Abstract
α- or 4-Substituted curcumin analogues are scarce. We describe herein the syntheses and crystal structures of the first α-halogenated curcumin derivatives: (1E,6E)-1,7-bis (4-hydroxy-3-methoxyphenyl)-4-bromo-5-hydroxy-1,3,6-heptatriene-3-one or (4-bromocurcumin) (1) and (1E,6E)-1,7-bis (4-hydroxy-3-methoxyphenyl)-4-chloro-5-hydroxy-1,3,6-heptatriene-3-one or (4-chlorocurcumin) (2). We note that the key step in the successful synthesis of the bromo-analog is the use of slightly acidic media to favor the diketo form of curcumin prior to carrying out the reaction. Both newly prepared compounds assume the keto–enol form in the solid state and crystallize in the monoclinic space group P21/c with four molecules in the unit cell each with slightly different dimensions. Inter- and intra- molecular hydrogen bonds were observed in the two structures. Most significant observed features were the inter-molecular O…O distances of 2.842 and 2.840 Å and intra-molecular O…O distances of 2.460 and 2.451 Å for bromo-or (1) and chloro- or (2) derivatives, respectively. No close halogen…halogen contacts were observed in either of the two structures. Both molecules are nearly planar with torsion angles of 0.54 and 1.16 °C between the planes of two terminal phenyl groups for (1) and (2), respectively. π-Stacks were observed in both structures with interplanar distances of 3.367 and 3.454 Å for the bromo- and chloro- compounds, respectively. Hirshfeld surface analysis confirms quantitively a picture of the inter- and intra-molecular interactions in both compounds compared with polymorph I (the most common form) of curcumin. UV–Vis absorption spectra are shifted to higher wavelengths with lmax of 475 and 477 nm for compounds 1 and 2, respectively, compared with 442 nm in dichloromethane solutions. The newly synthesized molecules will open the door for numerous possible synthetic modifications of the α-carbon to prepare valuable analogues of curcumin with more favorable solubility profiles.
1. Introduction
Curcumin, or (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptane-3,5-dione), is a yellow-orange polyphenolic compound found in turmeric. Curcumin has been the subject of numerous studies since the 1990s and has been shown to exhibit antioxidant [1,2] anti-inflammatory [3,4] and anticancer [5,6] activities.
Curcumin is characterized by the presence of an α, β-unsaturated β-diketone moiety. It exists predominantly in its diketo form in neutral and acidic solutions while the more stable keto–enol form exists predominantly under alkaline conditions. While both the phenols and the α, β-unsaturated diketo groups are responsible for the antioxidant properties of curcumin, the α, β-unsaturated diketo moiety is thought to be responsible for the anticancer activities of the molecule. Furthermore, the keto–enol tautomerism plays a critical role in biological activity, influencing molecular recognition, stability, and reactivity in enzyme catalysis and drug interactions [7]. Thus, simple structural modifications of curcumin at the α-carbon offer an attractive path to alter the steric, coordination sphere, and other properties of the molecule that may lead to enhanced solubility and biological activity [8]. In this work, we report the syntheses and crystal structures of two novel analogues of curcumin, where the hydrogen at the α-carbon or 4-position is replaced by either a bromine or a chlorine atom (Figure 1). The newly synthesized molecules will open the door for numerous possible synthetic modifications at the α-carbon of to prepare valuable analogues of curcumin with more favorable solubility profiles.
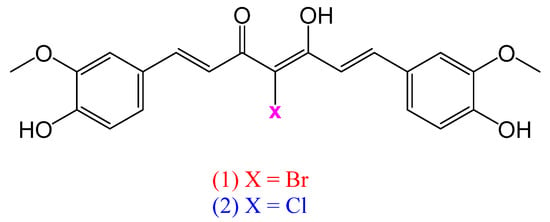
Figure 1.
Structures of 4-bromocurcumin (1) and 4-chlorocurcumin (2).
Several chemically modified curcumin derivatives have been synthesized mainly by substitution on the phenyl rings and by condensation/addition reactions such as semicarbazone and oxime derivatives [9,10]. Many curcumin metal complexes have also been prepared and characterized for a variety of applications including photodynamic therapy [11,12]. Reports on the α-functionalization of curcumin (or as is known the 4- position) are scarce [13,14,15]. Recently, however 4-alkylated curcumins were synthesized following a modified Pedersen procedure [16] and their keto–enol equilibrium constants were determined in various solvents using NMR spectroscopy [17]. Furthermore, two polymorphs of the 4-cyclobutylcurcumin were isolated and their single crystal structures determined [17].
To address low solubility and bioavailability of curcumin, researchers have explored structural modifications of curcumin through co-crystallization and co-amorphous systems [17]. Co-amorphous systems, as demonstrated by studies, help to stabilize curcumin in amorphous form by combining it with other molecules, enhancing solubility and stability. Additionally, co-crystals of curcumin involve the crystallization of curcumin with suitable co-formers. This technique creates new crystalline forms that improve the dissolution profile and bioavailability of curcumin. Furthermore, studies such as that by Görbitz et al., provide insights into modifying specific molecular sites on curcumin, allowing for more targeted enhancements of its chemical stability and interaction potential. Together, these approaches represent innovative pathways to optimize curcumin for therapeutic use [18,19,20,21].
Examining the Cambridge Structural Database reveals that curcumin is known to exist in three polymorphs (forms I, II, and III) with all polymorphs displaying the keto–enol forms. The most common polymorph of curcumin (form I) is where the molecule is nearly planar and crystallizes in the monoclinic space group P2/n whereas the other two uncommon forms are both orthorhombic with the space groups of Pca21 and Pbca for forms II and III, respectively [22,23,24,25,26,27,28,29]. There are also several reported crystal structures of curcumin derivatives including metal complexes [30,31,32]. Both solid state and solution NMR investigations of curcumin [33,34,35] in addition to curcumin co-crystals [36,37,38,39] were also reported. Surprisingly, the co-crystallization of curcumin with 4,4′-bipyridine N,N′-dioxide has resulted in the diketo form [17]. Furthermore, a comprehensive recent review of crystal engineering using curcumin was published [35].
2. Materials and Methods
All reagents were purchased from Sigma-Aldrich and used as received except curcumin, which was recrystallized from ethanol. The key in the successful synthesis of the bromo-analog was to carry out the reaction in slightly acidic media. Thus Amberlyst-15 was added before the bromination reaction. Chlorination of curcumin was successfully carried out using N-chlorosuccinimide in the absence of Amberlyst-15. Crystal structures were determined using a Rigaku MicroMax-007HF X-ray generator (Rigaku Corp., Tokyo Japan) in the Penn State Huck Institutes of Life Sciences. Data collection: SMART v5.625; cell refinement: SAINT V6.36A; data reduction: SAINT V6.36A; program(s) used to solve structure: SHELXS; program(s) used to refine structure: SHELXL 2018/3; molecular graphics: Olex2 1.3-ac4; software used to prepare material for publication: Olex2 1.3-ac4 [40,41,42,43,44]. Mo Kα radiation was used, λ = 0.71073 Å. The software packages used in this work were: Origin (Version 2024b (10.15); Crystal Explorer 21.5 [45,46] ChemDraw 16.0, Mercury 4.2, and Paint 2024). Spartan 16 was used to carry out the density Functional Theory calculations [47]. Hirshfeld surface analysis was performed using Crystal Explorer 21.5. Elemental analysis was carried out at Microanalysis Inc. (Wilmington, DE USA). Solubility was determined by preparing a saturated solution for each compound. We gradually added each compound to 1 mL of water at room temperature until it no longer dissolved, indicating saturation. The concentration was measured by using absorbance in UV–Vis after filtration. (See Supplementary Material).
2.1. Synthesis of (1E,6E)-1,7-Bis(4-Hydroxy-3-Methoxyphenyl) Hepta-4-Bromo-1,6-Diene-5-Hydroxy-3-One (1)
Curcumin (1.9865 g, 5.39 mmol) was dissolved in anhydrous acetyl acetate (60 mL) with heating at 40 °C for about 10 min. The solution was then briefly cooled in an ice bath and Amberlyst 15 (0.58 g) was added followed by N-bromosuccinimide (1.2182 g, 6.85 mmol). The ice bath was removed after 30 min and stirring continued at an ambient temperature overnight. The Amberlyst was removed, and the crude product crystallized in acetonitrile to yield bright orange crystals (16%). This product was then recrystallized to form an acetonitrile–hexane mixture (50:50) to obtain suitable crystals for the X-ray study. Anal. Calc’d (C21H19BrO6): C, 56.39; H, 4.28; Br, 17.86. Found: C, 56.88; H, 4.16, 17.63. Exact mass (NaC21H19BrO6): 469.026270; Found (EI, M+1): 469.0237. (See Supplementary Material).
2.2. Synthesis of (1E,6E)-1,7-Bis(4-Hydroxy-3-Methoxyphenyl) Hepta-4-Chloro-1,6-Diene-5-Hydroxy-3-One (2)
Curcumin (2.7444 g, 7.45 mmol) was dissolved in anhydrous acetonitrile with heating at 40 °C. The solution was briefly cooled in an ice bath before N-chlorosuccinimide (1.2081 g, 9.05 mmol) was added. Stirring was allowed to continue overnight at room temperature. The red crude powder was filtered and recrystallized in acetonitrile to give yellow crystals (33%). Anal. Calc’d (C21H19ClO6): C, 62.62; H, 4.75; Cl, 8.80. Found: C, 62.30; H, 4.77; Cl, 8.58. Exact mass: 402.0870. Found (EI, M+1): 403.0940.
3. Results and Discussion
The title compounds were successfully prepared by having curcumin react with N-bromosuccinimide or N-chlorosuccinimide with cooling in anhydrous solvents. While bromination requires Amberlyst 15, chlorination of curcumin can be achieved without the use of catalyst. Orange colored single crystals of α-bromocurcumin (1) were grown by slow evaporation from acetonitrile–hexane mixture (50:50) and a suitable crystal was selected for X-ray analysis. 4-Chlorocurcumin (2) was crystallized from acetonitrile as yellow needles. Table 1 shows a summary of the crystal data for 1 and 2. See Supplementary Material for elemental analysis and MS.

Table 1.
Crystal data and structure refinement for 1 and 2.
We first note that the two structures are isostructural with slightly different unit cell dimensions and solid-state structures. Both 4-bromocurcumin and 4-chloromocurcumin crystallize in the monoclinic space group P21/c, with four molecules in a unit cell. Unit cell dimensions for 1 bromo- and (2 chloro-) analogs are: a = 6.837, (16.7520) Å; b = 7.323, (7.27831) Å; c = 15.939, (15.9369) Å, β = 99.396, (100.0131)°, V = 1938.9, (1913.53) Å3. Both molecules assume the keto–enol form and are almost planar. The torsion between the phenyl rings of the mono brominated curcumin is 0.54°. The chloro- derivative is slightly less planar, having torsion between the phenyl rings of 1.16 °C.
Examining the two structures we first note that there are notable inter- and intra- molecular hydrogen bonds in both structures like those reported in polymorph I of the parent compound (Table 2). While the closest inter- O…O interactions involving the methoxy oxygens of two adjacent molecules for the bromo- and chloro- derivatives are 2.842 and 2.840 Å, respectively, compared with 2.666 in curcumin, both compounds exhibit short intra-O…O distances around the keto– enol center: 2.460 Å in (1) and 2.451 Å in (2) compared with 2.441; 2.498, and 2.490 in polymorphs I, II, and III of curcumin (Table 3). The bromo curcumin displayed slightly longer intra-O…O interactions, we note that both of the C-O and C=O bonds are longer compared to the chloro compound. As one might expect, with the presence of six oxygen atoms in each of these molecules, halogen…halogen interactions seem to play little or no role in solid state packing. No close halogen…halogen contacts were observed in either one of the two structures, with closest Br…Br distance of 5.772 Å and closest Cl…Cl distance of 5.926 Å. This might be plausible considering the many competing inter- and intra-molecular interactions involving other functional groups present and play a stronger role in steering the structure. This is not always the case as there are examples where halogen…halogen interactions play a significant role in solid-state structures of molecular materials even in the presence of other functional groups as shown in halogenated oligothiophenes for example [48,49,50,51,52,53].
We also note that the p-stack distance in the bromo compound (1) is slightly longer than the p-distance in the choro derivative (2), 3.367 and 3.362 Å, respectively (Table 2; Figure 2b and Figure 3b). Table 2 provides a summary of the main structural features observed in the structures of the two title compounds whereas Table 3 compares the major structural features reported herein with the three known polymorphs of curcumin with emphasis on main geometrical features around the keto–enol center. Plots of stacking distance and C-H…p distances as a function of the various torsion angles (shown in Figure 4) are provided in the Supplementary Material.
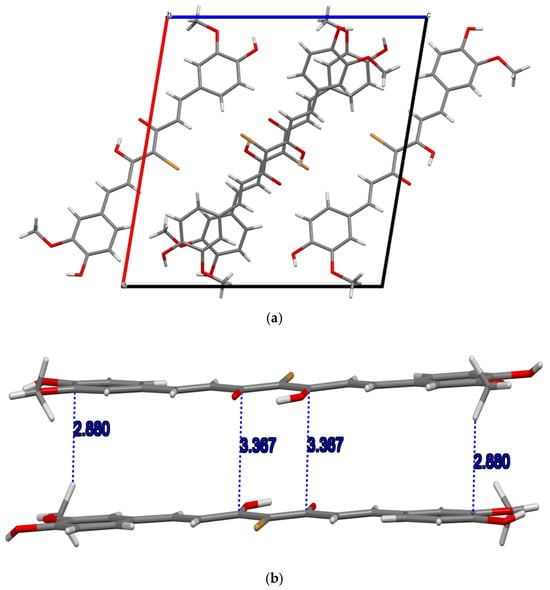
Figure 2.
(a) Packing in the unit cell of 4-bromocurcumin 1 along the b-axis; (b) p-stacks within the unit cell.
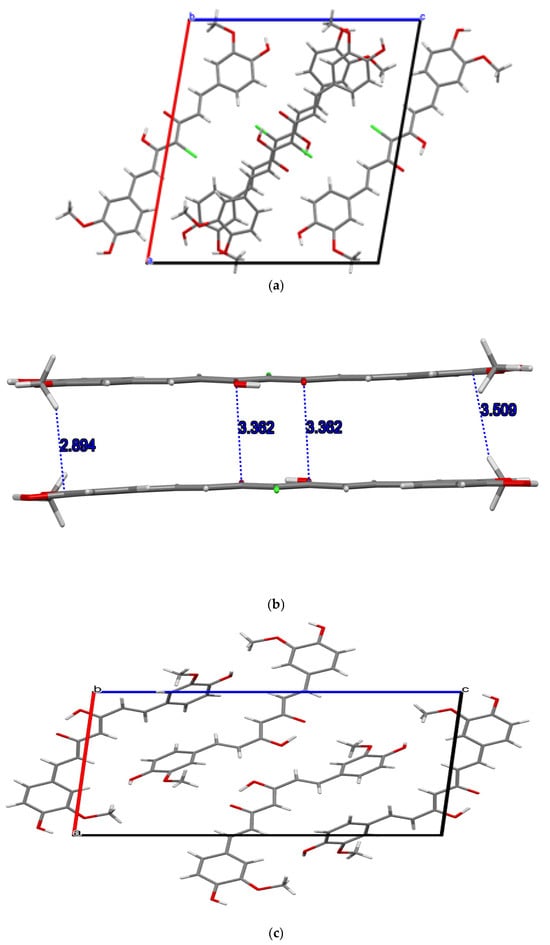
Figure 3.
(a) Packing in the unit cell of 4-chlorocurcumin 2 along the b-axis; (b) π-stacks within the unit cell; (c) unit cell of curcumin along the b-axis (shortest C-H...π distance is 4.387).

Table 2.
Summary of main inter- and intra-molecular interactions compounds (1) and (2) compared with the most common polymorph of curcumin.
Table 2.
Summary of main inter- and intra-molecular interactions compounds (1) and (2) compared with the most common polymorph of curcumin.
| Structural Feature | 1 | 2 | Curc Form I | Curc Form II | Curc Form III |
|---|---|---|---|---|---|
| Closest O…O inter- | 2.842 | 2.840 | 2.666 | 2.625; 2.834 | 2.666; 2.975 |
| Closest O…O intra- | 2.460 | 2.451 | 2.441 | 2.498; 2.527 | 2.490 |
| π-stack distances | 3.367 | 3.362 | NA | 3.306 | 3.817 |
| intra- O-H…O=C | 1.714 | 1.703 | 1.724 | 1.616; 1.676 | 1.724 |
| C-H..p interactions | 2.880 | 2.984 | 2.864 | 3.306; 3.365 | 4.387 |
| Closest Br…Br | 5.772 | - | - | - | - |
| Closest Cl…Cl | - | 5.926 | - | - | - |
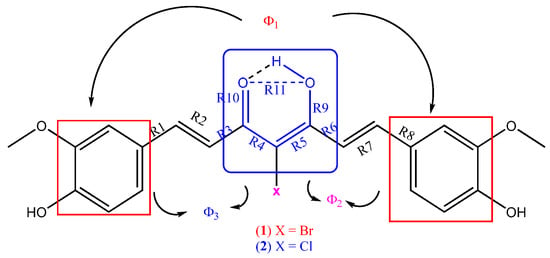
Figure 4.
Structural features used in Table 3.

Table 3.
Comparison of the main structural features shown in (Figure 4) in 1 and 2 compared with three forms of Curcumin [25,26,27].
Table 3.
Comparison of the main structural features shown in (Figure 4) in 1 and 2 compared with three forms of Curcumin [25,26,27].
| 1 | 2 | Curcumin Form I P21/n | Curcumin Form II Pca21 | Curcumin Form III P b c a | |
|---|---|---|---|---|---|
| O…O intra (R11) | 2.460 | 2.451 | 2.441 | 2.498 | 2.490 |
| Φ1 | 0.54 | 1.16 | 16.01 | 16.51 | 47.07 |
| Φ2 | 4.63 | 4.14 | 0.85 | 5.07 | 39.87 |
| Φ3 | 4.71 | 4.17 | 12.42 | 8.44 | 0.23 |
| R1 | 1.455 | 1.456 | 1.447 | 1.462 | 1.463 |
| R2 | 1.315 | 1.332 | 1.325 | 1.330 | 1.333 |
| R3 | 1.459 | 1.459 | 1.454 | 1.448 | 1.455 |
| R4 | 1.416 | 1.416 | 1.432 | 1.372 | 1.403 |
| R5 | 1.415 | 1.403 | 1.356 | 1.415 | 1.397 |
| R6 | 1.456 | 1.455 | 1.466 | 1.465 | 1.456 |
| R7 | 1.328 | 1.334 | 1.330 | 1.330 | 1.339 |
| R8 | 1.455 | 1.462 | 1.457 | 1.462 | 1.454 |
| R9 | 1.295 | 1.300 | 1.334 | 1.312 | 1.298 |
| R10 | 1.283 | 1.288 | 1.277 | 1.273 | 1.303 |
| O-H…O angle a | 150.24 | 150.63 | 152.20 | 155.04 | 154.36 |
| C-X | 1.898 | 1.736 | na | na | na |
| Methoxy groups configuration | syn | syn | syn | syn | anti |
| CCDC numbers | 2,258,982 | 2,324,288 | 807,904 | 807,905 | 807,906 |
a around the keto–enol. na; not applicable
Hirshfeld surface analysis was performed on the title compounds and compared with curcumin (form I), using Crystal Explorer 21. 5 software (Figure 5) [35]. It generates a 2D quantitative representation of different types of interactions in the unit cell. Distinct differences when comparing curcumin with the halo derivatives indicate the presence of cyclic hydrogen bonding (the areas between the sharp H bonding donor and acceptor; p-stacking the wings and green triangle in the center with sharper wings and more intense green triangles indicative of close p stacking distances) (Figure 5). The solubility of both new compounds in water was slightly lower than that of curcumin around 5 mg/mL for 1 and 2, respectively, compared to 6.6 mg/mL for curcumin [27,29]. This is further confirmed by studying the O…H/O…H interactions using Hirshfeld surface analysis (Figure 5) which revealed 21.2% for both compounds compared with 28.8% for curcumin which explains the solubility pattern observed. This is by interpreting a higher % of O…H/O…H interactions resulting in higher solubility.
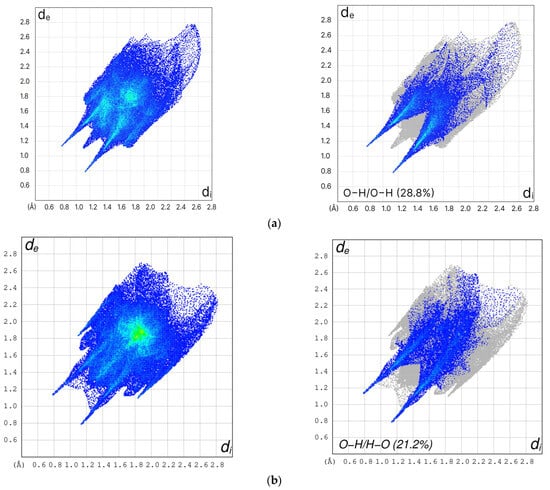
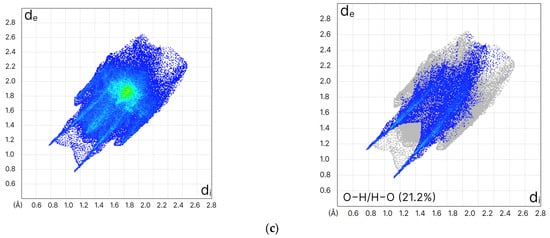
Figure 5.
Two dimensional fingerprints and O…H/O…H interactions of (a) curcumin form I (b) Curc-Br and (c) Curc-Cl.
The electronic spectra of curcumin and its derivatives were studied in detail. These studies demonstrated curcumin’s solvatochromic nature and sensitivity to hydrogen bonding and environmental polarity showcasing its potential for use in solvatochromic applications and molecular probes [54,55,56]. In the UV–Vis spectrum of the new compounds and compared with curcumin, three primary absorption maxima (λmax) are observed in each (Table 4). This is reflective of the molecules’ electronic structure. The highest λmax is usually attributed to the π → π* transition, which occurs at lower energy due to the extensive conjugation across the phenolic rings and the α,β-unsaturated β-diketone linker, leading to absorption in the visible region and imparting curcumin’s characteristic yellow color and the halo derivatives’ orange colors. The additional λmax values arise from n → π* transitions, involving the excitation of non-bonding electrons on oxygen atoms to anti-bonding π* orbitals. These transitions generally appear at shorter wavelengths. Absorption spectra showed bathochromic shift (red shift) of 33 and 35 nm for compounds 1 and 2, respectively, compared with the parent compound (Figure 6). This is consistent with the slight shortening in the carbon halogen bonds observed in their respective crystal structures indicating some electron-donating character of the halogen atoms.

Table 4.
DSC and spectroscopic data for curcumin and α-halo curcumins.
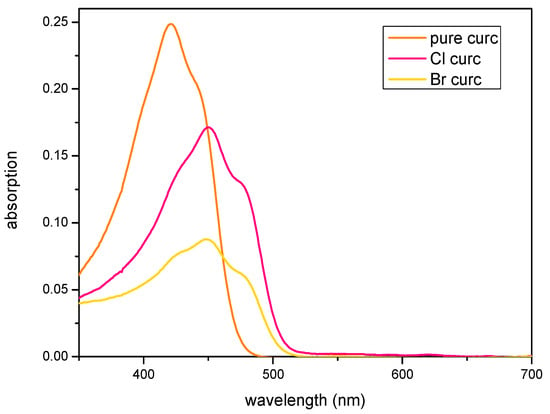
Figure 6.
UV–Vis absorption spectra of title compounds compared with curcumin in dichloromethane.
Surprisingly, the melting point of the bromo derivative is significantly lower than its chloro-counterpart one (Table 4).
Density functional theory (DFT) calculations using the Spartan 18 suite of programs and the B3LYP/6-311+G(d,p)//B3LYP/6-31G(d) level of theory [47] (ground state gas phase) indicated slight lowering of the LUMO level of the halogenated compounds resulting in the lowering of the calculated band gaps while also lowering the calculated dipole moments for the new compounds. The main points we obtained from these calculations are: lower band gaps of the halo derivatives, consistent with the UV–Vis data, and also the lower dipole moments associated with the observed lower solubility. The summary of the results of these calculations are depicted in Table 5. The lower solubility of the new compounds is consistent with the calculated dipole moment.

Table 5.
DFT calculated properties for 1, 2 and curcumin.
4. Conclusions
We have successfully synthesized and structurally characterized the first two α-halogenated curcumins: 4-bromocurcumin and 4-chlorocurcumin. The new molecules were studied by single crystal structural analysis, UV–Vis, DFT, and Hirshfeld surface analyses. These molecules are more thermally stable, albeit less soluble, in water than the unsubstituted curcumin. Competing inter- and intra-molecular interactions were compared with known closely related structures, especially the three known forms of curcumin. It is interesting to note that the introduction of the halogens at the α-position result in more planar structures and shorter π-stacking distances than any known curcumin derivatives reported to date. We assume that these molecules will be used as precursors to prepare a wide range of curcumin derivatives with more favorable solubility and bioavailability profiles.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst14121041/s1, UV–Vis spectra; MS and elemental analysis of compounds 1 and 2.
Author Contributions
Conceptualization, P.-T.T.P.; formal analysis, P.-T.T.P. and M.M.B.; writing—review and editing, P.-T.T.P. and M.M.B.; supervision, project administration, funding acquisition, P.-T.T.P. and M.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded internally. Research and Development Grant Penn State Scranton (P.-T.T.P.) and VP for Research, Office of Research at Alfaisal University (IRG-2020 and IRG-2024) (M.M.B.).
Data Availability Statement
Data and possibly samples can be obtained from corresponding authors.
Acknowledgments
The authors acknowledge support from Penn State Scranton in the form of a Research and Development Grant and partial support from the SIG S10 of the National Institutes of Health under award number 1S10OD028589-01 and 1S10RR023439-01 and Neela Yennawar for the X-ray instrumentation. The authors also acknowledge Hemant P. Yennawar of the X-ray facility at Penn State University, University Park. We also highly appreciate J. Steed’s help in sharing his group’s manual on using the Crystal Explorer program. M. Bader acknowledges financial support from the VP for Research, Office of Research at Alfaisal University (IRG-2020 and IRG-2024). The help of undergraduate students at Alfaisal University, Samar M. Al Rifai and Sarah H. Younas, with Figure 5 and Figure 6 is highly appreciated. We thank Edreese Alsharaeh and Mohanraj Krishnan for their help with DSC measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dairam, A.; Fogel, R.; Santy, D.; Limson, J.L. Antioxidant and Iron-Binding Properties of Curcumin, Capsaicin, and S-Allylcysteine Reduce Oxidative Stress in Rat Brain Homogenate. J. Agric. Food Chem. 2008, 56, 3350–3356. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.A. Currying favor for the heart. J. Clin. Investig. 2008, 118, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Kotian, V.; Koland, M.; Mutalik, S. Nanocrystal-Based Topical Gels for Improving Wound Healing Efficacy of Curcumin. Crystals 2022, 12, 1565. [Google Scholar] [CrossRef]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Shishodia, S.S.; Chaturvedi, M.M.; Aggarwal, B.B. Role of curcumin in cancer therapy. Curr. Prob. Cancer 2007, 31, 243–305. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Z. The Effect of Curcumin on Breast Cancer Cells. J. Breast Cancer 2013, 16, 133–137. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Chemical and structural features influencing the biological activity of curcumin. Curr. Pharm. Des. 2013, 19, 2093–2100. [Google Scholar]
- Huang, M.T.; Lysz, T.; Ferraro, T.; Abidl, T.F.; Laskin, J.D.; Conney, A.H. Inhibitory Effects of Curcumin on in Vitro Lipoxygenase and Cyclooxygenase Activities in Mouse Epidermis. Cancer Res. 1991, 51, 813–819. [Google Scholar]
- Dutta, S.; Padhye, S.; Priyadarsini, K.I.; Newton, C. Antioxidant and antiproliferative activity of curcumin semicarbazone derivative. Bioorg. Med. Chem. 2005, 15, 2738–2744. [Google Scholar] [CrossRef]
- Simoni, D.; Rizzi, M.; Rondanin, R.; Baruchello, R.; Marchetti, P.; Invidiata, F.P.; Labbozzetta, M.; Poma, P.; Carina, V.; Notarbartolo, M. Antitumor effects of curcumin and structurally modified b-diketone analogs on multidrug resistant cancer cells. Bioorg. Med. Chem. Lett. 2008, 18, 845–849. [Google Scholar] [CrossRef]
- Raduly, F.; Rădițoiu, V.; Fierăscu, R.; Rădițoiu, A.; Nicolae, C.; Purcar, V. Influence of Organic-Modified Inorganic Matrices on the Optical Properties of Palygorskite–Curcumin-Type Hybrid Materials. Crystals 2022, 12, 1005. [Google Scholar] [CrossRef]
- Pavel, O.; Şerban, A.; Zăvoianu, R.; Bacalum, E.; Bîrjega, R. Curcumin Incorporation into Zn3Al Layered Double Hydroxides—Preparation, Characterization and Curcumin Release. Crystals 2020, 10, 244. [Google Scholar] [CrossRef]
- Taguchi, H.; Yanagisawa, D.; Morikawa, S.; Hirao, K.; Shirai, N.; Tooyama, I. Synthesis and Tautomerism of Curcumin Derivatives and Related Compounds. Aust. J. Chem. 2015, 68, 224–229. [Google Scholar] [CrossRef]
- Lin, L.; Shi, Q.; Su, C.Y.; Shih, C.C.Y.; Lee, K.H. Antitumor agents 247 New 4-ethoxycarbonylethyl curcumin analogs as potential antiandrogenic agents. Bioorg. Med. Chem. 2006, 14, 2527–2534. [Google Scholar] [CrossRef]
- Lin, L.; Shi, Q.; Nyarko, A.K.; Bastow, K.F.; Wu, C.C.; Su, C.Y.; Shih, C.C.Y.; Lee, K.H. Antitumor agents. 250. Design and synthesis of new curcumin analogues as potential anti-prostate cancer agents. J. Med. Chem. 2006, 49, 3963–3972. [Google Scholar] [CrossRef]
- Pedersen, U.; Rasmussen, P.B.; Lawesson, S.O. Synthesis of Naturally Occurring Curcuminoids and Related-Compounds. Liebigs Ann. Chem. 1985, 1985, 1557–1569. [Google Scholar] [CrossRef]
- Osifová, Z.; Reiberger, R.; Cisarova, I.; Machara, A.; Dracinsky, M. Diketo−Ketoenol Tautomers in Curcuminoids: Synthesis, Separation of Tautomers, and Kinetic and Structural Studies. J. Org. Chem. 2022, 87, 10309–10318. [Google Scholar] [CrossRef]
- Ziaee, A.; Raei, M.; Poorghorban, M.; Salehi, E.; Soltanmohammadi, F.; Fathi, Z. Enhancing Curcumin Bioavailability via Co-Amorphous Systems: Characterization and In Vitro Performance. LWT 2022, 161, 114091. [Google Scholar] [CrossRef]
- Roy, S.; Priya, S.; Panda, A.; Dasgupta, S.; Ghosh, D.; Pathak, D.; Haldar, S. Curcumin Co-Crystals as Enhanced Bioavailability and Dissolution Rate Forms: Crystal Engineering and Solid-State Characterization. J. Supercrit. Fluids 2021, 167, 105190. [Google Scholar] [CrossRef]
- Caro Garrido, C.; Vandooren, M.; Robeyns, K.; Debecker, D.; Luis, P.; Leyssens, T. Combining a Drug and a Nutraceutical: A New Cocrystal of Praziquantel and Curcumin. Crystals 2024, 14, 181. [Google Scholar] [CrossRef]
- Tylik, E.; Szymańska, I.; Kasprzyk, A.; Tyszczuk-Rotko, K.; Lis, T.; Paneth, P.; Szafran, W.; Borowicz, P. Engineering Curcumin Co-Crystals to Improve Physicochemical Properties: Comparative Studies on Bioavailability Enhancement. Cryst. Growth Des. 2023, 23, 3925–3933. [Google Scholar] [CrossRef]
- Görbitz, C.H.; Haaland, A.; Jørgensen, K.A.; Krane, J.; Seip, R. Crystal Structure and α-Functionalization of Curcumin Derivatives: A Structural Analysis. Acta Chem. Scand. 1986, 40, 420–429. [Google Scholar] [CrossRef][Green Version]
- Tonnesen, H.H.; Karlsen, J.; Mostad, A. Structural Studies of Curcuminoids. 1. The Crystal Structure of Curcumin. Acta Chem. Scand. B 1982, 36, 475–479. [Google Scholar] [CrossRef]
- Parimita, S.P.; Ramshankar, Y.V.; Suresh, S.; Row, T.N.G. Redetermination of curcumin: (1E,4Z,6E)-5-hydroxy-1,7-bis (4-hy droxy-3-methoxyphenyl)hepta-1,4,6-trien-3-one. Acta Crystallogr. E 2007, 63, O860–O862. [Google Scholar] [CrossRef]
- Sanphui, P.; Goud, N.R.; Khandavilli, U.B.R.; Bhanoth, S.; Nangia, A. New polymorphs of curcumin. Chem. Commun. 2011, 47, 5013–5015. [Google Scholar] [CrossRef]
- Matlinska, M.A.; Wasylishen, R.E.; Bernard, G.M.; Terskikh, V.V.; Brinkmann, A.; Michaelis, V.K. Capturing Elusive Polymorphs of Curcumin: A Structural Characterization and Computational Study. Cryst. Growth Des. 2018, 18, 5556–5563. [Google Scholar] [CrossRef]
- Liu, J.; Svard, M.; Hippen, P.; Rasmuson, A.C. Solubility and Crystal Nucleation in Organic Solvents of Two Polymorphs of Curcumin. J. Pharm. Sci. 2015, 104, 2183–2189. [Google Scholar] [CrossRef]
- Yuan, L.; Lorenz, H. Solvate Formation of Bis(demethoxy)curcumin: Screening and Characterization. Crystals 2018, 8, 407. [Google Scholar] [CrossRef]
- Ishtiaq, M.; Asghar, S.; Khan, I.; Iqbal, M.; Khalid, S. Development of the Amorphous Solid Dispersion of Curcumin: A Rational Selection of Polymers for Enhanced Solubility and Dissolution. Crystals 2022, 12, 1606. [Google Scholar] [CrossRef]
- Halevas, E.; Arvanitidou, M.; Mavroidi, B.; Hatzidimitriou, A.G.; Politopoulos, K.; Alexandratou, E.; Pelecanou, M.; Sagnou, M. A novel curcumin gallium complex as photosensitizer in photodynamic therapy: Synthesis, structural and physicochemical characterization, photophysical properties and in vitro studies against breast cancer cells. J. Mol. Struct. 2021, 1240, 130485. [Google Scholar] [CrossRef]
- Kazantzis, K.T.; Koutsonikoli, K.; Mavroidi, B.; Zachariadis, M.; Alexiou, P.; Pelecanou, M.; Politopoulos, K.; Alexandratou, E.; Sagnou, M. Curcumin derivatives as photosensitizers in photo dynamic therapy: Photophysical properties and in vitro studies with prostate cancer cells. Photochem. Photobiol. Sci. 2020, 19, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, U.; Kumar, B.; Garai, A.; Bhattacharyya, A.; Kumar, A.; Banerjee, S.; Kondaiah, P.; Chakravarty, A.R. Curcumin “Drug” Stabilized in Oxidovanadium(IV)-BODIPY Conjugates for Mitochondria-Targeted Photocytotoxicity. Inorg. Chem. 2017, 56, 12457–12468. [Google Scholar] [CrossRef] [PubMed]
- Sandusky, P.; Alworth, W.L. NMR Study of the Solution Structure of Curcumin, Payton, Florastina. J. Nat. Prod. 2007, 70, 143–146. [Google Scholar]
- Dai, Y.; Terskikh, V.; Brinmkmann, A.; Wu, G. Solid-State 1H, 13C, and 17O NMR Characterization of the Two Uncommon Polymorphs of Curcumin. Cryst. Growth Des. 2020, 20, 7484–7491. [Google Scholar] [CrossRef]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.E.S.; Lee, D.-K.; Ramamoorthy, A. Determining the Effects of Lipophilic Drugs on Membrane Structure by Solid-State NMR Spectroscopy: The Case of the Antioxidant Curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef]
- Heffernan, C.; Ukrainczyk, M.; Zeglinski, J.; Hodnett, B.K.; Rasmuson, A.C. Influence of Structurally Related Impurities on the Crystal Nucleation of Curcumin. Cryst. Growth Des. 2018, 18, 4715–4723. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S.V. Crystal Engineering of Curcumin with Salicylic Acid and Hydroxyquinol as Coformers. Cryst. Growth Des. 2017, 17, 3974–3988. [Google Scholar] [CrossRef]
- Su, H.M.; He, H.M.; Tian, Y.Y.; Zhao, N.; Sun, F.X.; Zhang, X.M.; Jiang, Q.; Zhu, G.S. Syntheses and characterizations of two curcumin-based cocrystals. Inorg. Chem. Commun. 2015, 55, 92–95. [Google Scholar] [CrossRef]
- Sanphui, P.; Bolla, G. Curcumin, a Biological Wonder Molecule: A Crystal Engineering Point of View. Cryst. Growth Des. 2018, 18, 5690–5711. [Google Scholar] [CrossRef]
- SMART v5.625: Bruker. SMART (Control and Integration Software), Version 5.625; Bruker AXS Inc.: Madison, WI, USA, 2001.
- SAINT V6.36A: Bruker. SAINT (Data Integration and Reduction Software), Version 6.36A; Bruker AXS Inc.: Madison, WI, USA, 2001.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement, and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17: A program for Hirshfeld surface analysis, visualization, and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2017, 50, 1260–1274. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Shao, Y.; Molnar, L.F.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.T.; Gilbert, A.T.; Slipchenko, L.V.; Levchenko, S.V.; O’Neill, D.P.; et al. Advances in Methods and Algorithms in a Modern Quantum Chemistry Program Package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, J.-Y.; Pei, J. Control of π–π Stacking via Crystal Engineering in Organic Conjugated Small Molecule Crystals. Cryst. Growth Des. 2018, 18, 7–15. [Google Scholar] [CrossRef]
- Bader, M.M. 2,5-Bis(5-bromo-2-thienyl) thiophene. Acta Cryst. 2009, E65, o2119. [Google Scholar] [CrossRef]
- Pham, P.; Bader, M.M. Structural Studies on Some Oligothiophenes and Ethylenedioxy-thiophenes. MRS Online Proc. Libr. 2015, 1799, 19–28. [Google Scholar] [CrossRef]
- Pham, P.; Bader, M.M. Inter- and Intramolecular Interactions in Some Bromo- and Tricyanovinyl-Substituted Thiophenes and Ethylene dioxythiophenes. Cryst. Growth Des. 2014, 14, 916–922. [Google Scholar] [CrossRef]
- Pham, P.; Bader, M.M. Thiophenes Endowed with Electron-Accepting Groups: A Structural Study. Cryst. Growth Des. 2024, 24, 906–912. [Google Scholar] [CrossRef]
- Pham, P.; Young, V.G., Jr.; Bader, M.M. The impact of vinylene bridges and side chain alkyl groups on the solid-state structures of tricyanovinyl-substituted thiophenes. CrystEngComm 2018, 20, 128–132. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Studies on Curcumin and Curcuminoids: X. Spectral Properties of Curcumin. Spectrochim. Acta A 1985, 41, 1069–1074. [Google Scholar]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of Curcumin in Buffer Solutions and Characterization of its Degradation Products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, K. Solvent Effects on the UV-Visible Absorption Spectra of Curcumin and its Derivatives. J. Mol. Struct. 2006, 834–836, 140–145. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).