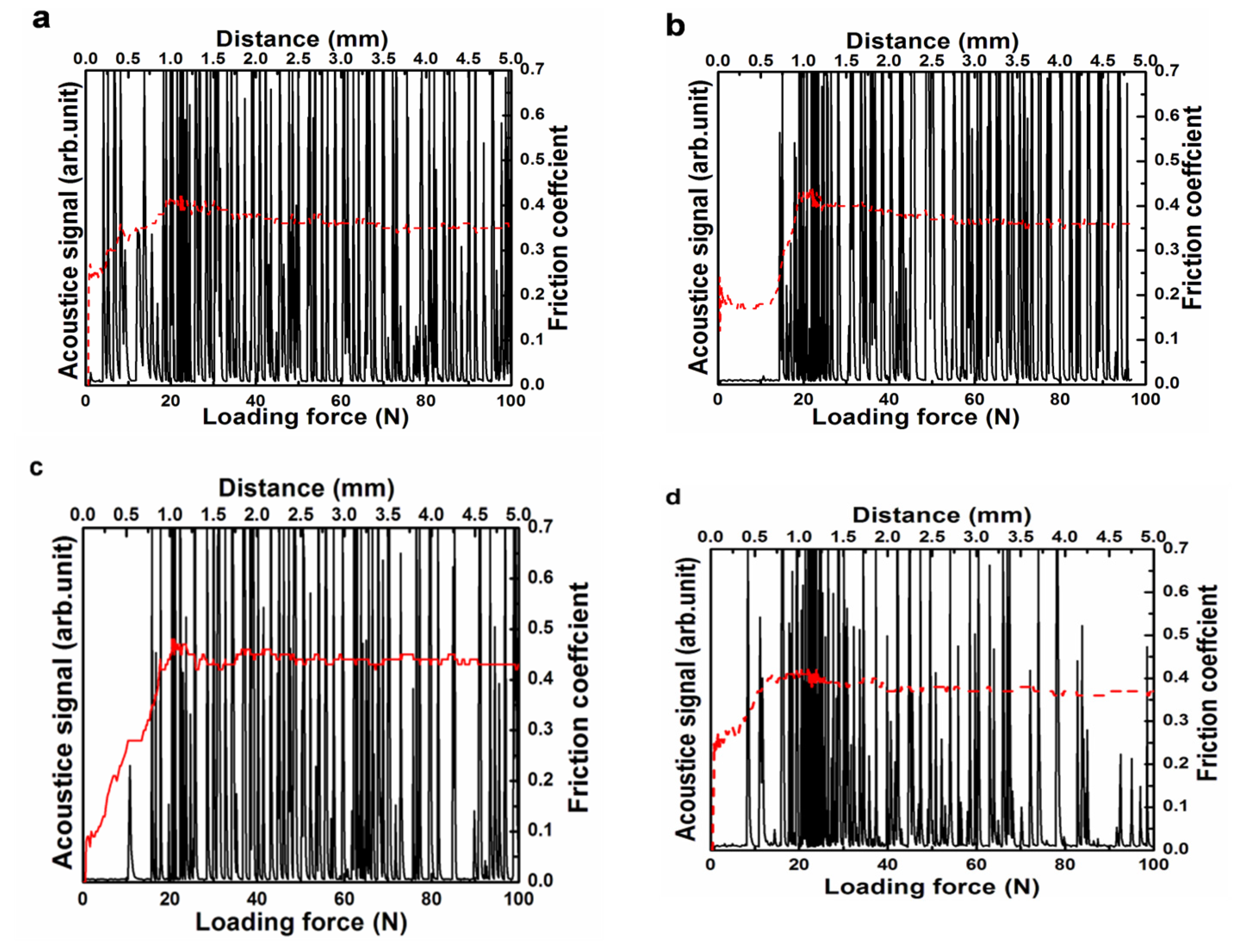
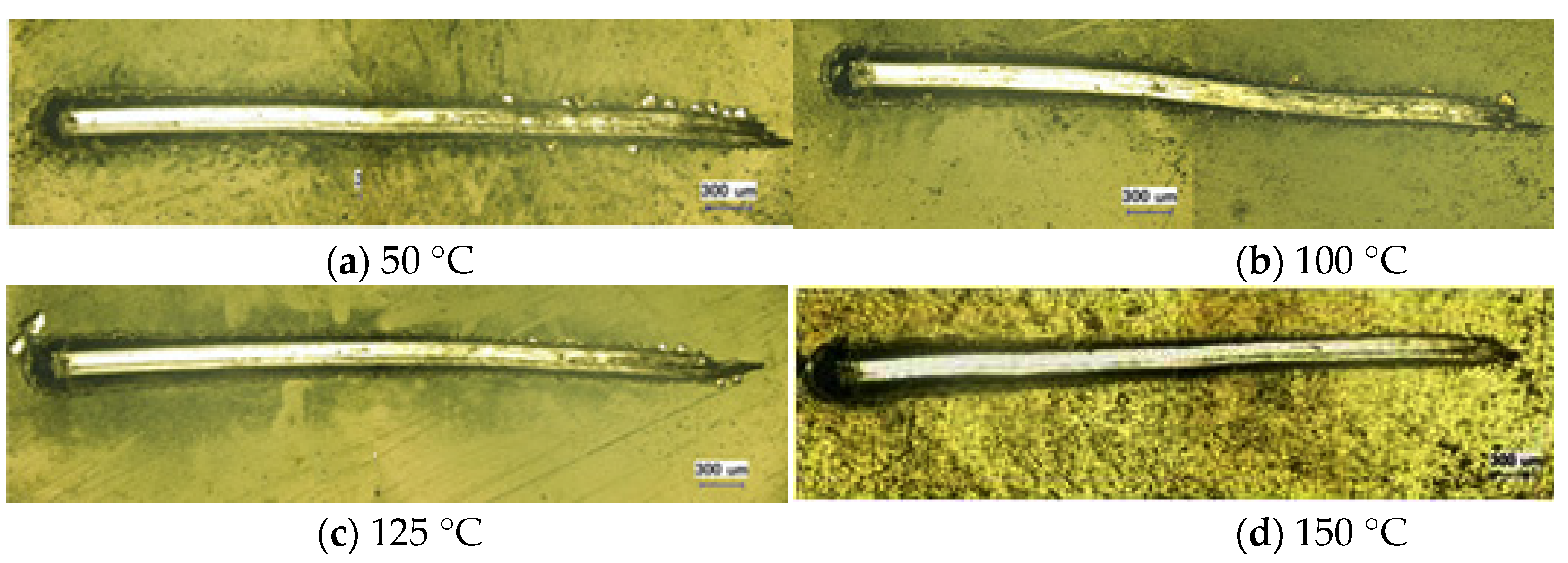
3.1. Sectional Morphology of DLC Films and Substrates at Different Temperatures
3.2. Membrane Substrate Adhesion at Different Temperatures
4. Discussion
4.1. Discussion of the Relationship Between Deposition Temperature and Film Thickness
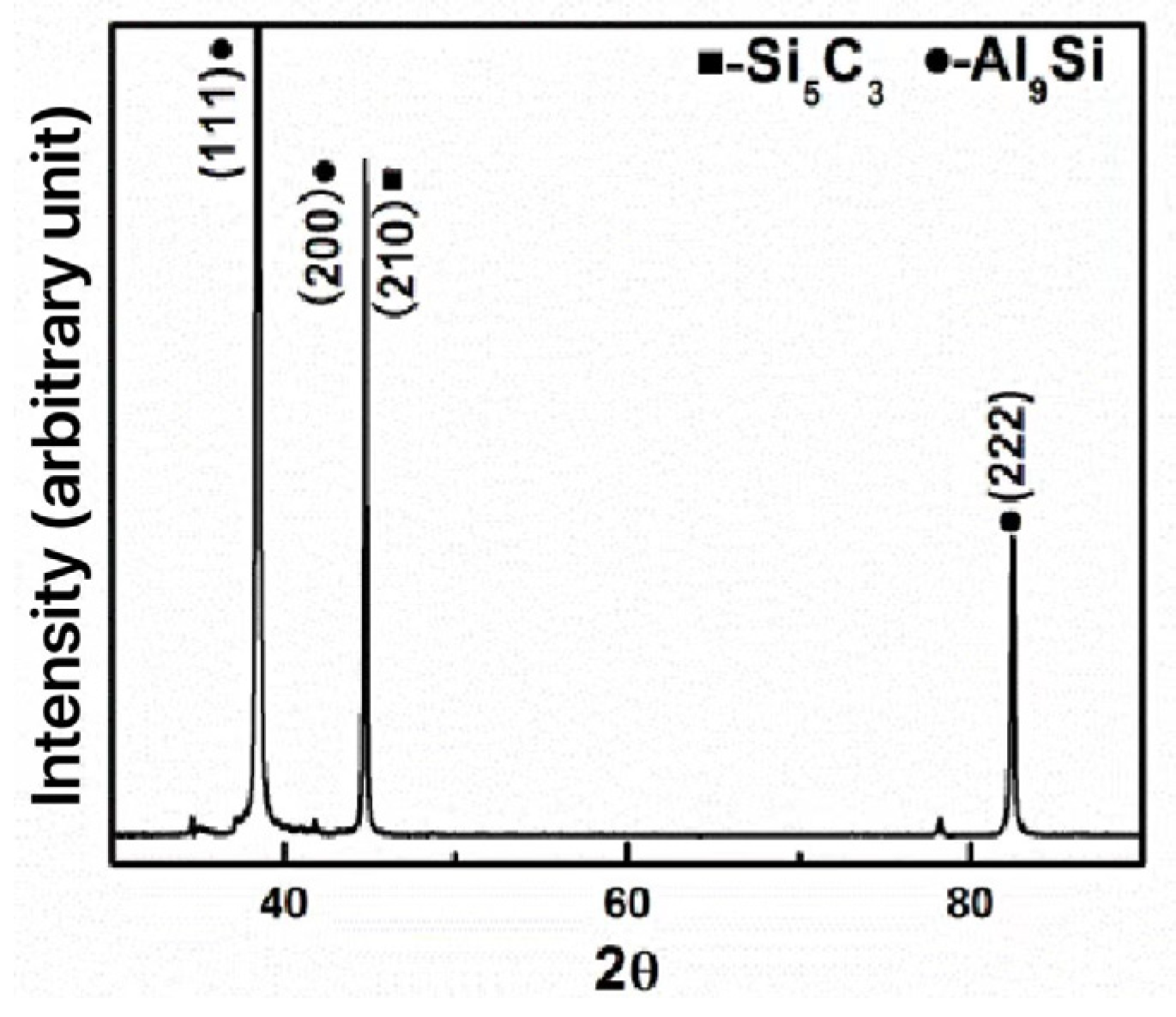
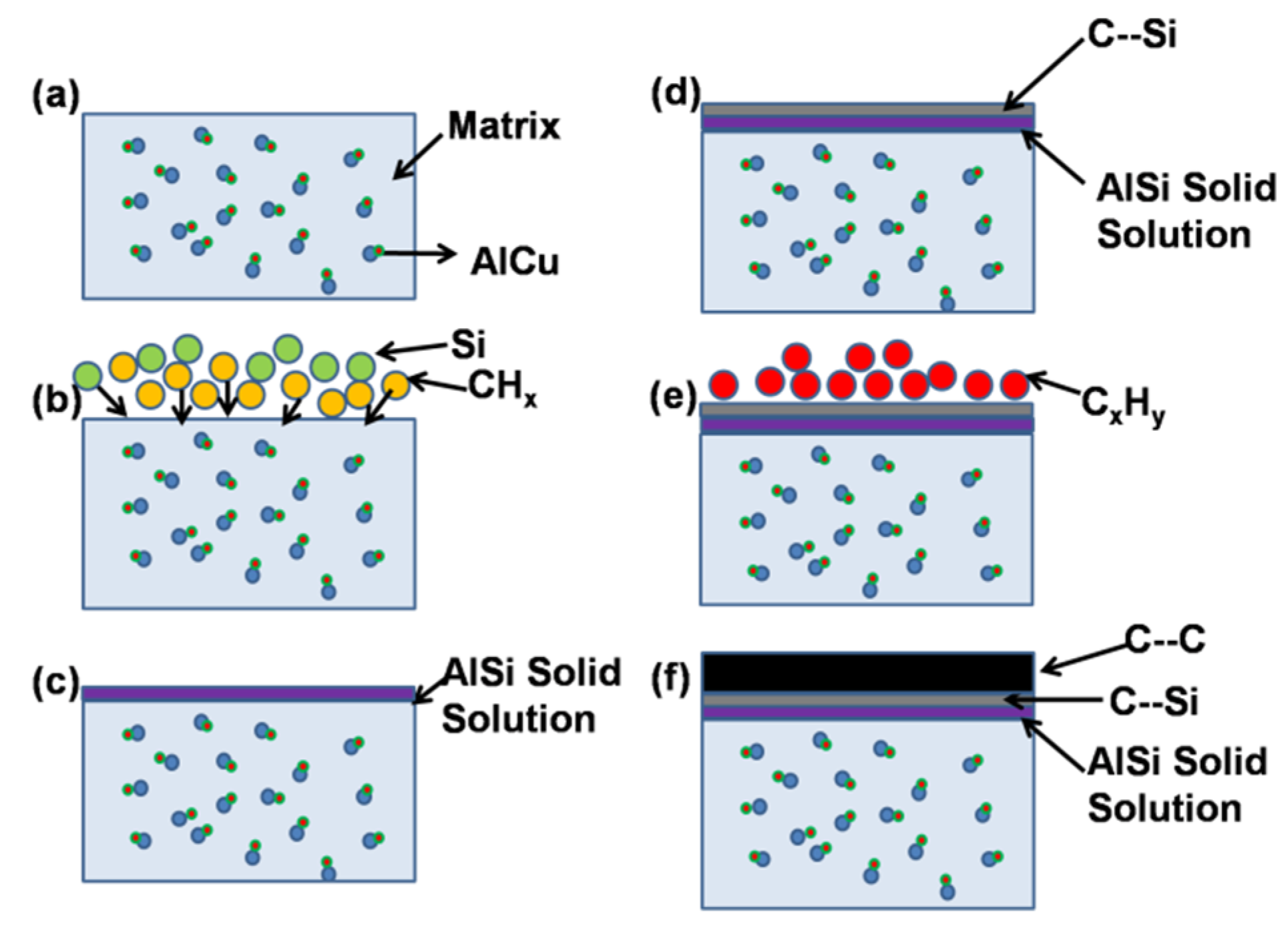
4.2. Discussion on Formation Mechanism of DLC Thin Films
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, M.T.; Wilson, P.; Mahesh, R.; Ramesh, C.; Sagayaraj, P. Chemo-resistive detection of hydrogen in argon using Pd nanoparticles on TiO2 nanotubes prepared via rapid breakdown anodization. Mater. Res. Express 2019, 7, 114221. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Chen, Y.L.; Zhang, Y.; Bian, G.X. The long-term corrosion performance and adhesion properties of 7B04 aluminum alloy/anodic film/epoxy primer system in acidic NaCl solution. Mater. Corros. 2021, 73, 93–105. [Google Scholar] [CrossRef]
- Ding, M.Y.; Yu, M.; Hua, J.W.; Chen, H. Effect of inclusion on performance of A6N01 aluminum alloy joints. Mater. Express 2020, 10, 1395–1403. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Li, Z.X.; Mariusz, B.; Senkara, J. Quantitative analysis of the solute redistribution and precipitate of Al-Mg-Si series alloys resistance spot welds after post-weld heat treatment. J. Mater. Res. Technol. 2021, 15, 5906–5919. [Google Scholar] [CrossRef]
- Qian, S.H.; Zhang, T.M.; Chen, Y.H.; Xie, J.L.; Chen, Y.; Lin, T.S.; Li, H.X. Effect of ultrasonic impact treatment on microstructure and corrosion behavior of friction stir welding joints of 2219 aluminum alloy. J. Mater. Res. Technol. 2022, 18, 1631–1642. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Wu, K.; Yang, G.; Wu, B.; Qin, L.Y.; Wu, H.; Yang, C.Y. Microstructure and mechanical properties of wire arc additively manufactured 205A high strength aluminum alloy: The comparison of as-deposited and T6 heat-treated samples. Mater. Charact. 2022, 189, 111990. [Google Scholar] [CrossRef]
- Ji, L.; Wang, H.; Shi, M. Analysis of corrosion resistance of lightweight aluminum alloy sheet in the friction-stir joint area. Mater. Express 2020, 10, 1352–1357. [Google Scholar] [CrossRef]
- Zhang, P.; Zuo, Y. Effects of pore parameters on performance of anodic film on 2024 aluminum alloy. Mater. Chem. Phys. 2019, 231, 9–20. [Google Scholar] [CrossRef]
- Lin, S.-C.; Wang, C.-C.; Tien, C.-L.; Tung, F.-C.; Wang, H.-F.; Lai, S.-H. Fabrication of Aluminum Oxide Thin-Film Devices Based on Atomic Layer Deposition and Pulsed Discrete Feed Method. Micromachines 2023, 14, 279. [Google Scholar] [CrossRef]
- Hsu, H.-Y.; Yen, J.-S.; Lin, C.-Y.; Liu, C.-W.; Aranganadin, K.; Lin, C.-R.; Sun, J.-S.; Lin, M.-C. Extraordinary Field Emission of Diamond Film Developed on a Graphite Substrate by Microwave Plasma Jet Chemical Vapor Deposition. Appl. Sci. 2023, 13, 2531. [Google Scholar] [CrossRef]
- Milenov, T.; Trifonov, D.; Kalchevski, D.A.; Kolev, S.; Avramova, I.; Russev, S.; Genkov, K.; Avdeev, G.; Dimov, D.; Karaivanova, D.M.; et al. Study of the Chemical Vapor Deposition of Nano-Sized Carbon Phases on {001} Silicon. Materials 2023, 16, 7190. [Google Scholar] [CrossRef] [PubMed]
- Shang, P.; Ma, Y.; Zhang, Z.; Sun, P.; Liu, H.; Shi, H.; Lin, Q.; Xue, T.; Ji, Y. The Effects of Hemisphere Dome Orientation on the Structure of Diamond-like Carbon Film Prepared Using Ion Beam Assisted Deposition. Materials 2023, 16, 1773. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.P.; Lee, K.-I.; You, H.J.; Jang, S.O.; Choi, Y.S. Scanning Deposition Method for Large-Area Diamond Film Synthesis Using Multiple Microwave Plasma Sources. Nanomaterials 2022, 12, 1959. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. Rev. 2002, 37, 129–181. [Google Scholar] [CrossRef]
- Roy, M.E.; Whiteside, L.A.; Xu, J.; Katerberg, B.J. Diamond-like carbon coatings enhance the hardness and resilience of bearing surfaces for use in joint arthroplasty. Acta Biomater. 2010, 6, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, Q.; Kong, X. Optical and electrical properties evolution of diamond-like carbon thin films with deposition temperature. Chin. Phys. Lett. 2009, 26, 027802. [Google Scholar]
- Roman, E.; Kalin, M.; Vizintin, J. The effect of temperature on the tribological mechanisms and reactivity of hydrogenated amorphous diamond-like carbon coatings under oil-lubricated conditions. Thin Solid Film. 2007, 515, 3644–3652. [Google Scholar]
- Huang, L.; Yuan, J.T.; Wang, Z.H.; Yu, B.B. Effect of the temperature on diamond-like carbon (DLC) thin film based on LIS. Appl. Mech. Mater. 2013, 421, 212–216. [Google Scholar] [CrossRef]
- Bhargava, S.; Bist, H.D.; Narlikar, A.V.; Samanta, S.B.; Narayan, J.; Tripathi, H.B. Effect of substrate temperature and heat treatment on the microstructure of diamondlike carbon films. J. Appl. Phys. 1996, 79, 1917–1925. [Google Scholar] [CrossRef]
- Mei, X.; Ma, T. Effects of substrate temperature on the chemical structure of diamond-like carbon films deposited by high-intensity pulsed ion beam ablation. Chem. J. Chin. Univ. 2003, 24, 1262–1265. [Google Scholar]
- Ji, X.; Hao, J.; Xu, Z. Effect of deposition temperature on surface morphology and perfor mance of diamond-like-carbon coatings. Mater. Mech. Eng. 2014, 38, 40–45. [Google Scholar]
- Mößner, C.; Grant, P.; Tran, H.; Clarke, G.; Lockwood, D.J.; Labbe, H.J.; Mason, B.; Sproule, I. Characterization of diamond-like carbon by raman spectroscopy, xps and optical constants. Thin Solid Film. 1998, 317, 397–401. [Google Scholar] [CrossRef]
- Dirk, D.; Taylor, C.E.; Ladner, E.P. Novel Techniques for the Conversion of Methane Hydrates. Stud. Surf. Sci. Catal. 2001, 136, 543–548. [Google Scholar]
- Dongari, N.; Zhang, Y.; Reese, J.M. Molecular free path distribution in rarefied gases. J. Phys. D Appl. Phys. 2011, 44, 125502. [Google Scholar] [CrossRef]
- Lutisan, J.; Cvengro, J. Mean free path of molecules on molecular distillation. Chem. Eng. J. Biochem. Eng. J. 1995, 56, 39–50. [Google Scholar] [CrossRef]
- Alexopoulos, N.D. On the corrosion-induced mechanical degradation for different artificial aging conditions of 2024 aluminum alloy. Mater. Sci. Eng. A 2009, 520, 40–48. [Google Scholar] [CrossRef]
- Zanatta, A.; Chambouleyron, I. Local electronegativity and chemical shift in Si and Ge based molecules and alloys. Solid State Commun. 1995, 95, 207–210. [Google Scholar] [CrossRef]
- Ohshita, J.; Tanaka, D.; Matsukawa, J.; Mizumo, T.; Yoshida, H.; Ooyama, Y.; Harima, Y. Hybridization of Carbon Nanotubes with Si–π Polymers and Attachment of Resulting Hybrids to TiO2 Surface. Chem. Lett. 2011, 40, 87–89. [Google Scholar] [CrossRef]
- De Nicola, F.; Hines, P.; De Crescenzi, M.; Motta, N. Moth-eye effect in hierarchical carbon nanotube anti-reflective coatings. Carbon 2016, 108, 262. [Google Scholar] [CrossRef]
| Cu | Mg | Mn | Fe | Zn | Si | Ti | Cr | Al |
|---|---|---|---|---|---|---|---|---|
| 3.81 | 1.4 | 0.4 | 0.4 | 0.14 | 0.22 | 0.05 | 0.05 | Balance |
| No. | Deposition Time/h | Pulse Bias/V | Experimental Temperature/°C | Ar:C2H2/(sccm) |
|---|---|---|---|---|
| 1# | 5 | −3300 | 50 | 20:60 |
| 2# | 5 | −3300 | 100 | 20:60 |
| 3# | 5 | −3300 | 125 | 20:60 |
| 4# | 5 | −3300 | 150 | 20:60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Li, T.; Shang, B.; Guo, L.; Zhang, T.; Han, W. The Influence of Deposition Temperature on the Microscopic Process of Diamond-like Carbon (DLC) Film Deposition on a 2024 Aluminum Alloy Surface. Crystals 2024, 14, 950. https://doi.org/10.3390/cryst14110950
Yang L, Li T, Shang B, Guo L, Zhang T, Han W. The Influence of Deposition Temperature on the Microscopic Process of Diamond-like Carbon (DLC) Film Deposition on a 2024 Aluminum Alloy Surface. Crystals. 2024; 14(11):950. https://doi.org/10.3390/cryst14110950
Chicago/Turabian StyleYang, Li, Tong Li, Baihui Shang, Lili Guo, Tong Zhang, and Weina Han. 2024. "The Influence of Deposition Temperature on the Microscopic Process of Diamond-like Carbon (DLC) Film Deposition on a 2024 Aluminum Alloy Surface" Crystals 14, no. 11: 950. https://doi.org/10.3390/cryst14110950
APA StyleYang, L., Li, T., Shang, B., Guo, L., Zhang, T., & Han, W. (2024). The Influence of Deposition Temperature on the Microscopic Process of Diamond-like Carbon (DLC) Film Deposition on a 2024 Aluminum Alloy Surface. Crystals, 14(11), 950. https://doi.org/10.3390/cryst14110950




