Revealing the Interaction Between Dislocations and LPSO-Precipitates Structure in a Mg-Y-Al Alloy at Different Temperatures
Abstract
:1. Introduction
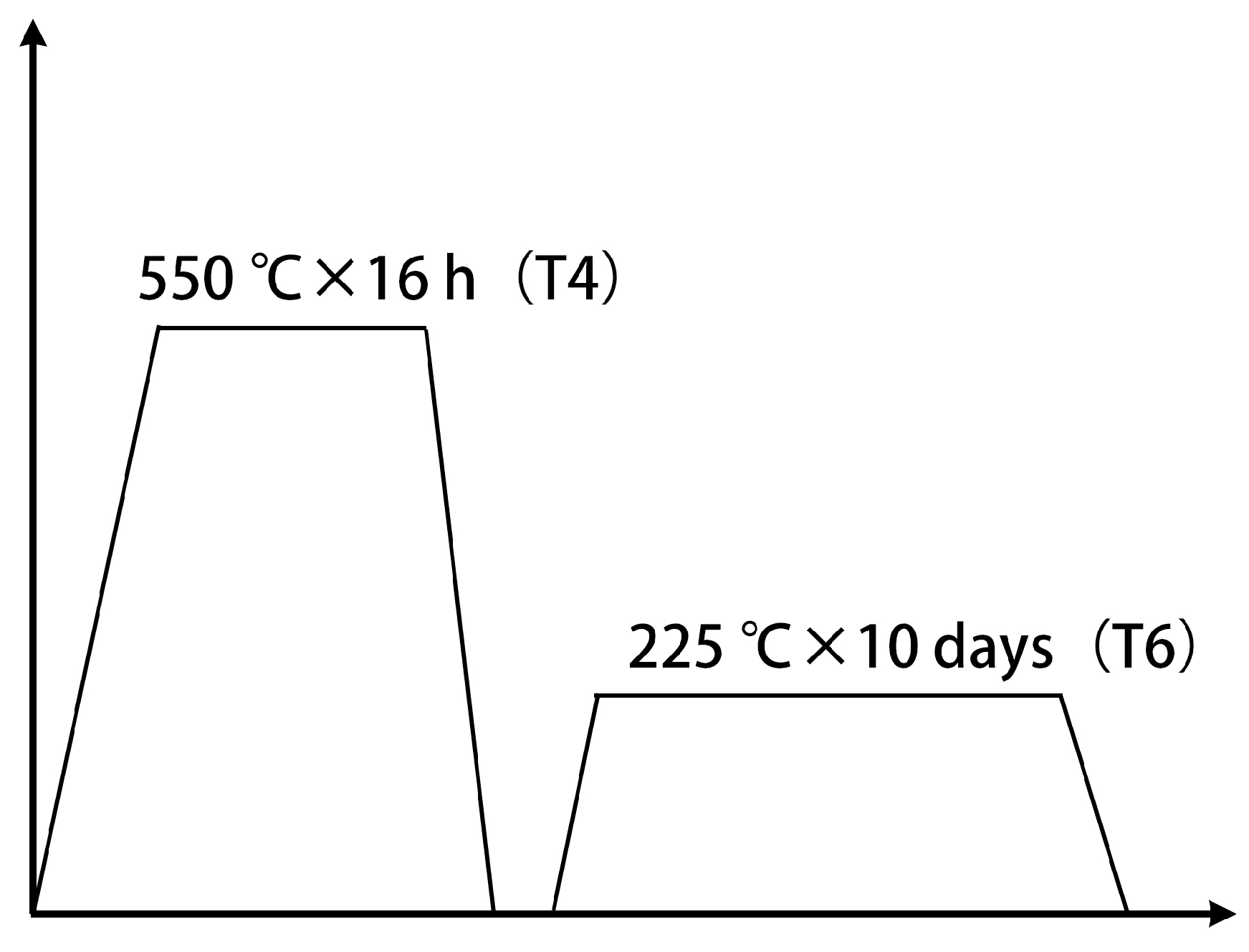
2. Materials and Methods
3. Results and Discussion
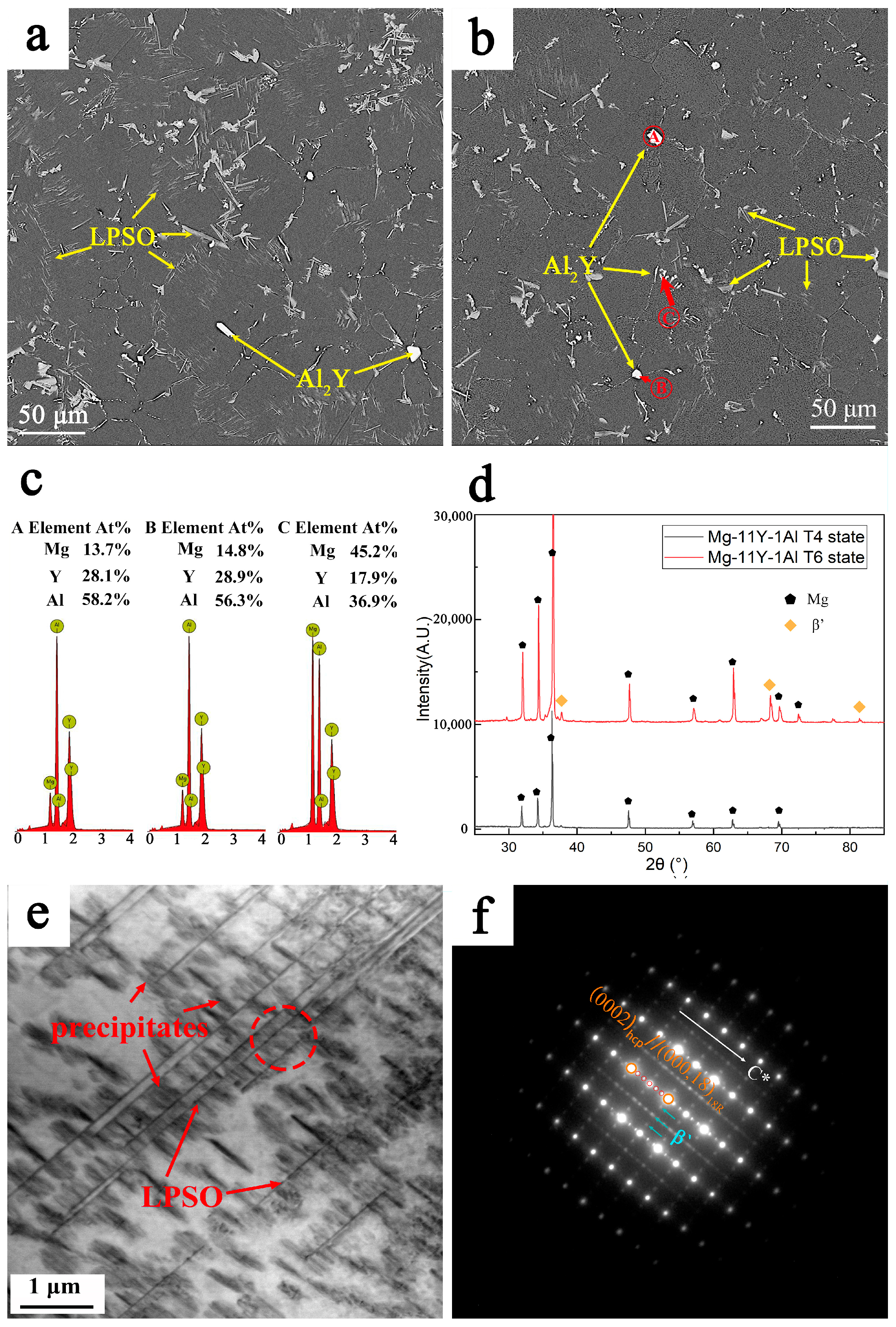
3.1. Microstructures of Mg-11Y-Al Alloys in T4 and T6 States
3.2. Mechanical Properties of Mg-11Y-Al Alloys in T4 and T6 States
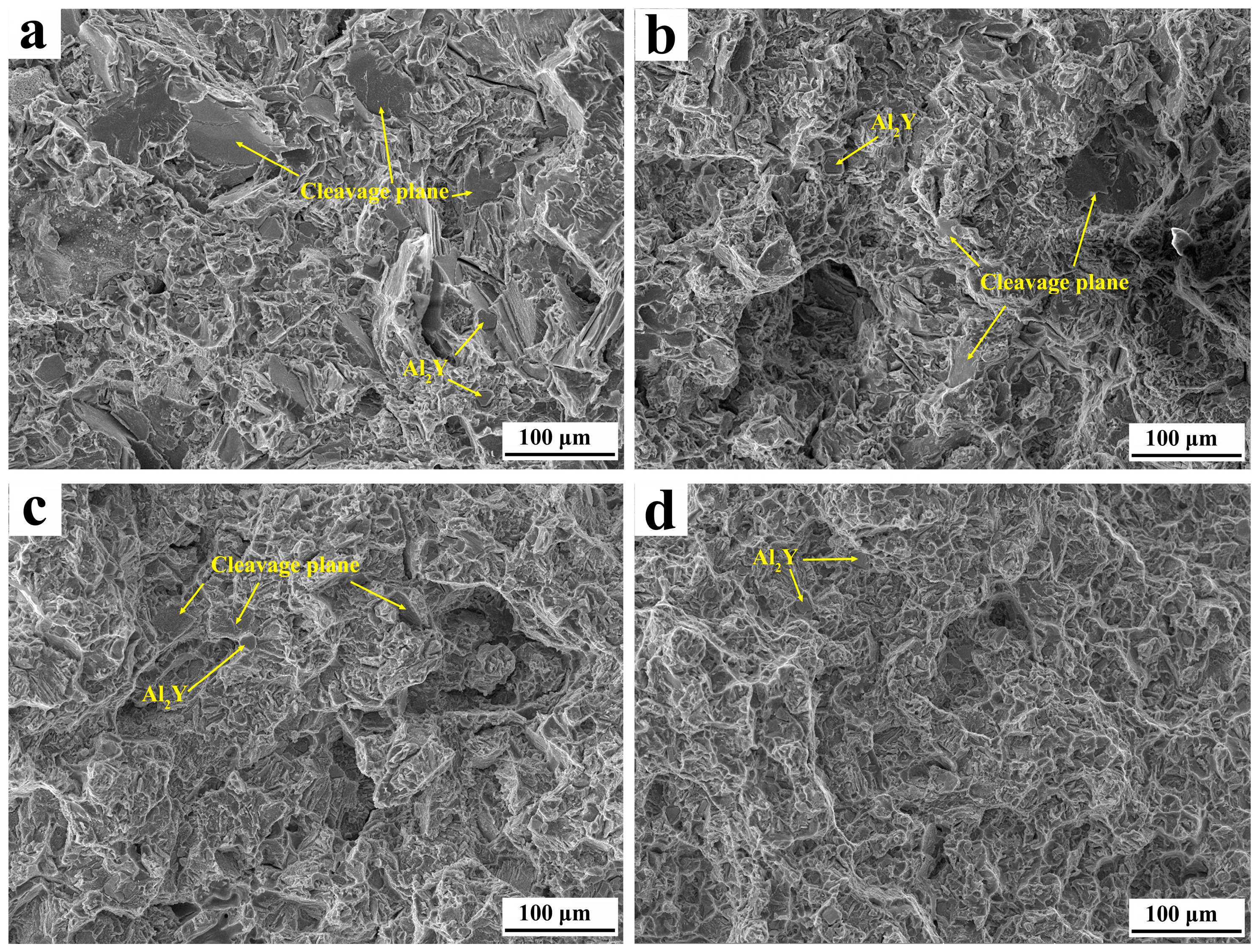
3.3. Fracture Morphology of the T4 and T6 State Mg-11Y-Al Alloy
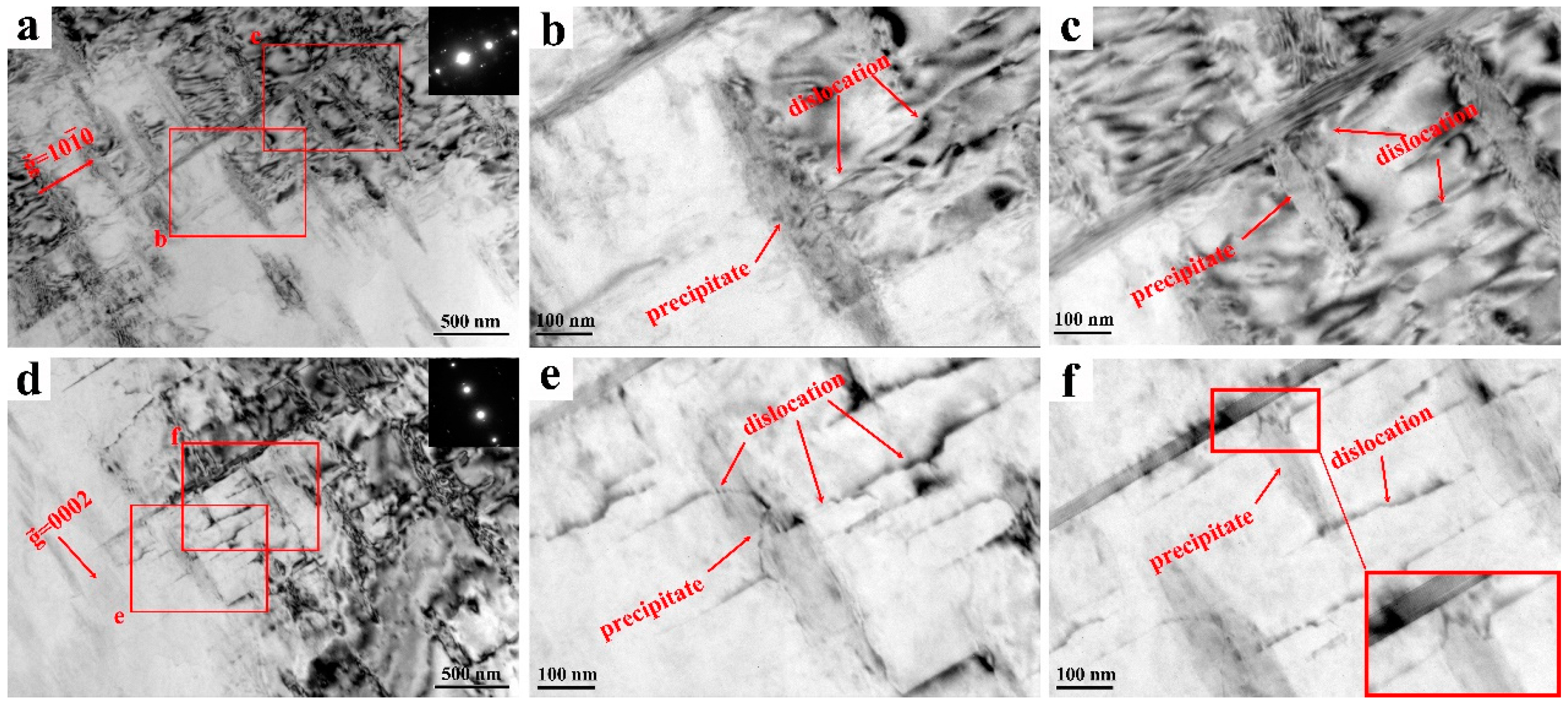
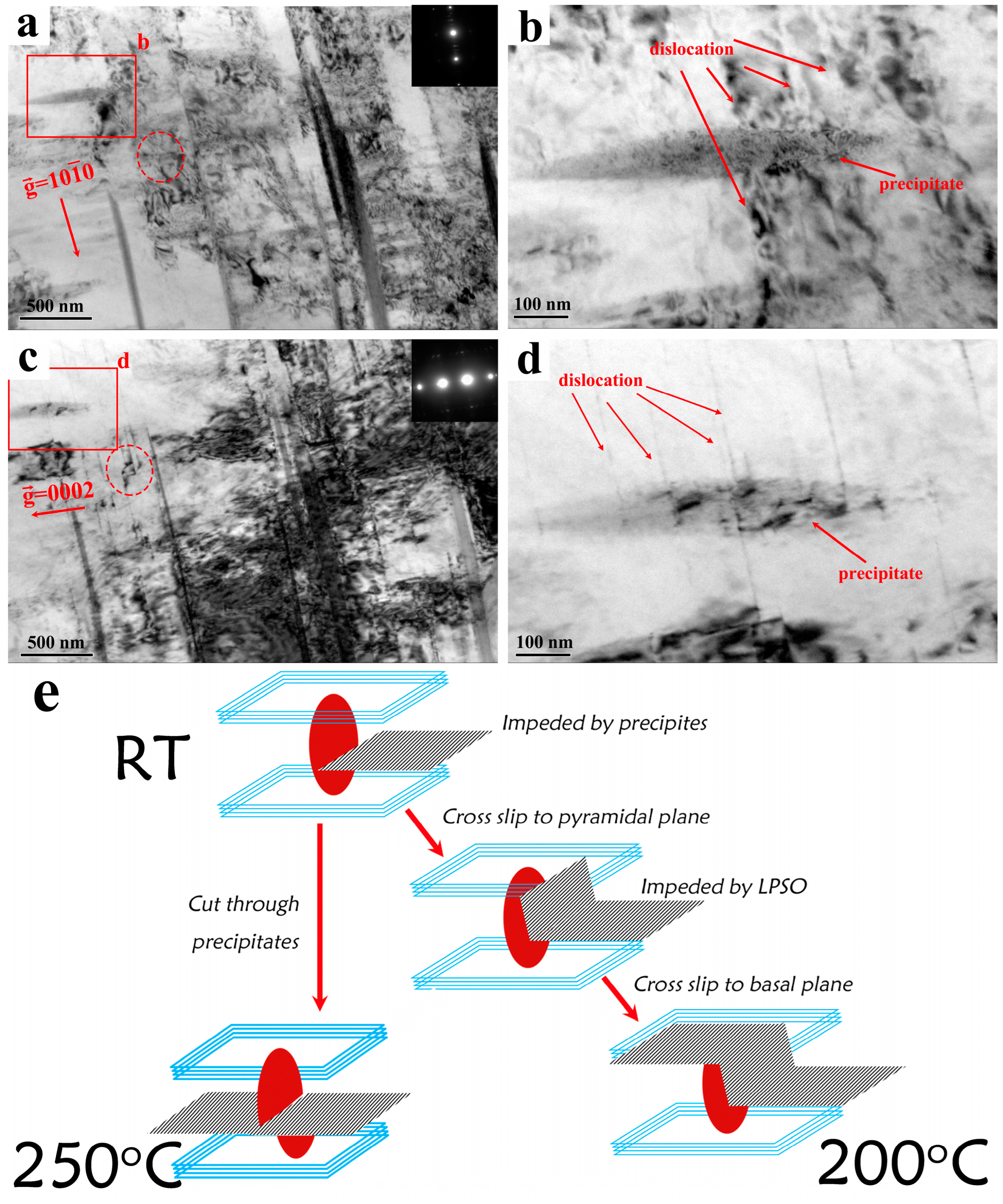
3.4. TEM Observation of the Mg-11Y-Al Alloy in T4 and T6 States and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pollock, T.M. Weight Loss with Magnesium Alloys. Science 2010, 328, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Nie, J.F.; Xu, S.W.; Davies, C.H.J.; Birbilis, N. Super-formable pure magnesium at room temperature. Nat. Commun. 2017, 8, 972. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, J.; You, Z.; Liu, S.; Guan, K.; Wu, R.; Wang, J.; Feng, J. Towards developing Mg alloys with simultaneously improved strength and corrosion resistance via RE alloying. J. Magnes. Alloy. 2020, 9, 41–56. [Google Scholar] [CrossRef]
- Nie, J.-F. Precipitation and Hardening in Magnesium Alloys. Metall. Mater. Trans. 2012, 43, 3891–3939. [Google Scholar] [CrossRef]
- He, S.M.; Zeng, X.Q.; Peng, L.M.; Gao, X.; Nie, J.F.; Ding, W.J. Microstructure and strengthening mechanism of high strength Mg–10Gd–2Y–0.5Zr alloy. J. Alloys Compd. 2007, 427, 316–323. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Y.; Cao, F.; Qiu, D.; Yang, Y.; Wang, J.; Zhang, H.; Ying, T.; Ding, W.; Zeng, X. Towards development of a high-strength stainless Mg alloy with Al-assisted growth of passive film. Nat. Commun. 2022, 13, 5838. [Google Scholar] [CrossRef]
- Gao, X.; He, S.M.; Zeng, X.Q.; Peng, L.M.; Ding, W.J.; Nie, J.F. Microstructure evolution in a Mg–15Gd–0.5Zr (wt.%) alloy during isothermal aging at 250 °C. Mater. Sci. Eng. A 2006, 431, 322–327. [Google Scholar] [CrossRef]
- Xie, D.; Pan, H.; Pan, Z.; Fu, T.; Zeng, Z.; Xie, H.; Ren, Y.; Tang, W.; Qin, G. Achieving outstanding heat-resistant properties in Mg alloy via constructing stable solute-network. Mater. Res. Lett. 2023, 11, 374–382. [Google Scholar] [CrossRef]
- Hagihara, K.; Okamoto, T.; Izuno, H.; Yamasaki, M.; Matsushita, M.; Nakano, T.; Kawamura, Y. Plastic deformation behavior of 10H-type synchronized LPSO phase in a Mg–Zn–Y system. Acta Mater. 2016, 109, 90–102. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Morton, A.J.; Nie, J.F. The 18R and 14H long-period stacking ordered structures in Mg–Y–Zn alloys. Acta Mater. 2010, 58, 2936–2947. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.; Luo, Q.; Liu, B.; Gu, Q.; Li, Q. A 12R long-period stacking-ordered structure in a Mg-Ni-Y alloy. J. Mater. Sci. Technol. 2018, 34, 2235–2239. [Google Scholar] [CrossRef]
- Issa, A.; Saal, J.E.; Wolverton, C. Formation of high-strength β′ precipitates in Mg–RE alloys: The role of the Mg/β″ interfacial instability. Acta Mater. 2015, 83, 75–83. [Google Scholar] [CrossRef]
- Nishijima, M.; Yubuta, K.; Hiraga, K. Characterization of β′ Precipitate Phase in Mg2 at%Y Alloy Aged to Peak Hardness Condition by High-Angle Annular Detector Dark-Field Scanning Transmission Electron Microscopy (HAADF-STEM). Mater. Trans. 2006, 48, 84–87. [Google Scholar] [CrossRef]
- Liu, C.Q.; Chen, H.W.; Liu, H.; Zhao, X.J.; Nie, J.F. Metastable precipitate phases in Mg–9.8 wt%Sn alloy. Acta Mater. 2018, 144, 590–600. [Google Scholar] [CrossRef]
- Shi, Z.; Song, G.; Atrens, A. Influence of the β phase on the corrosion performance of anodised coatings on magnesium–aluminium alloys. Corros. Sci. 2005, 47, 2760–2777. [Google Scholar] [CrossRef]
- Wu, Z.; Ahmad, R.; Yin, B.; Sandlöbes, S.; Curtin, W.A. Mechanistic origin and prediction of enhanced ductility in magnesium alloys. Science 2018, 359, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Zheng, W.; Li, Q.; Yu, M.; Ying, T.; Zeng, X. CALPHAD-guided design of Mg-Y-Al alloy with improved strength and ductility via regulating the LPSO phase. Acta Mater. 2024, 263, 119521. [Google Scholar] [CrossRef]
- Zhu, Q.; Shang, X.; Zhang, H.; Qi, X.; Li, Y.; Zeng, X. Influence of Al2Y particles on mechanical properties of Mg-11Y-1Al alloy with different grain sizes. Mater. Sci. Eng. A 2022, 831, 142166. [Google Scholar] [CrossRef]
- Qiu, D.; Zhang, M.X.; Taylor, J.A.; Kelly, P.M. A new approach to designing a grain refiner for Mg casting alloys and its use in Mg–Y-based alloys. Acta Mater. 2009, 57, 3052–3059. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, Y.; Ding, Z.; Wang, J.; Liu, Y.; Zhang, H.; Xie, T.; Wang, M.; Zhu, H.; Ying, T.; et al. Unveiling precipitation behavior in Mg-Y based alloys. Mater. Des. 2021, 202, 109570. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.Q.; Zhu, Y.M.; Chen, H.W.; Bourgeois, L.; Nie, J.F. Revisiting building block ordering of long-period stacking ordered structures in Mg–Y–Al alloys. Acta Mater. 2018, 152, 96–106. [Google Scholar] [CrossRef]
- Li, Y.X.; Qiu, D.; Rong, Y.H.; Zhang, M.X. Effect of long-period stacking ordered phase on thermal stability of refined grains in Mg-RE-based alloys. Philos. Mag. 2014, 94, 1311–1326. [Google Scholar] [CrossRef]
- Muzamil, M.; Wu, J.; Samiuddin, M.; Majeed, A.; Zhang, Z. The response of heat-treatable filler on non-heat-treatable aluminum alloy substrate against age hardening cycle for intelligent development of surface welded joints using TIG welding process. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 229. [Google Scholar] [CrossRef]
- Muzamil, M.; Akhtar, M.; Samiuddin, M.; Mehdi, M. Effect of heat treatment on impact resistance of AU5GT and AS7G06 aluminum alloys. J. Mech. Sci. Technol. 2016, 30, 4543–4548. [Google Scholar] [CrossRef]
- Wu, J.; Lu, S.; Tian, J.; Chiu, Y. In-situ TEM study of <c+a> dislocations in Mg–Y alloys. Mater. Sci. Eng. A 2024, 897, 146320. [Google Scholar]
- Li, N.; Yang, L.; Wang, C.; Monclús, M.A.; Shi, D.; Molina-Aldareguía, J.M. Deformation mechanisms of basal slip, twinning and non-basal slips in Mg–Y alloy by micropillar compression. Mater. Sci. Eng. A 2021, 819, 141408. [Google Scholar] [CrossRef]
- Janik, V.; Yin, D.D.; Wang, Q.D.; He, S.M.; Chen, C.J.; Chen, Z.; Boehlert, C.J. The elevated-temperature mechanical behavior of peak-aged Mg–10Gd–3Y–0.4Zr Alloy. Mater. Sci. Eng. A 2011, 528, 3105–3112. [Google Scholar] [CrossRef]
- Chen, R.; Sandlöbes, S.; Zeng, X.; Li, D.; Korte-Kerzel, S.; Raabe, D. Room temperature deformation of LPSO structures by non-basal slip. Mater. Sci. Eng. A 2017, 682, 354–358. [Google Scholar] [CrossRef]
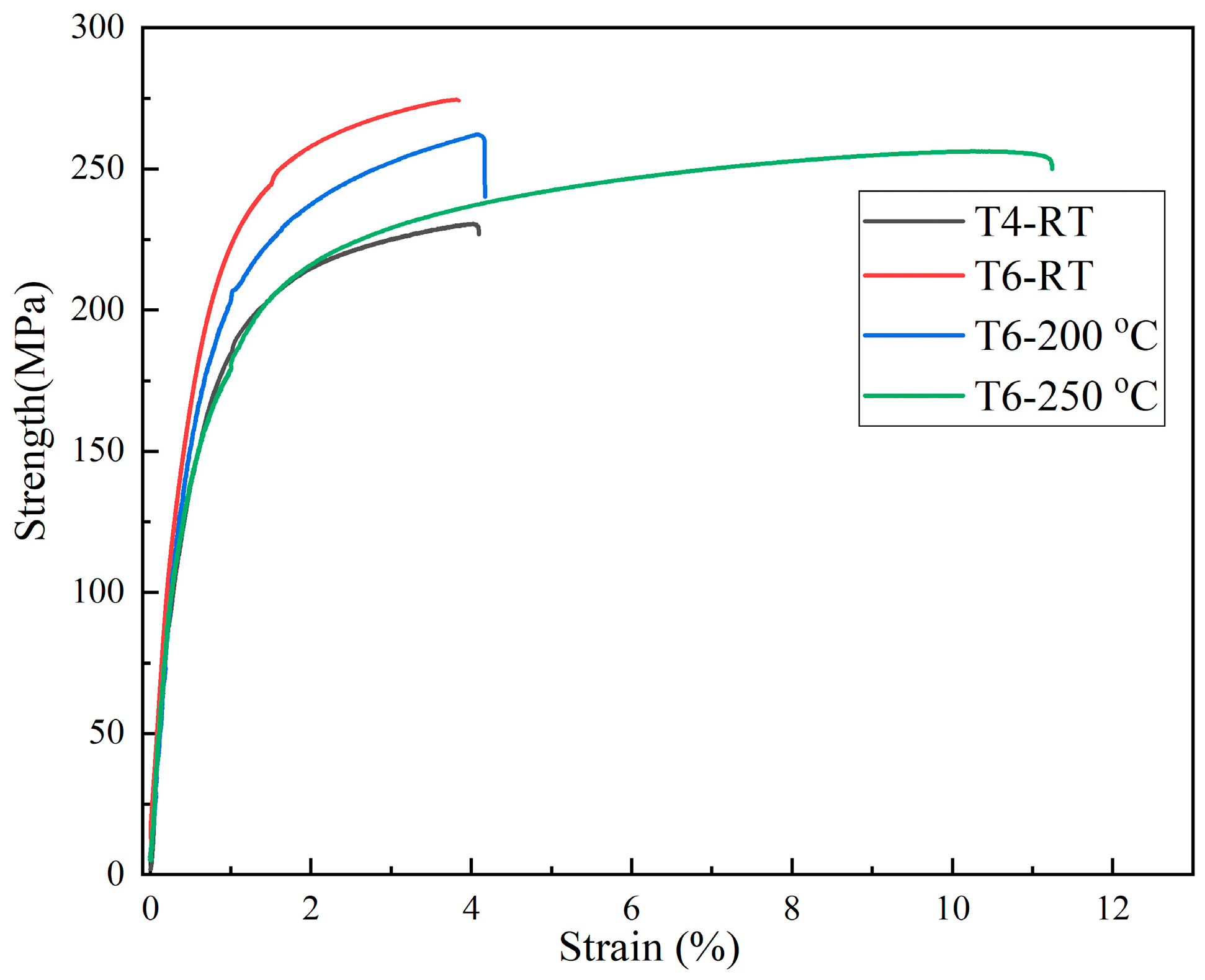

| Yield Strength/MPa | Tensile Strength/MPa | Elongation/% | |
|---|---|---|---|
| T4-RT | 151 | 225 | 4.1 |
| T6-RT | 198 | 275 | 3.9 |
| T6-200 °C | 172 | 262 | 4.1 |
| T6-250 °C | 152 | 256 | 11.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Li, Y.; Zhang, H.; Wang, J.; Jiang, H.; Zhao, J. Revealing the Interaction Between Dislocations and LPSO-Precipitates Structure in a Mg-Y-Al Alloy at Different Temperatures. Crystals 2024, 14, 1018. https://doi.org/10.3390/cryst14121018
Zhu Q, Li Y, Zhang H, Wang J, Jiang H, Zhao J. Revealing the Interaction Between Dislocations and LPSO-Precipitates Structure in a Mg-Y-Al Alloy at Different Temperatures. Crystals. 2024; 14(12):1018. https://doi.org/10.3390/cryst14121018
Chicago/Turabian StyleZhu, Qingchun, Yangxin Li, Huan Zhang, Jie Wang, Hongxiang Jiang, and Jiuzhou Zhao. 2024. "Revealing the Interaction Between Dislocations and LPSO-Precipitates Structure in a Mg-Y-Al Alloy at Different Temperatures" Crystals 14, no. 12: 1018. https://doi.org/10.3390/cryst14121018
APA StyleZhu, Q., Li, Y., Zhang, H., Wang, J., Jiang, H., & Zhao, J. (2024). Revealing the Interaction Between Dislocations and LPSO-Precipitates Structure in a Mg-Y-Al Alloy at Different Temperatures. Crystals, 14(12), 1018. https://doi.org/10.3390/cryst14121018





