Surface Modification of Bi2Te3 Nanoplates Deposited with Tin, Palladium, and Tin/Palladium Using Electroless Deposition
Abstract
1. Introduction
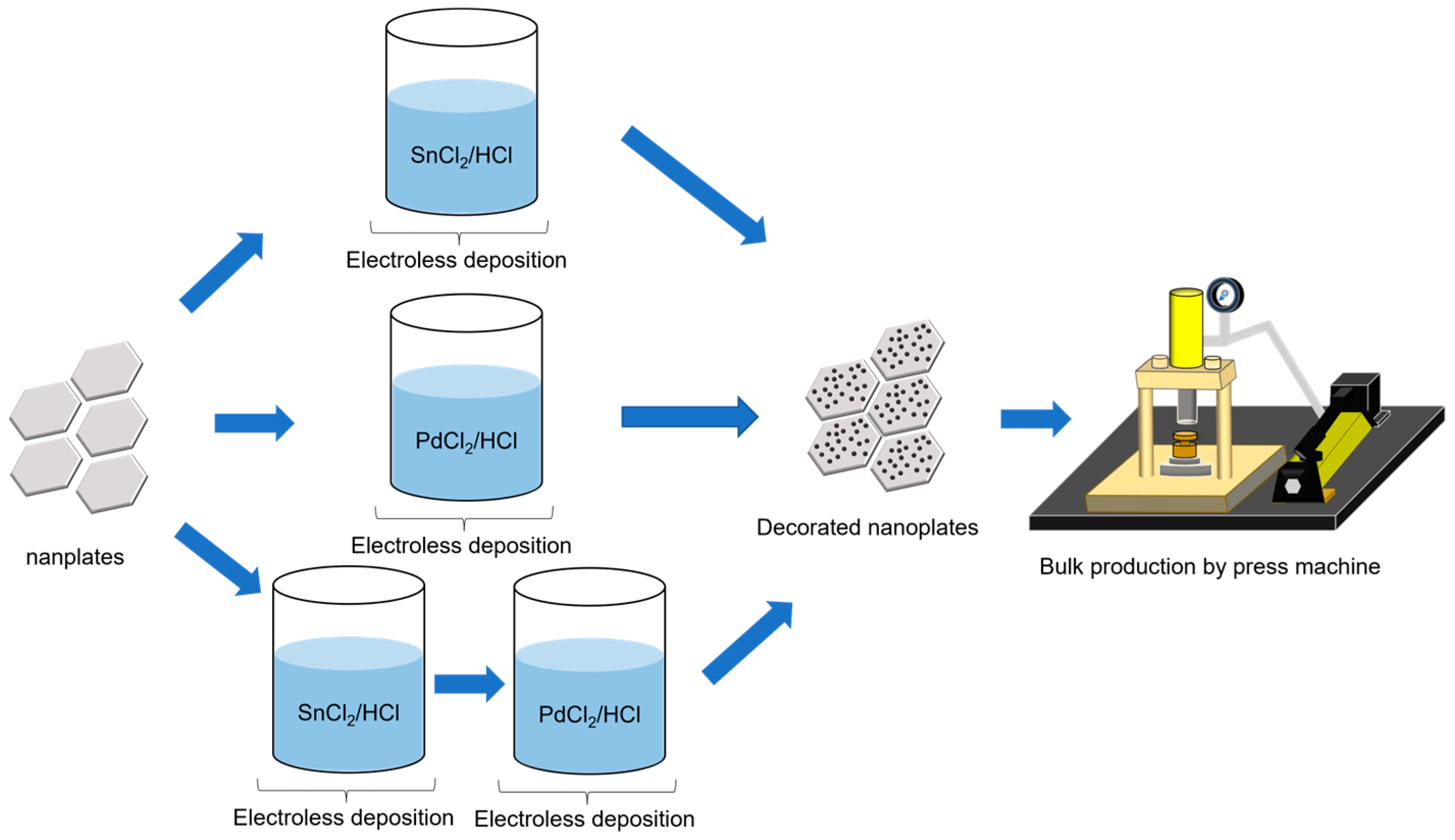
2. Materials and Methods
3. Results and Discussion
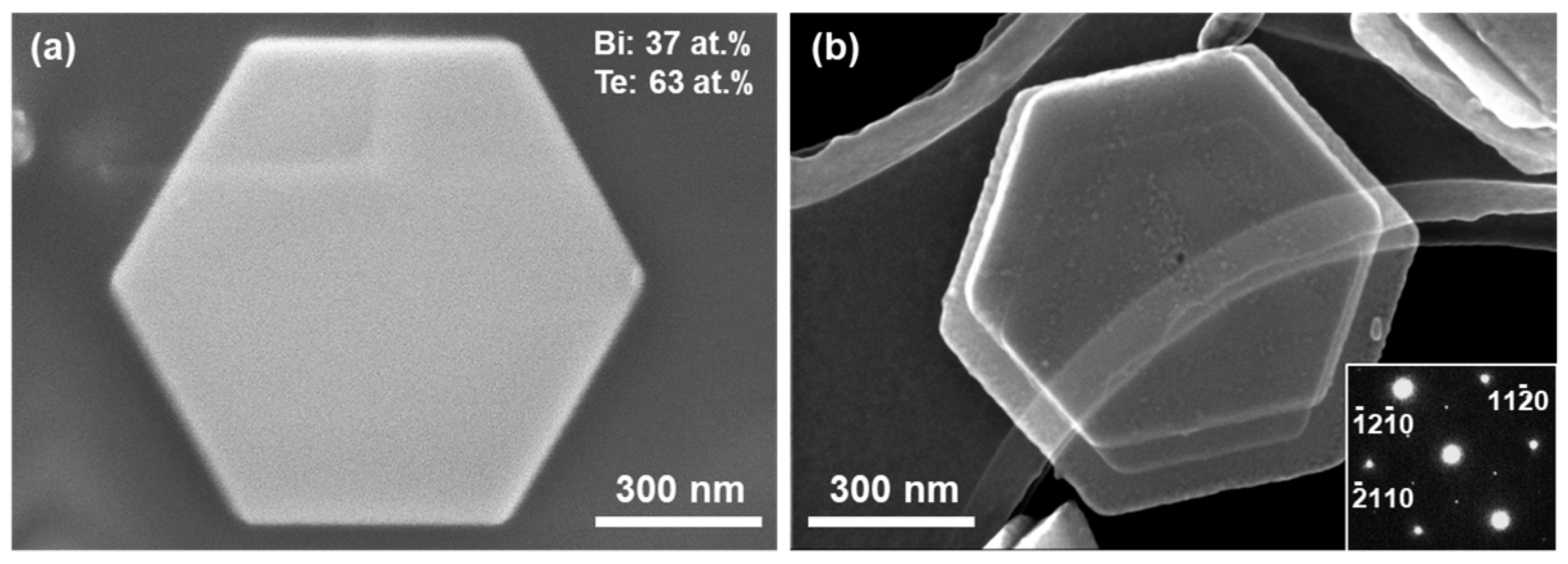
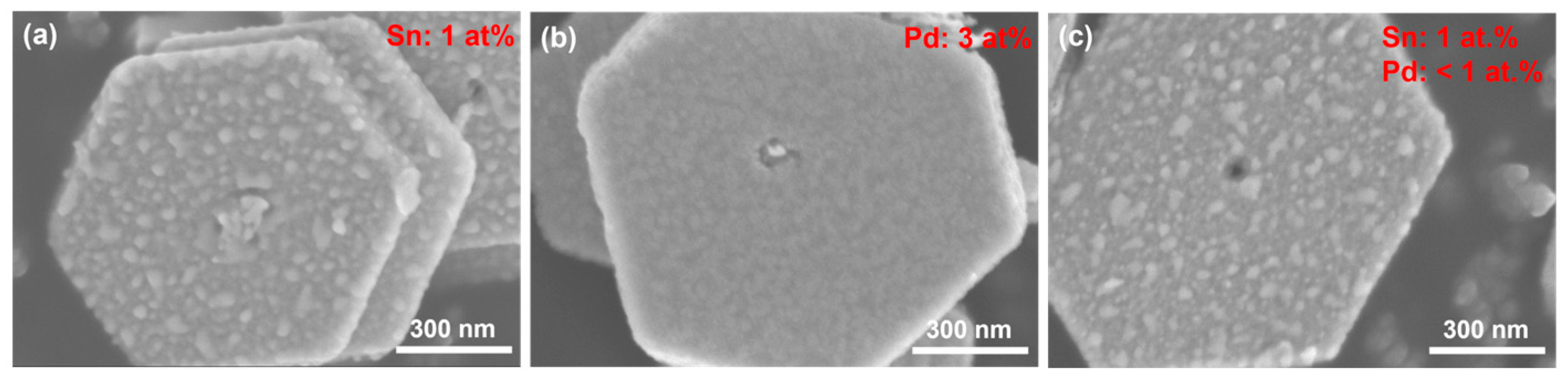
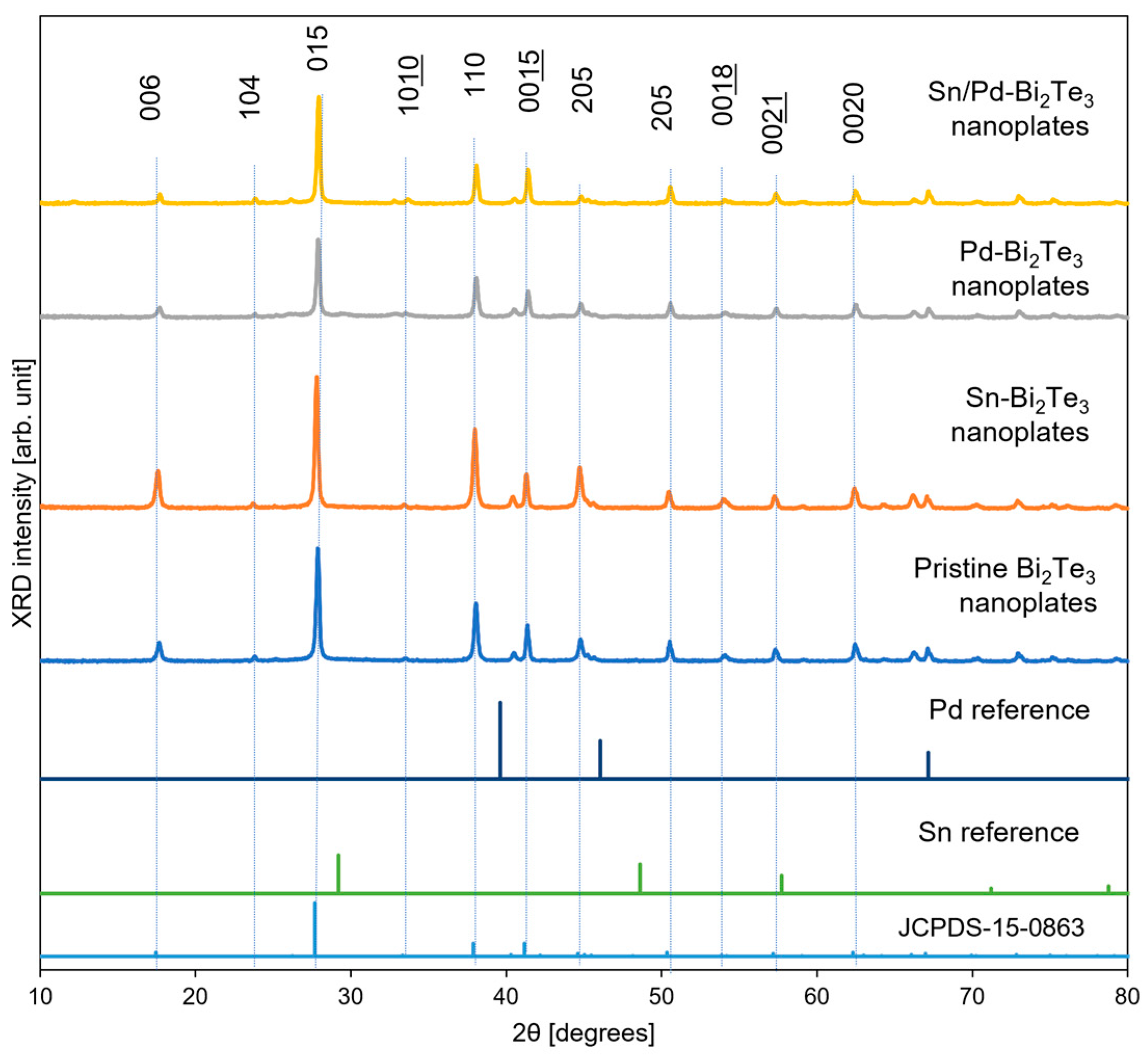
3.1. Structural Characteristics of Bi2Te3 Nanoplates Deposited with Different Metals
3.2. Thermal Conductivity of Bi2Te3 Nanoplates Deposited with Different Metals
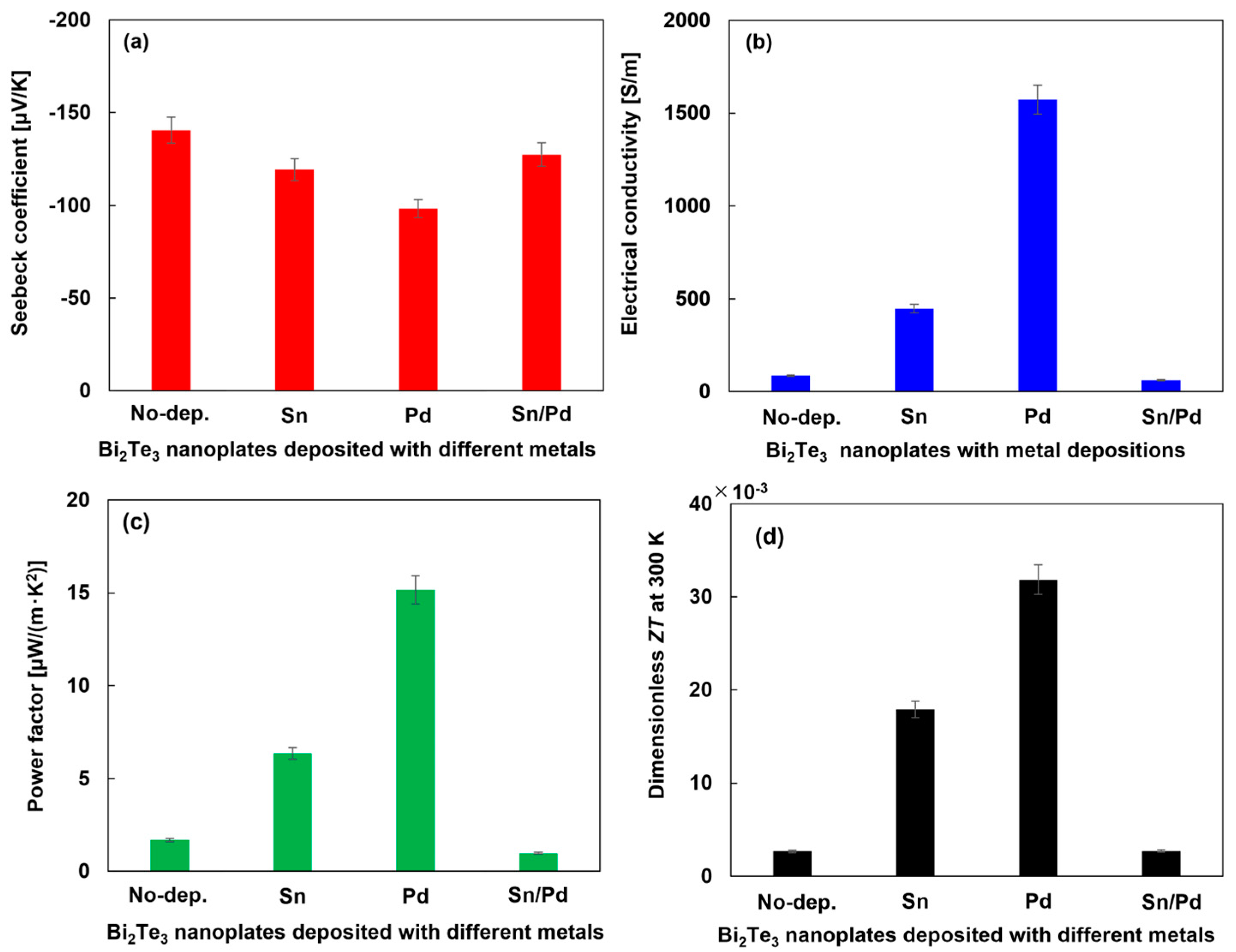
3.3. Thermoelectric Properties of Bi2Te3 Nanoplates Deposited with Different Metals
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landaluce, H.; Arjona, L.; Perallos, A.; Falcone, F.; Angulo, I.; Muralter, F. Review of IoT sensing applications and challenges using RFID and wireless sensor networks. Sensors 2020, 20, 2495. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, M.H.; Kim, S.; Kuruoğlu, N. Energy harvesting techniques for wireless sensor networks/radio-frequency identification: A review. Symmetry 2019, 11, 865. [Google Scholar] [CrossRef]
- Guo, C.F.; Sun, T.; Cao, F.; Liu, Q.; Ren, Z. Metallic nanostructures for light trapping in energy-harvesting devices. Light Sci. Appl. 2014, 3, 161. [Google Scholar]
- Matiko, J.W.; Grabham, N.J.; Beeby, S.P.; Tudor, M.J. Review of the application of energy harvesting in buildings. Meas. Sci. Technol. 2014, 25, 012002. [Google Scholar] [CrossRef]
- Beeby, S.P.; Tudor, M.J.; White, N.M. Energy harvesting vibration sources for microsystems applications. Meas. Sci. Technol. 2006, 17, R175–R195. [Google Scholar] [CrossRef]
- Wei, C.; Jing, X. A comprehensive review on vibration energy harvesting: Modeling and realization. Renew. Sustain. Energy Rev. 2017, 74, 1–18. [Google Scholar] [CrossRef]
- Leonov, V. Thermoelectric energy harvesting of human body heat for wearable sensors. IEEE Sens. J. 2013, 13, 2284–2291. [Google Scholar] [CrossRef]
- Zhou, A.; Al-Furjan, M.S.H.; Zou, J.; Liu, W. A review on heat and mechanical energy harvesting from human—Principles, prototypes and perspectives. Renew. Sustain. Energy Rev. 2018, 82, 3582–3609. [Google Scholar] [CrossRef]
- Akinaga, H. Recent advances and future prospects in energy harvesting technologies. Jpn. J. Appl. Phys. 2020, 59, 110201. [Google Scholar] [CrossRef]
- Almoneef, T.S.; Erkmen, F.; Ramahi, O.M. Harvesting the energy of multi-polarized electromagnetic waves. Sci. Rep. 2017, 7, 14656. [Google Scholar] [CrossRef]
- Ioffe, A.F.; Stil’bans, L.S.; Iordanishvili, E.K.; Stavitskaya, T.S.; Gelbtuch, A.; Vineyard, G. Semiconductor thermoelements and thermoelectric cooling. Phys. Today 1959, 12, 42. [Google Scholar] [CrossRef]
- Champier, D. Thermoelectric generators: A review of applications. Energy Convers. Manag. 2017, 140, 167–181. [Google Scholar] [CrossRef]
- Zoui, M.A.; Bentouba, S.; Stocholm, J.G.; Bourouis, M. A Review on thermoelectric generators: Progress and applications. Energies 2020, 13, 3606. [Google Scholar] [CrossRef]
- Chiba, T.; Amma, Y.; Takashiri, M. Heat source free water floating carbon nanotube thermoelectric generators. Sci. Rep. 2021, 11, 14707. [Google Scholar] [CrossRef] [PubMed]
- Jaziri, N.; Boughamoura, A.; Müller, J.; Mezghani, B.; Fares, T.F.; Ismail, M. A comprehensive review of Thermoelectric Generators: Technologies and common applications. Energy Rep. 2020, 6, 264–287. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, S.; Hua, T.; Huang, B.; Liu, S.; Tao, X. Fiber-based thermoelectric generators: Materials, device structures, fabrication, characterization, and applications. Adv. Enrrgy Mater. 2018, 8, 1700524. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and old concepts in thermoelectric materials. Angew. Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar] [CrossRef]
- Chen, G.; Dresselhaus, M.S.; Dresselhaus, G.; Fleurial, J.-P.; Caillat, T. Recent developments in thermoelectric materials. Int. Mater. Rev. 2003, 48, 45–66. [Google Scholar] [CrossRef]
- Gomez-Romero, R. Hybrid organic–inorganic materials—In search of synergic activity. Adv. Mater. 2001, 13, 163–174. [Google Scholar] [CrossRef]
- Amma, Y.; Miura, K.; Nagata, S.; Nishi, T.; Miyake, S.; Miyazaki, K.; Takashiri, M. Ultra-long air-stability of n-type carbon nanotube films with low thermal conductivity and all-carbon thermoelectric generators. Sci. Rep. 2022, 12, 21603. [Google Scholar] [CrossRef]
- Judeinstein, P.; Sanchez, C. Hybrid organic–inorganic materials: A land of multidisciplinarity. J. Mater. Chem. 1996, 6, 511–525. [Google Scholar] [CrossRef]
- Van Bommel, K.J.C.; Friggeri, A.; Shinkai, S. Organic templates for the generation of inorganic materials. Angew. Chem. 2003, 42, 980–999. [Google Scholar] [CrossRef] [PubMed]
- Goldsmid, H.J. The electrical conductivity and thermoelectric power of bismuth telluride. Proc. Phys. Soc. 1958, 71, 633–646. [Google Scholar] [CrossRef]
- Soleimani, Z.; Zoras, S.; Ceranic, B.; Shahzad, S.; Cui, Y. A review on recent developments of thermoelectric materials for room-temperature applications. Sustain. Energy Technol. Assess. 2020, 37, 100604. [Google Scholar] [CrossRef]
- Yim, W.M.; Rosi, F.D. Compound tellurides and their alloys for Peltier cooling—A review. Solid-State Electron. 1972, 15, 1121–1134. [Google Scholar] [CrossRef]
- Peng, J.; Tikhonov, E. Predicted bismuth—Tellurium under high pressures. Phase Transit. 2023, 96, 328–336. [Google Scholar] [CrossRef]
- Masut, R.A.; André, C.; Vasilevskiy, D.; Turenne, S. Charge transport anisotropy in hot extruded bismuth telluride: Scattering by acoustic phonons. J. Appl. Phys. 2020, 128, 115106. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Z.; Tian, Z.; Zhang, Y.; Li, X.; Song, H. High thermoelectric performance of large size Bi2Te2.7Se0.3 alloy ingots. J. Electron. Mater. 2023, 52, 6682–6689. [Google Scholar] [CrossRef]
- Trung, N.H.; Sakamoto, K.; Toan, N.V.; Ono, T. Synthesis and evaluation of thick films of electrochemically deposited Bi2Te3 and Sb2Te3 thermoelectric materials. Materials 2017, 10, 154. [Google Scholar] [CrossRef]
- Goldsmid, H.J. Conversion Efficiency and Figure-of-Merit. In CRC Handbook of Thermoelectrics; Rowe, D.M., Ed.; CRC Press: New York, NY, USA, 1995; pp. 19–42. [Google Scholar]
- Hicks, L.D.; Dresselhaus, M.S. Effect of quantum-well structure on the thermoelectric figure of merit. Phys. Rev. B 1993, 47, 12727–12731. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, R.; Siivola, E.; Colpitts, T.; O’Quinn, B. Thin-film thermoelectric devices with high room-temperature figures of merit. Nature 2001, 413, 597–602. [Google Scholar] [CrossRef]
- Peranio, N.; Eibl, O.; Nurnus, J. Structural and thermoelectric properties of epitaxially grown BiTe thin films and superlattices. J. Appl. Phys. 2006, 100, 114306. [Google Scholar] [CrossRef]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.; Minnich, A.; Yu, B.; Yan, X.; Wang, D.; Muto, A.; Vashaee, D.; et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, J.; Yu, D.; He, J.; Liu, Z.; Xu, B.; Tian, Y. Enhanced thermoelectric figure of merit in nanocrystalline Bi2Te3 bulk. J. Appl. Phys. 2009, 105, 094303. [Google Scholar] [CrossRef]
- Kudo, S.; Hagino, H.; Takana, S.; Miyazaki, K.; Takashiri, M. Determining the thermal conductivities of nanocrystalline bismuth telluride thin films using the differential 3ω method while accounting for thermal contact resistance. J. Electron. Mater. 2015, 44, 2021–2025. [Google Scholar] [CrossRef]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The surface science of nanocrystals. Nat. Mater. 2016, 15, 141–153. [Google Scholar] [CrossRef]
- Nakamoto, T.; Yokoyama, S.; Takamatsu, T.; Harata, K.; Motomiya, K.; Takahashi, H.; Miyazaki, Y.; Tohji, K. Aqueous chemical synthesis and consolidation of size-controlled Bi2Te3 nanoparticles for low-cost and high-performance thermoelectric materials. J. Electron. Mater. 2019, 48, 2700–2711. [Google Scholar] [CrossRef]
- Welch, C.M.; Compton, R.G. The use of nanoparticles in electroanalysis: A review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef]
- Liu, J.L.; Wang, H.; Li, X.; Chen, H.; Zhang, Z.K.; Pan, W.W.; Luo, G.Q.; Yuan, C.L.; Ren, Y.L.; Lei, W. High performance visible photodetectors based on thin two-dimensional Bi2Te3 nanoplates. J. Alloys Compd. 2019, 798, 656–664. [Google Scholar] [CrossRef]
- Tiwari, J.; Tiwari, R.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Wu, Z.; Mu, E.; Che, Z.; Liu, Y.; Sun, F.; Wang, X.; Hu, Z. Nanoporous (00l)-oriented Bi2Te3 nanoplate film for improved thermoelectric performance. J. Alloys Compd. 2020, 828, 154239. [Google Scholar] [CrossRef]
- Lu, W.; Ding, Y.; Chen, Y.; Wang, Z.L.; Fang, J. Bismuth telluride hexagonal nanoplatelets and their two-step epitaxial growth. J. Am. Chem. Soc. 2005, 127, 10112–10116. [Google Scholar] [CrossRef] [PubMed]
- Hollar, C.; Lin, Z.; Kongara, M.; Varghese, T.; Karthik, C.; Schimpf, J.; Eixenberger, J.; Davis, P.H.; Wu, Y.; Duan, X.; et al. High-performance flexible bismuth telluride thin film from solution processed colloidal nanoplates. Adv. Mater. Technol. 2020, 5, 2000600. [Google Scholar] [CrossRef] [PubMed]
- Mamur, H.; Bhuiyan, M.R.A.; Korkmaz, F.; Nil, M. A review on bismuth telluride (Bi2Te3) nanostructure for thermoelectric applications. Renew. Sustain. Energy Rev. 2018, 82, 4159–4169. [Google Scholar] [CrossRef]
- Ganesh, S.H.; Prabhu, A.N. A Review on doped/composite bismuth chalcogenide compounds for thermoelectric device applications: Various synthesis techniques and challenges. J. Electron. Mater. 2022, 51, 2014–2042. [Google Scholar]
- Hegde, G.S.; Prabhu, A.N.; Gao, Y.H.; Kuo, Y.K.; Reddy, V.R. Potential thermoelectric materials of indium and tellurium co-doped bismuth selenide single crystals grown by melt growth technique. J. Alloys Compd. 2021, 866, 158814. [Google Scholar] [CrossRef]
- Jin, Q.; Jiang, S.; Zhao, Y.; Wang, D.; Qiu, J.; Tang, D.-M.; Tan, J.; Sun, D.-M.; Hou, P.-X.; Chen, X.-Q.; et al. Flexible layer-structured Bi2Te3 thermoelectric on a carbon nanotube scaffold. Nat. Mater. 2018, 18, 62–68. [Google Scholar] [CrossRef]
- Chiba, T.; Yabuki, H.; Takashiri, M. High thermoelectric performance of flexible nanocomposite films based on Bi2Te3 nanoplates and carbon nanotubes selected using ultracentrifugation. Sci. Rep. 2023, 3, 3010. [Google Scholar] [CrossRef]
- Kango, S.; Klia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Li, F.; Song, J.; Yang, H.; Gan, S.; Zhang, Q.; Han, D.; Ivaska, A.; Niu, L. One-step synthesis of graphene/SnO2 nanocomposites and its application in electrochemical supercapacitors. Nanotechnology 2009, 20, 455602. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.; Thorkelsson, K.; Bai, P.; Rancatore, B.; Xu, T. Toward functional nanocomposites: Taking the best of nanoparticles, polymers, and small molecules. Chem. Soc. Rev. 2013, 42, 2654–2678. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-S.; Jang, I.; Rhyee, J.-S.; Hong, S.-J.; Jong Yoo, S.; Park, I.K. Enhanced thermoelectric performance of Mo nanoparticle decorated n-type Bi2Te2.7Se0.3 powder composites. Appl. Surf. Sci. 2021, 548, 149200. [Google Scholar] [CrossRef]
- Min, Y.; Park, G.; Kim, B.; Giri, A.; Zeng, J.; Roh, J.W.; Kim, S.I.; Lee, K.H.; Jeong, U. Synthesis of multi-shell nanoplates by consecutive epitaxial growth of Bi2Se3 and Bi2Te3 nanoplates and enhanced thermoelectric properties. ACS Nano 2015, 9, 6843–6853. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Luo, Y.; Liu, Y.; Shi, J.; Xiong, R.; Wang, Z. Synergistical tuning interface barrier and phonon propagation in Au–Sb2Te3 nanoplate for boosting thermoelectric performance. J. Phys. Chem. Lett. 2019, 10, 4903–4909. [Google Scholar] [CrossRef] [PubMed]
- Kohashi, K.; Yamamoto, H.; Okano, Y.; Kaneko, K.; Miyake, S.; Takashiri, M. Low-dimensional heterostructures of tin nanoparticle-decorated Bi2Te3 nanoplates for reducing lattice thermal conductivity. Ceram. Int. 2024, 50, 764–771. [Google Scholar] [CrossRef]
- Chernov, A.A. Modern Crystallography III: Crystal Growth; Springer: Berlin, Germany, 1984; pp. 85–103. [Google Scholar]
- Cui, X.; Hutt, D.A.; Scurr, D.J.; Conway, P.P. The evolution of Pd∕Sn catalytic surfaces in electroless copper deposition. J. Electrochem. Soc. 2011, 158, D172. [Google Scholar] [CrossRef]
- Lin, W.P.; Wesolowski, D.E.; Lee, C.C. Barrier/bonding layers on bismuth telluride (Bi2Te3) for high temperature thermoelectric modules. J. Mater. Sci. Mater. Electron. 2011, 22, 1313–1320. [Google Scholar] [CrossRef]
- Takashiri, M.; Kai, S.; Wada, K.; Takasugi, S.; Tomita, K. Role of stirring assist during solvothermal synthesis for preparing single-crystal bismuth telluride hexagonal nanoplates. Mater. Chem. Phys. 2016, 173, 213–218. [Google Scholar] [CrossRef]
- Wada, K.; Tomita, K.; Takashiri, M. Fabrication of bismuth telluride nanoplates via solvothermal synthesis using different alkalis and nanoplate thin films by printing method. J. Cryst. Growth 2017, 468, 194–198. [Google Scholar] [CrossRef]
- Yamazaki, H.; Eguchi, R.; Takashiri, M. Investigation of phase transition from critical nucleus to Bi2Te3 nanoplate based on screw dislocation-driven spiral growth by solvothermal synthesis. Cryst. Res. Technol. 2021, 56, 2100153. [Google Scholar] [CrossRef]
- Yae, S.; Nasu, N.; Matsumoto, K.; Hagihara, T.; Fukumuro, N.; Matsuda, H. Nucleation behavior in electroless displacement deposition of metals on silicon from hydrofluoric acid solutions. Electrochim. Acta 2007, 53, 35–41. [Google Scholar] [CrossRef]
- Nagata, S.; Nishi, T.; Miyake, S.; Azuma, N.; Hatori, K.; Awano, T.; Ohta, H. Development of novel thermal diffusivity analysis by spot periodic heating and infrared radiation thermometer method. Materials 2020, 13, 4848. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.J.; Jenkins, R.J.; Butler, C.P.; Abbott, G.L. Flash method of determining thermal diffusivity, heat capacity, and thermal conductivity. J. Appl. Phys. 1961, 32, 1679–1684. [Google Scholar] [CrossRef]
- Takashiri, M. Thin Films of Bismuth-Telluride-Based Alloys. In Thermoelectric Thin Films; Mele, P., Narducci, D., Ohta, M., Biswas, K., Morante, J., Saini, S., Endo, T., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–30. [Google Scholar]
- Hatsuta, N.; Takemori, D.; Takashiri, M. Effect of thermal annealing on the structural and thermoelectric properties of electrodeposited antimony telluride thin films. J. Alloys Compd. 2016, 685, 147–152. [Google Scholar] [CrossRef]
- Yamauchi, K.; Mori, R.; Yamaguchi, M.; Takashiri, M. Thermoelectric properties including thermal conductivity of electrodeposited bismuth selenide thin films fabricated using different acid solutions. J. Alloys Compd. 2019, 792, 222–229. [Google Scholar] [CrossRef]
- Al-Alam, P.; Pernot, G.; Isaiev, M.; Lacroix, D.; Vos, M.D.; Stein, N.; Osenberg, D.; Philippe, L. Lattice thermal conductivity of Bi2Te3 and SnSe using Debye-Callaway and Monte Carlo phonon transport modeling: Application to nanofilms and nanowires. Phys. Rev. B 2019, 100, 115304. [Google Scholar] [CrossRef]
- He, S.; Lehmann, S.; Bahrami, A.; Nielsch, K. Current state-of-the-art in the interface/surface modification of thermoelectric materials. Adv. Energy Mater. 2021, 11, 2101877. [Google Scholar] [CrossRef]
- Singkaselit, K.; Sakulkalavek, A.; Sakdanuphab, R. Effects of annealing temperature on the structural, mechanical and electrical properties of flexible bismuth telluride thin films prepared by high pressure RF magnetron sputtering. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 035002. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, S.-I.; Lim, J.-C.; Cho, J.Y.; Yang, H.; Kim, H.-S. Approach to determine the density-of-states effective mass with carrier concentration-dependent Seebeck coefficient. Adv. Funct. Mater. 2022, 32, 2203852. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, X.-S.; Nan, C.-W. Solvothermal preparation and thermoelectric properties of ternary Sn–Bi–Te alloy. Phys. Status Solidi A 2003, 199, 265–271. [Google Scholar] [CrossRef]
- Yu, F.; Xu, B.; Zhang, J.; Yu, D.; He, J.; Liu, Z.; Tian, Y. Structural and thermoelectric characterizations of high pressure sintered nanocrystalline Bi2Te3 bulks. Mater. Res. Bull. 2012, 47, 1432–1437. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohashi, K.; Okano, Y.; Tanisawa, D.; Kaneko, K.; Miyake, S.; Takashiri, M. Surface Modification of Bi2Te3 Nanoplates Deposited with Tin, Palladium, and Tin/Palladium Using Electroless Deposition. Crystals 2024, 14, 132. https://doi.org/10.3390/cryst14020132
Kohashi K, Okano Y, Tanisawa D, Kaneko K, Miyake S, Takashiri M. Surface Modification of Bi2Te3 Nanoplates Deposited with Tin, Palladium, and Tin/Palladium Using Electroless Deposition. Crystals. 2024; 14(2):132. https://doi.org/10.3390/cryst14020132
Chicago/Turabian StyleKohashi, Kaito, Yutaro Okano, Daiki Tanisawa, Keisuke Kaneko, Shugo Miyake, and Masayuki Takashiri. 2024. "Surface Modification of Bi2Te3 Nanoplates Deposited with Tin, Palladium, and Tin/Palladium Using Electroless Deposition" Crystals 14, no. 2: 132. https://doi.org/10.3390/cryst14020132
APA StyleKohashi, K., Okano, Y., Tanisawa, D., Kaneko, K., Miyake, S., & Takashiri, M. (2024). Surface Modification of Bi2Te3 Nanoplates Deposited with Tin, Palladium, and Tin/Palladium Using Electroless Deposition. Crystals, 14(2), 132. https://doi.org/10.3390/cryst14020132






