Phase Separation Investigation of Axitinib in Supersaturated Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of the Phase Separation Concentration of Axitinib
2.3. Detection of the Phase Separation Property of Axitinib
2.4. Detection of the Phase Separation Process of Axitinib
2.5. Detection of the Phase Separation Process of Axitinib in Presence of Different Polymers
3. Results and Discussion
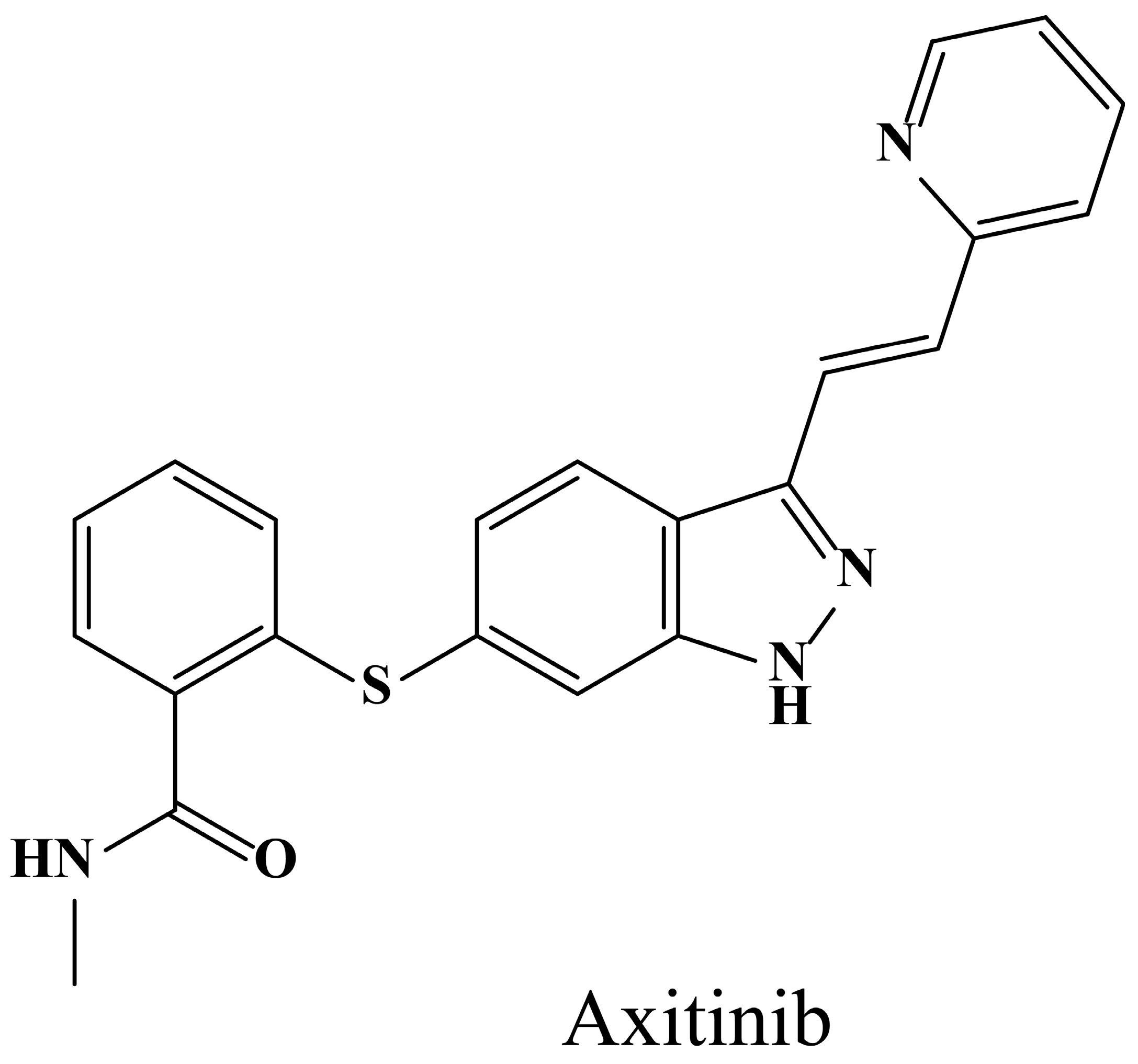
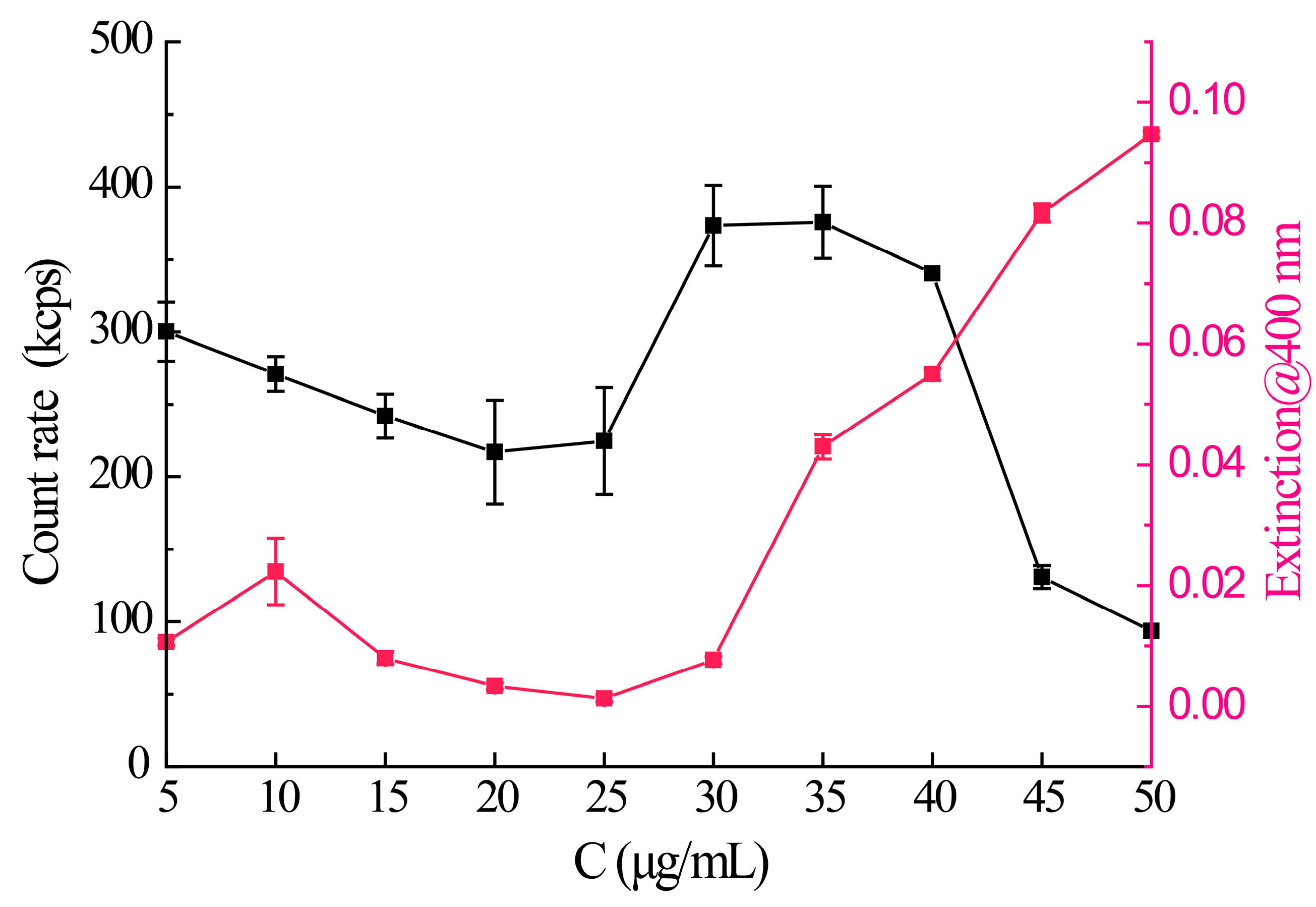
3.1. The Phase Separation Concentration of Axitinib
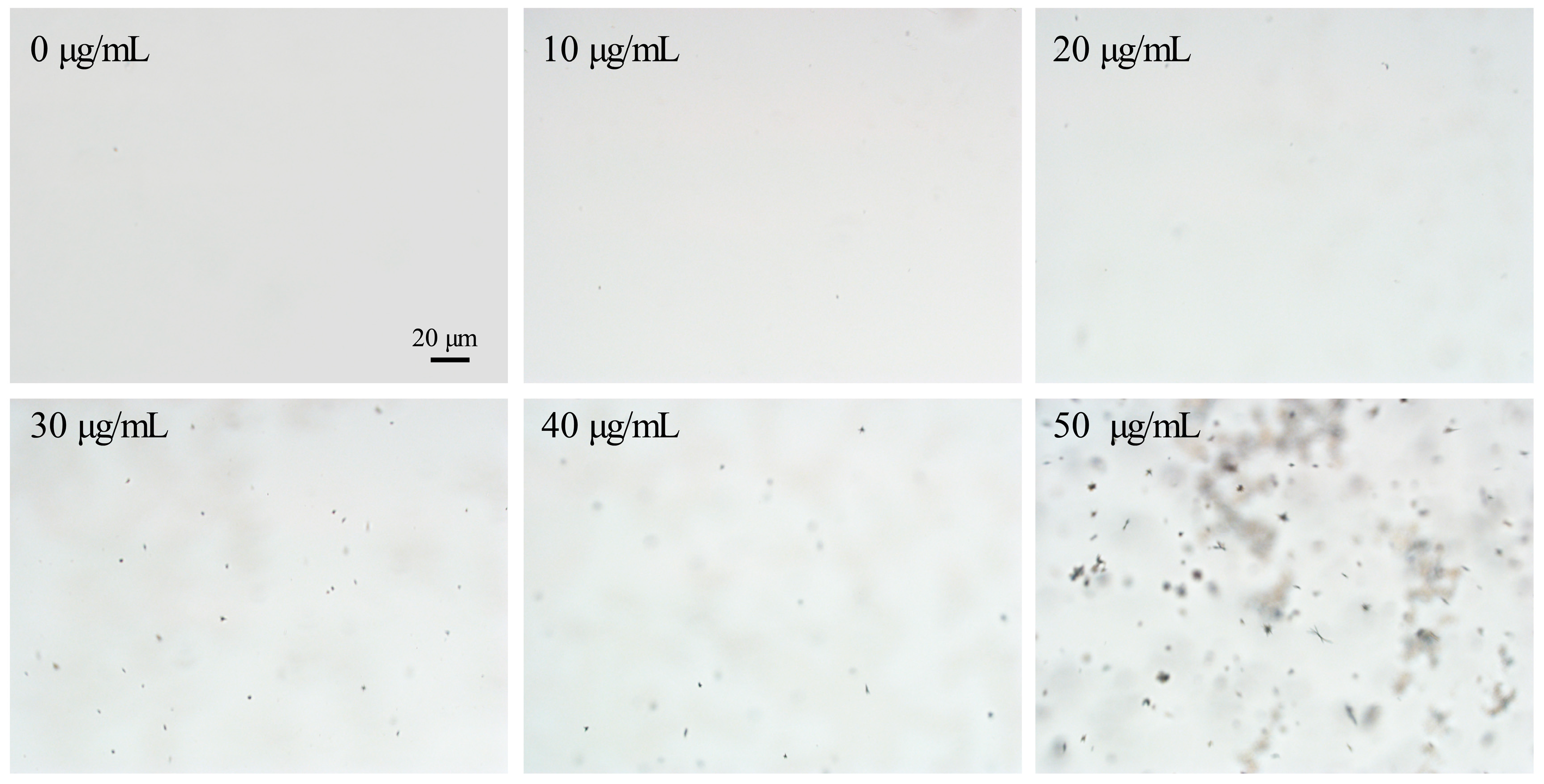
3.2. The Phase Separation Property of Axitinib
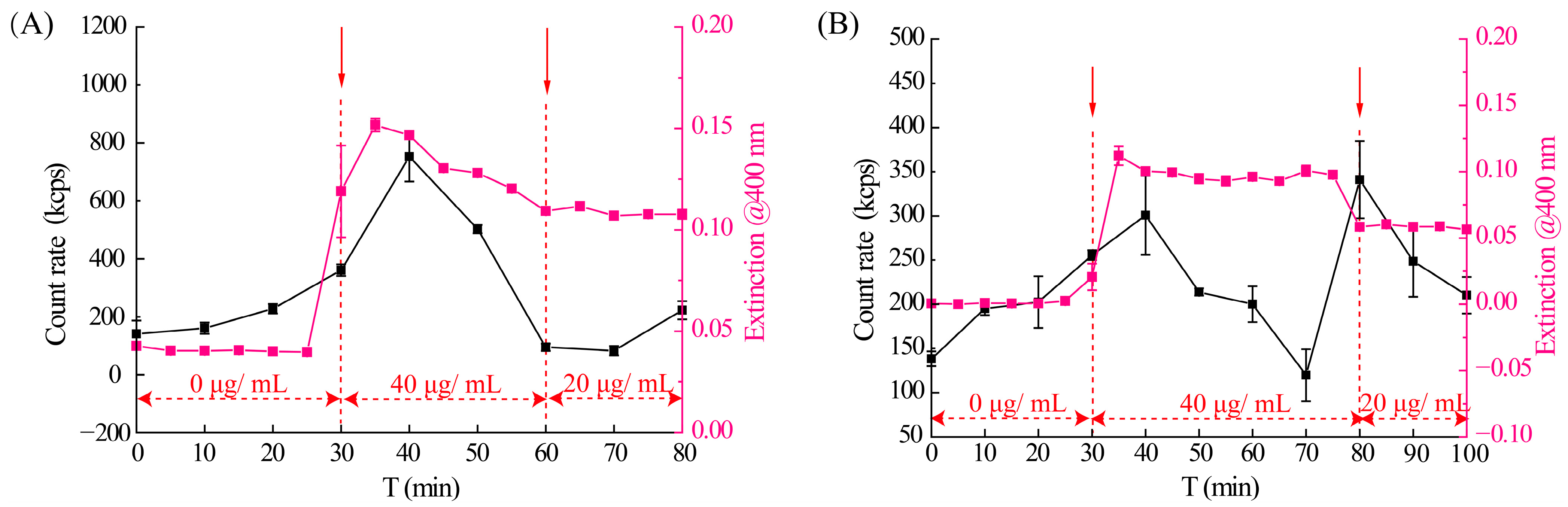
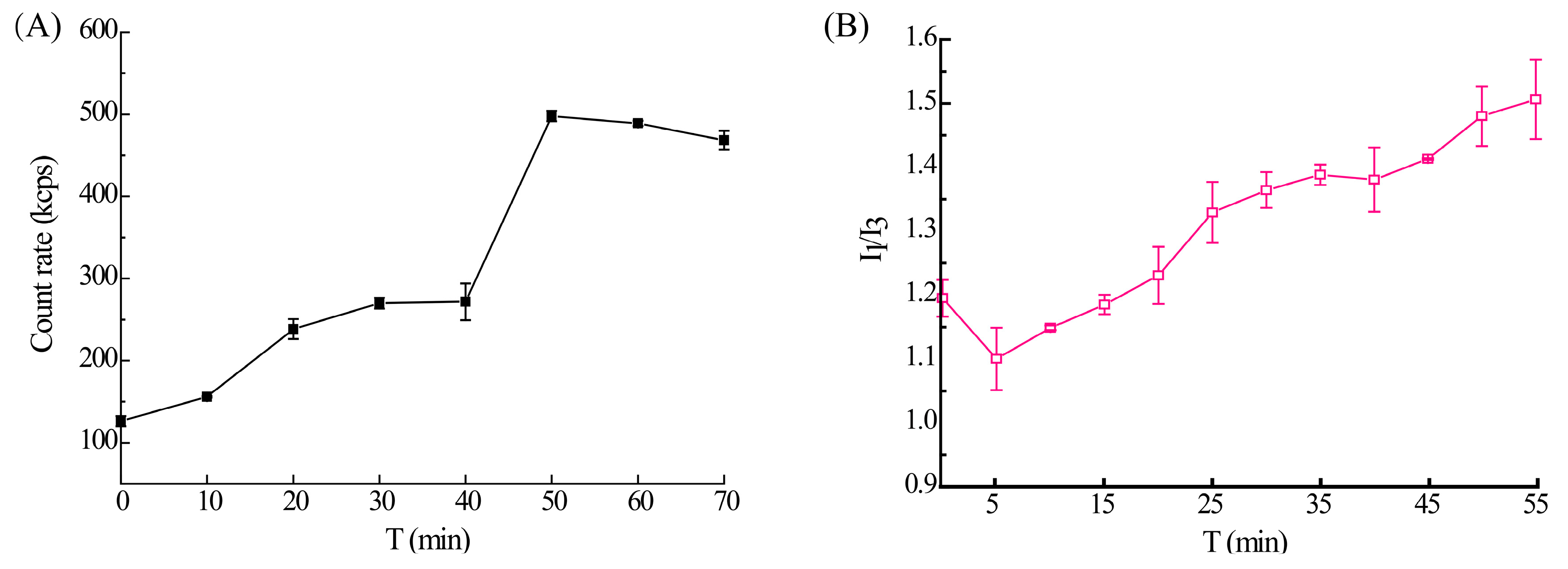
3.3. The Phase Separation Process of Axitinib
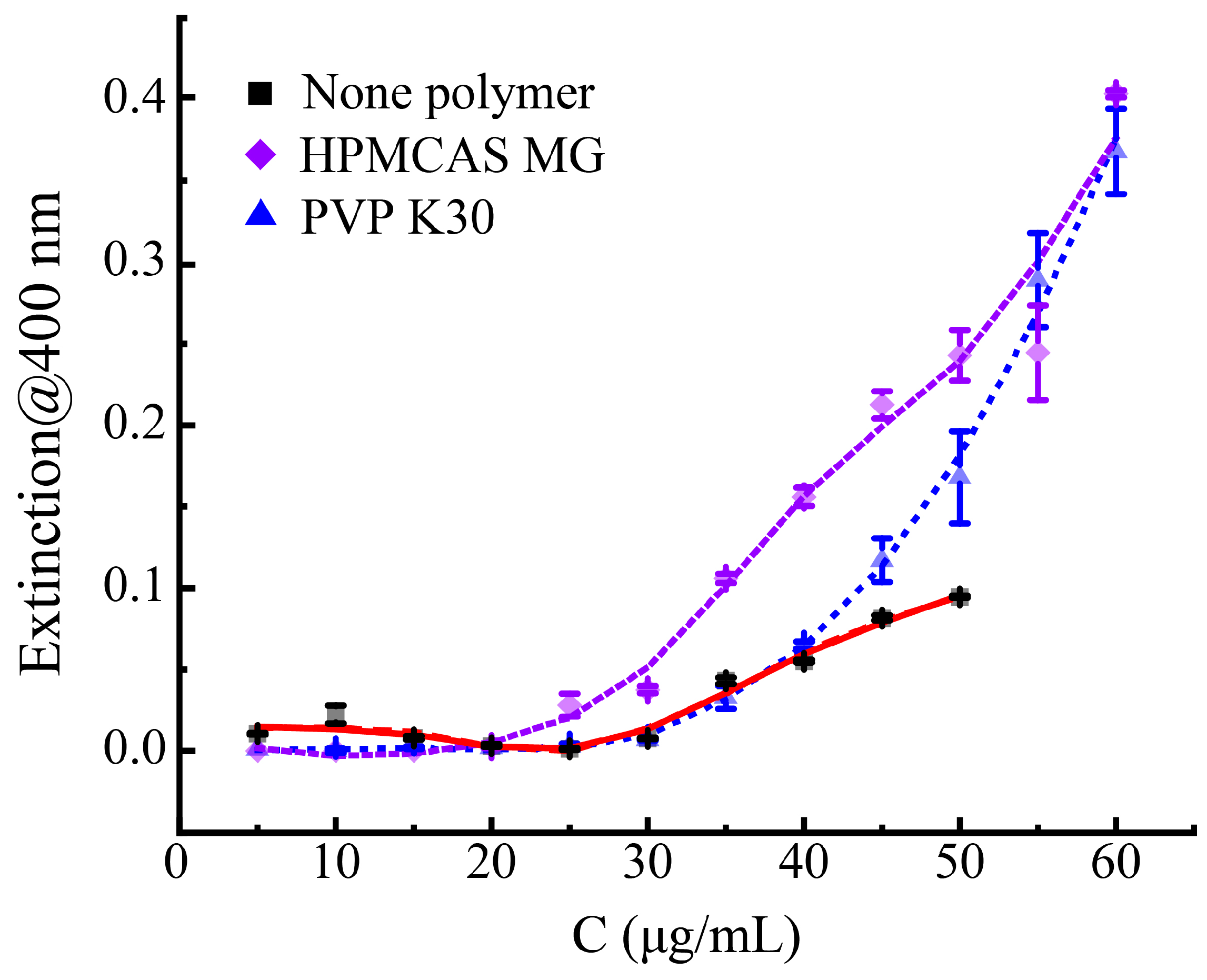
3.4. The Influence of Different Polymers on the Phase Separation Process of Axitinib
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Sullivan, A.; Long, B.; Verma, V.; Ryan, K.M.; Padrela, L. Solid-state and particle size control of pharmaceutical cocrystals using atomization-based techniques. Int. J. Pharm. 2022, 621, 121798. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, X.; Zhao, Y.; Gao, Y.; Cai, C.; Zhang, Q.; Ding, Z.; Fan, Z.; Zhang, H.; Liu, M.; et al. Effect of plasticizers on manufacturing ritonavir/copovidone solid dispersions via hot-melt extrusion: Preformulation, physicochemical characterization, and pharmacokinetics in rats. Eur. J. Pharm. Sci. 2019, 127, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Hiew, T.N.; Taylor, L.S. Combining drug salt formation with amorphous solid dispersions—A double edged sword. J. Control. Release 2022, 352, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Niu, W.; Liu, J.; Cui, Z.; Li, J.; Zhang, Z.; Ju, J. Preparation of β-cyclodextrin and hydroxypropyl-β-cyclodextrin inclusion complexes of baicalein and evaluation of their effects on dextran sulfate sodium-induced acute ulcerative colitis in mice. J. Drug Deliv. Sci. Technol. 2023, 87, 104714. [Google Scholar] [CrossRef]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.S.; Ramakrishna, S. Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Kim, J.S.; Din, F.u.; Cho, H.J.; Choi, Y.J.; Woo, M.R.; Cheon, S.; Ji, S.H.; Park, S.; Youn, Y.S.; Oh, K.T.; et al. Impact of carrier hydrophilicity on solid self nano-emulsifying drug delivery system and self nano-emulsifying granule system. Int. J. Pharm. 2023, 648, 123578. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, Y.; Guan, Q.; Xiao, S.; Dong, W.; Lian, S.; Zhang, H.; Liu, M.; Wang, Z.; Han, J. Amorphous solid dispersions of cyclosporine A with improved bioavailability prepared via hot melt extrusion: Formulation, physicochemical characterization, and in vivo evaluation. Eur. J. Pharm. Sci. 2022, 168, 106036. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Li, L.; Kang, K.; Peng, D.; Shi, Y.; Wang, P. Crystallization inhibitory effects of konjac glucomannan, sodium alginate and xanthan gum on curcumin in supersaturated solution. Int. J. Biol. Macromol. 2023, 245, 125489. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.R.; Ma, Y.; Lee, P.I. Impact of phase separation morphology on release mechanism of amorphous solid dispersions. Eur. J. Pharm. Sci. 2019, 136, 104955. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Sato, K.; Fukushima, M.; Miyazaki, A.; Yamamura, Y.; Sakuma, S. Phase separation of supersaturated solution created from amorphous solid dispersions: Relevance to oral absorption. Eur. J. Pharm. Biopharm. 2018, 132, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Tres, F.; Posada, M.M.; Hall, S.D.; Mohutsky, M.A.; Taylor, L.S. Mechanistic understanding of the phase behavior of supersaturated solutions of poorly water-soluble drugs. Int. J. Pharm. 2018, 543, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Indulkar, A.S.; Gao, Y.; Raina, S.A.; Zhang, G.G.Z.; Taylor, L.S. Impact of Monomeric versus Micellar Surfactant and Surfactant–Polymer Interactions on Nucleation–Induction Times of Atazanavir from Supersaturated Solutions. Cryst. Growth Des. 2020, 20, 62–72. [Google Scholar] [CrossRef]
- Zhao, P.; Han, W.; Shu, Y.; Li, M.; Sun, Y.; Sui, X.; Liu, B.; Tian, B.; Liu, Y.; Fu, Q. Liquid–liquid phase separation drug aggregate: Merit for oral delivery of amorphous solid dispersions. J. Control. Release 2023, 353, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.S.; Zhang, G.G.Z. Physical chemistry of supersaturated solutions and implications for oral absorption. Adv. Drug Deliv. Rev. 2016, 101, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Almeida e Sousa, L.; Reutzel-Edens, S.M.; Stephenson, G.A.; Taylor, L.S. Assessment of the Amorphous “Solubility” of a Group of Diverse Drugs Using New Experimental and Theoretical Approaches. Mol. Pharm. 2015, 12, 484–495. [Google Scholar] [CrossRef]
- Alonzo, D.E.; Gao, Y.; Zhou, D.; Mo, H.; Zhang, G.G.Z.; Taylor, L.S. Dissolution and Precipitation Behavior of Amorphous Solid Dispersions. J. Pharm. Sci. 2011, 100, 3316–3331. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Z.; Chen, Y.; Lu, J.; Li, Y.; Wang, S.; Wu, G.; Qian, F. Improving Oral Bioavailability of Sorafenib by Optimizing the “Spring” and “Parachute” Based on Molecular Interaction Mechanisms. Mol. Pharm. 2016, 13, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-W.; Yu, L.; Huang, J.; Hussain, M.A.; Derdour, L.; Qian, F.; de Villiers, M.M. A Method to Evaluate the Effect of Contact with Excipients on the Surface Crystallization of Amorphous Drugs. AAPS PharmSciTech 2014, 15, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Song, J.; Li, J.; Li, M.; Tian, B.; He, Z.; Liu, X.; Fu, Q. Co-amorphization of atorvastatin by lisinopril as a co-former for solubility improvement. Int. J. Pharm. 2021, 607, 120971. [Google Scholar] [CrossRef]
- Benet, L.Z. Predicting drug disposition via application of a Biopharmaceutics Drug Disposition Classification System. Basic Clin. Pharmacol. Toxicol. 2010, 106, 162–167. [Google Scholar] [CrossRef]
- Dodd, S.; Kollipara, S.; Sanchez-Felix, M.; Kim, H.; Meng, Q.; Beato, S.; Heimbach, T. Prediction of ARA/PPI Drug-Drug Interactions at the Drug Discovery and Development Interface. J. Pharm. Sci. 2019, 108, 87–101. [Google Scholar] [CrossRef]
- Dilli Babu, A.; Singh, S.; Thota, A.; Duhan, S.; Sainatham, C.; Gill, H.; Raghavakurup, L.; Tantry, U.; Bliden, K.; Gurbel, P. Systemic hypertension with VEGFR inhibitor (axitinib) therapy in cancer patients: A meta-analysis and systematic review of randomized controlled trials. IJC Heart Vasc. 2024, 52, 101415. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, Y.; Cai, D.; Guo, Q.; Wang, J.; Lei, L.; Li, X.; Shi, S. Penetrating-peptide-mediated non-invasive Axitinib delivery for anti-neovascularisation. J. Control. Release 2022, 347, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Loftsson, T.; Stefánsson, E. Drug-like properties of tyrosine kinase inhibitors in ophthalmology: Formulation and topical availability. Int. J. Pharm. 2024, 655, 124018. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Peng, F.; Zheng, Q.; Zeng, L.; Chen, H.; Li, X.; Huang, J. Micelle-solubilized axitinib for ocular administration in anti-neovascularization. Int. J. Pharm. 2019, 560, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lessmann, T.; Jones, S.A.; Voigt, T.; Weisbrod, S.; Kracker, O.; Winter, S.; Zúñiga, L.A.; Stark, S.; Bisek, N.; Sprogøe, K. Degradable Hydrogel for Sustained Localized Delivery of Anti-Tumor Drugs. J. Pharm. Sci. 2023, 112, 2843–2852. [Google Scholar] [CrossRef]
- Qu, H.; Li, Z.; Zhang, G.; Zhou, Z.; Wu, S. Tuning the physicochemical properties of axitinib by crystallization: Preparation, calculation and Structure-property relationship. J. Ind. Eng. Chem. 2023, 124, 570–578. [Google Scholar] [CrossRef]
- Ilevbare, G.A.; Taylor, L.S. Liquid–Liquid Phase Separation in Highly Supersaturated Aqueous Solutions of Poorly Water-Soluble Drugs: Implications for Solubility Enhancing Formulations. Cryst. Growth Des. 2013, 13, 1497–1509. [Google Scholar] [CrossRef]
- Praphanwittaya, P.; Saokham, P.; Jansook, P.; Loftsson, T. Aqueous solubility of kinase inhibitors: I the effect of hydrophilic polymers on their γ-cyclodextrin solubilization. J. Drug Deliv. Sci. Technol. 2020, 55, 101462. [Google Scholar] [CrossRef]
- Fan, X.; Zheng, W.; Singh, D.J. Light scattering and surface plasmons on small spherical particles. Light Sci. Appl. 2014, 3, 179. [Google Scholar] [CrossRef]
- Kong, Y.; Wang, W.; Wang, C.; Li, L.; Peng, D.; Tian, B. Supersaturation and phase behavior during dissolution of amorphous solid dispersions. Int. J. Pharm. 2023, 631, 122524. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Ma, Q.; Zhao, Y.; Jiang, X.; Zhang, H.; Liu, M.; Wang, Z.; Han, J. Cellulose derivatives as effective recrystallization inhibitor for ternary ritonavir solid dispersions: In vitro-in vivo evaluation. Carbohydr. Polym. 2021, 273, 118562. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Guan, Q.; Zhang, H.; Ding, Z.; Wang, Q.; Wang, Z.; Han, J.; Liu, M.; Zhao, Y. Impact of hypromellose acetate succinate and Soluplus® on the performance of β-carotene solid dispersions with the aid of sorbitan monolaurate: In vitro-in vivo comparative assessment. Int. J. Biol. Macromol. 2023, 253, 126639. [Google Scholar] [CrossRef] [PubMed]
- Ilevbare, G.A.; Liu, H.; Pereira, J.; Edgar, K.J.; Taylor, L.S. Influence of Additives on the Properties of Nanodroplets Formed in Highly Supersaturated Aqueous Solutions of Ritonavir. Mol. Pharm. 2013, 10, 3392–3403. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.-Q.; Wang, S.-R.; Zhang, Y.; Huo, J.-S.; Wang, L.-N.; Cai, J.-H.; Li, Z.-Q.; Xiang, B.; Qi, X.-R. Hot melt extrusion technology for improved dissolution, solubility and “spring-parachute” processes of amorphous self-micellizing solid dispersions containing BCS II drugs indomethacin and fenofibrate: Profiles and mechanisms. Eur. J. Pharm. Sci. 2019, 130, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Ilevbare, G.A.; Liu, H.; Edgar, K.J.; Taylor, L.S. Impact of Polymers on Crystal Growth Rate of Structurally Diverse Compounds from Aqueous Solution. Mol. Pharm. 2013, 10, 2381–2393. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Su, J.; Zhang, H.; Bu, R.; Ding, Z.; Zhang, N.; Zhao, Y. Phase Separation Investigation of Axitinib in Supersaturated Solution. Crystals 2024, 14, 1042. https://doi.org/10.3390/cryst14121042
Xu J, Su J, Zhang H, Bu R, Ding Z, Zhang N, Zhao Y. Phase Separation Investigation of Axitinib in Supersaturated Solution. Crystals. 2024; 14(12):1042. https://doi.org/10.3390/cryst14121042
Chicago/Turabian StyleXu, Jie, Jianshuo Su, Huaizhen Zhang, Rupeng Bu, Zhuang Ding, Ning Zhang, and Yanna Zhao. 2024. "Phase Separation Investigation of Axitinib in Supersaturated Solution" Crystals 14, no. 12: 1042. https://doi.org/10.3390/cryst14121042
APA StyleXu, J., Su, J., Zhang, H., Bu, R., Ding, Z., Zhang, N., & Zhao, Y. (2024). Phase Separation Investigation of Axitinib in Supersaturated Solution. Crystals, 14(12), 1042. https://doi.org/10.3390/cryst14121042





