RE0.01Sr0.99Co0.5Fe0.5O3 (RE = La, Pr, and Sm) Cathodes for SOFC
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
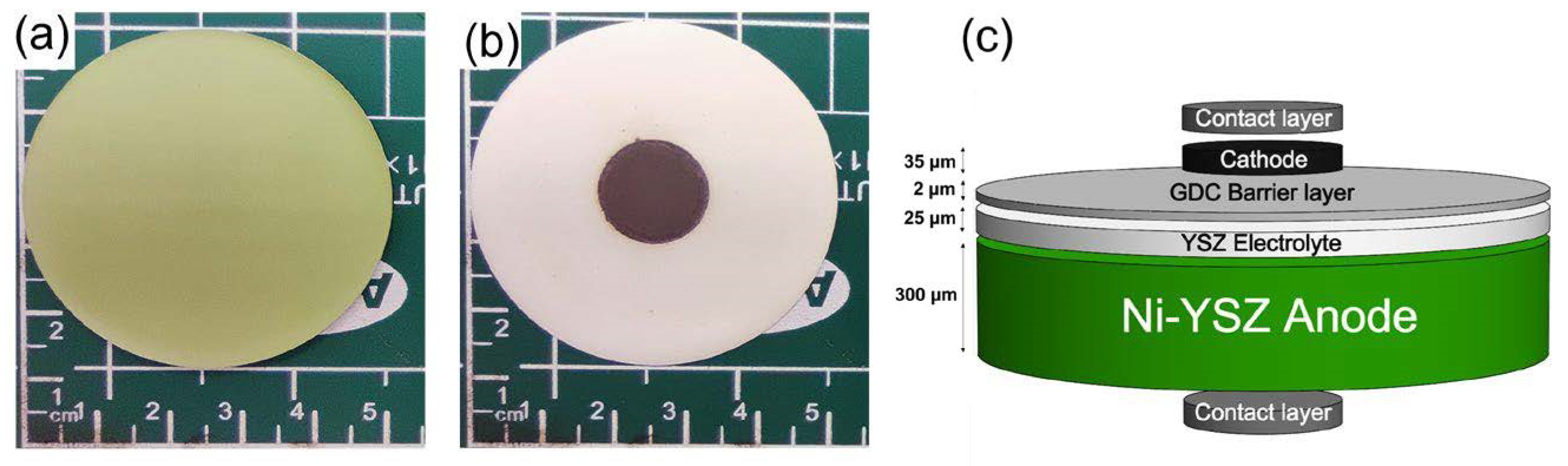
2.2. Material Synthesis and Cell Fabrication
2.3. Characterization
3. Results and Discussions
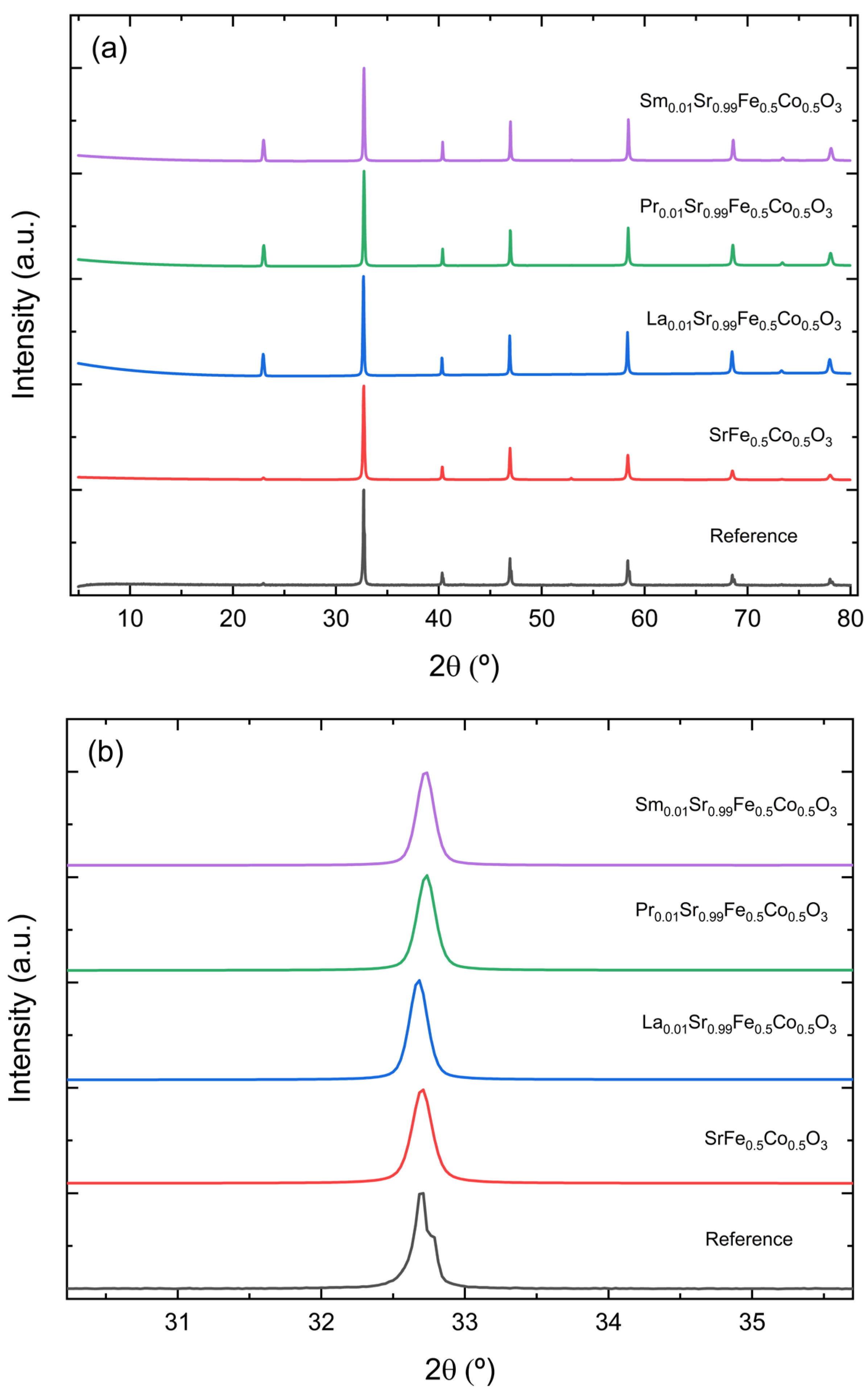
3.1. X-ray Powder Patterns
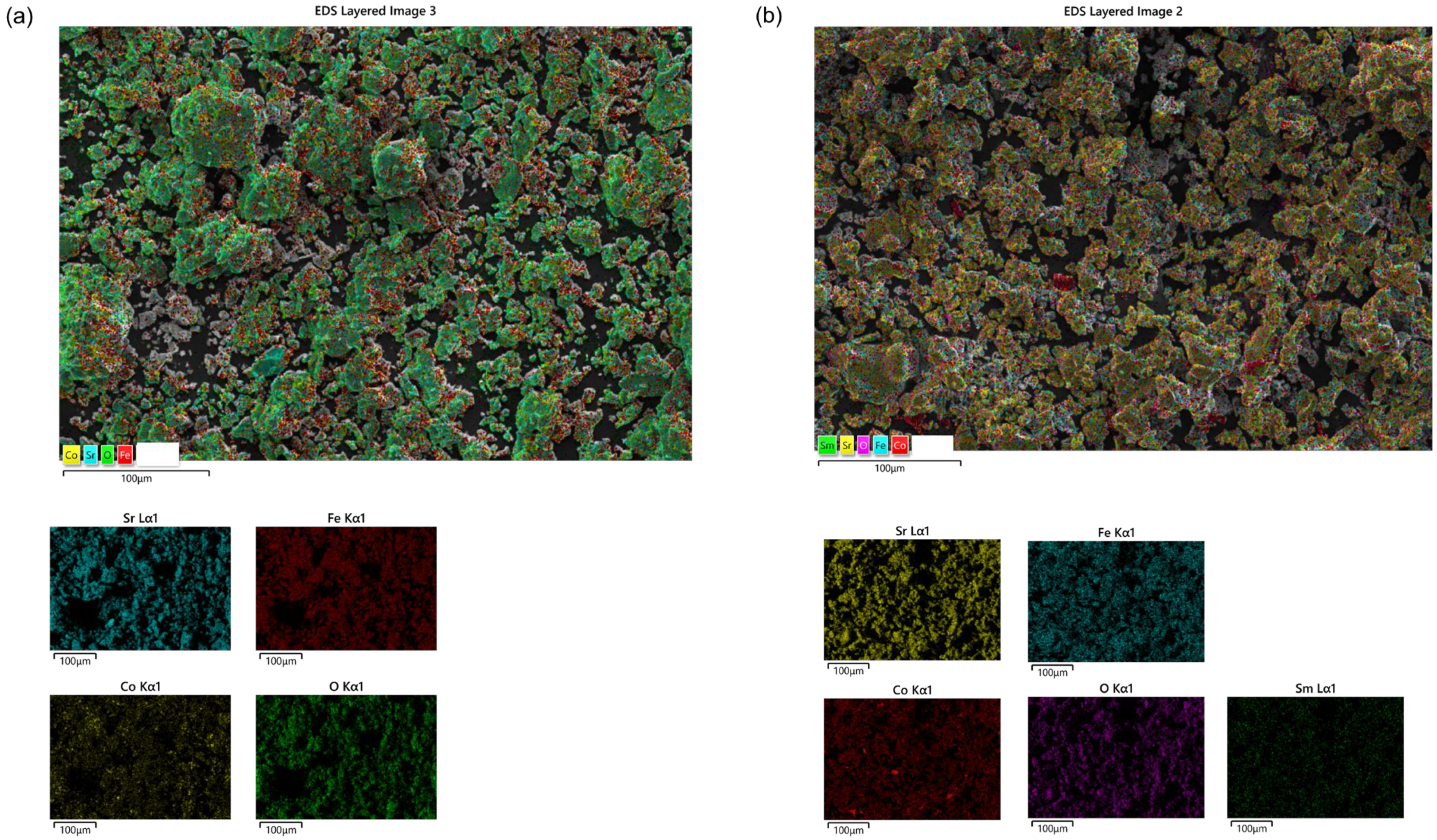
3.2. Morphological Analysis
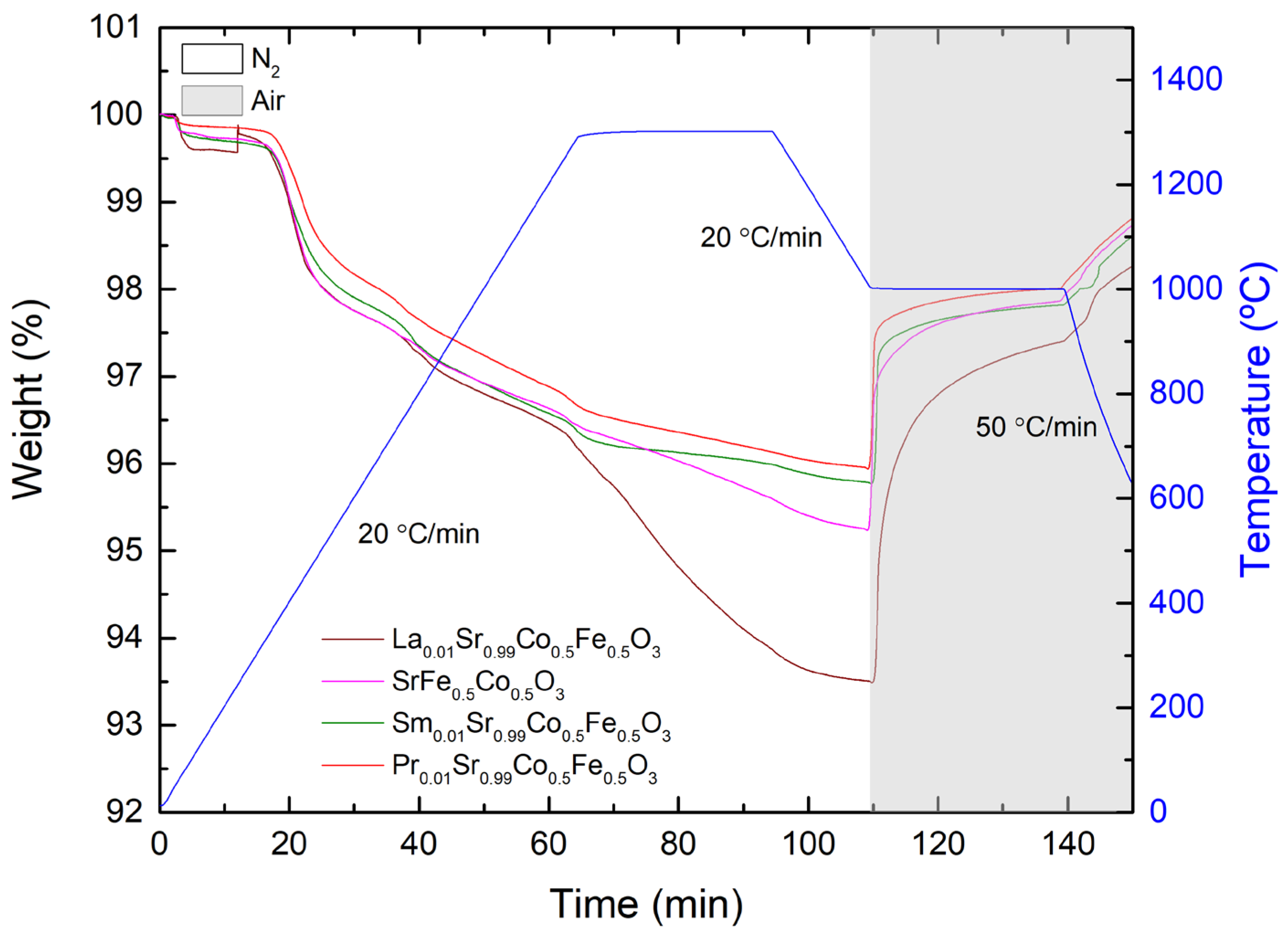
3.3. Thermogravimetric Analysis
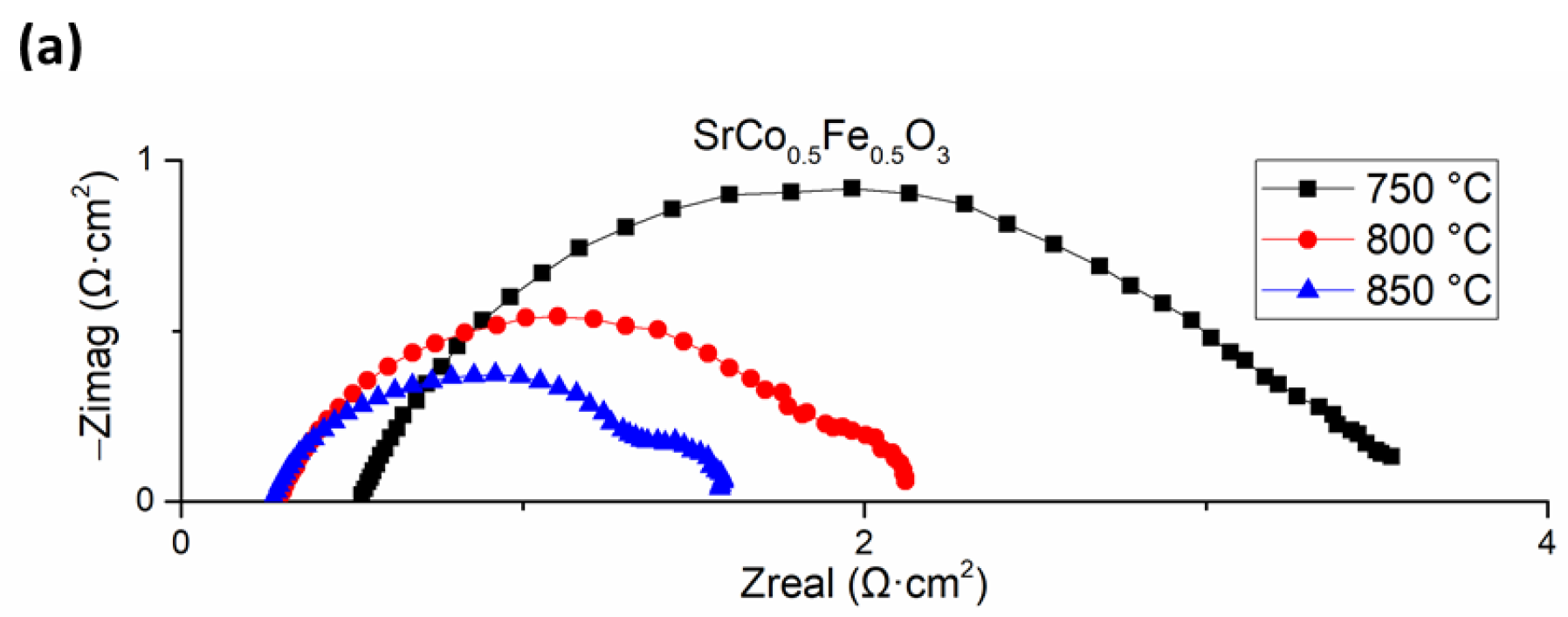
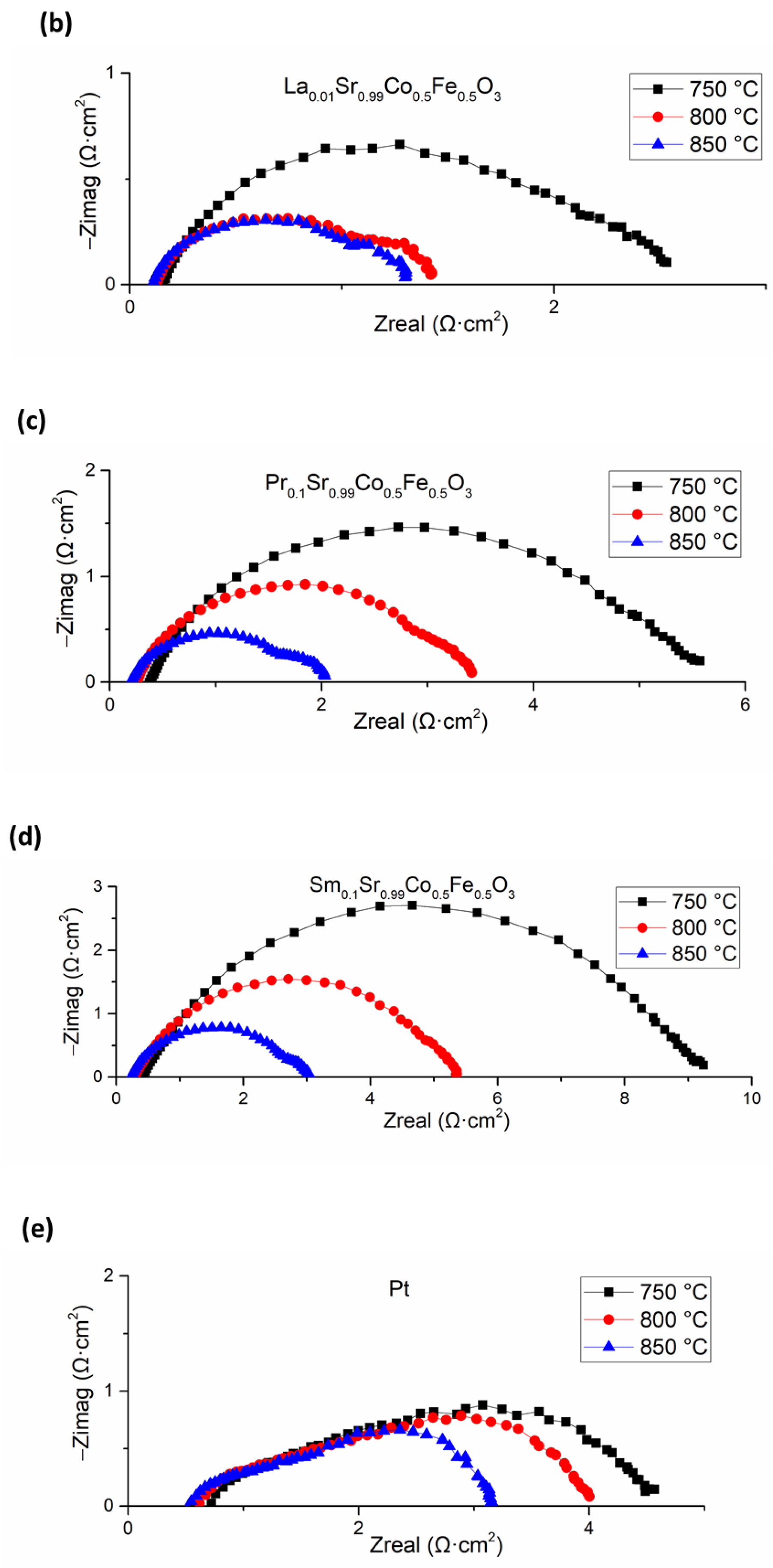
3.4. Cell Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Choudhury, A.; Chandra, H.; Arora, A. Application of Solid Oxide Fuel Cell Technology for Power Generation—A Review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Esposito, V.; Garbayo, I.; Linderoth, S.; Pryds, N. Solid-Oxide Fuel Cells. In Epitaxial Growth of Complex Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2015; pp. 443–478. [Google Scholar]
- Chen, D.; Chen, X. Luminescent Perovskite Quantum Dots: Synthesis, Microstructures, Optical Properties and Applications. J. Mater. Chem. C Mater. 2019, 7, 1413–1446. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]
- Bera, S.; Pradhan, N. Perovskite Nanocrystal Heterostructures: Synthesis, Optical Properties, and Applications. ACS Energy Lett. 2020, 5, 2858–2872. [Google Scholar] [CrossRef]
- Rodríguez-Romero, J.; Clasen Hames, B.; Galar, P.; Fakharuddin, A.; Suarez, I.; Schmidt-Mende, L.; Martínez-Pastor, J.P.; Douhal, A.; Mora-Seró, I.; Barea, E.M. Tuning Optical/Electrical Properties of 2D/3D Perovskite by the Inclusion of Aromatic Cation. Phys. Chem. Chem. Phys. 2018, 20, 30189–30199. [Google Scholar] [CrossRef]
- Yang, F.; Li, M.; Li, L.; Wu, P.; Pradal-Velázquez, E.; Sinclair, D.C. Defect Chemistry and Electrical Properties of Sodium Bismuth Titanate Perovskite. J. Mater. Chem. A Mater. 2018, 6, 5243–5254. [Google Scholar] [CrossRef]
- Schade, L.; Wright, A.D.; Johnson, R.D.; Dollmann, M.; Wenger, B.; Nayak, P.K.; Prabhakaran, D.; Herz, L.M.; Nicholas, R.; Snaith, H.J.; et al. Structural and Optical Properties of Cs2AgBiBr6 Double Perovskite. ACS Energy Lett. 2019, 4, 299–305. [Google Scholar] [CrossRef]
- Świerczek, K.; Yoshikura, N.; Zheng, K.; Klimkowicz, A. Correlation between Crystal and Transport Properties in LnBa0.5Sr0.5Co1.5Fe0.5O5+δ (Ln—Selected Lanthanides, Y). Solid. State Ion. 2014, 262, 645–649. [Google Scholar] [CrossRef]
- Smith, A.J.; Welch, A.J.E. Some Mixed Metal Oxides of Perovskite Structure. Acta Crystallogr. 1960, 13, 653–656. [Google Scholar] [CrossRef]
- Luo, P.; Liu, Z.; Xia, W.; Yuan, C.; Cheng, J.; Lu, Y. Uniform, Stable, and Efficient Planar-Heterojunction Perovskite Solar Cells by Facile Low-Pressure Chemical Vapor Deposition under Fully Open-Air Conditions. ACS Appl. Mater. Interfaces 2015, 7, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Sunarso, J.; Hashim, S.S.; Zhu, N.; Zhou, W. Perovskite Oxides Applications in High Temperature Oxygen Separation, Solid Oxide Fuel Cell and Membrane Reactor: A Review. Prog. Energy Combust. Sci. 2017, 61, 57–77. [Google Scholar] [CrossRef]
- Kaus, I.; Wiik, K.; Krogh, B.; Dahle, M.; Hofstad, K.H.; Aasland, S. Stability of SrFeO3-Based Materials in H2O/CO2-Containing Atmospheres at High Temperatures and Pressures. J. Am. Ceram. Soc. 2007, 90, 2226–2230. [Google Scholar] [CrossRef]
- Klug, M.T.; Osherov, A.; Haghighirad, A.A.; Stranks, S.D.; Brown, P.R.; Bai, S.; Wang, J.T.W.; Dang, X.; Bulović, V.; Snaith, H.J.; et al. Tailoring Metal Halide Perovskites through Metal Substitution: Influence on Photovoltaic and Material Properties. Energy Environ. Sci. 2017, 10, 236–246. [Google Scholar] [CrossRef]
- Giorgi, G.; Fujisawa, J.I.; Segawa, H.; Yamashita, K. Cation Role in Structural and Electronic Properties of 3D Organic-Inorganic Halide Perovskites: A DFT Analysis. J. Phys. Chem. C 2014, 118, 12176–12183. [Google Scholar] [CrossRef]
- Troncoso, L.; Gardey, M.C.; Fernández-Díaz, M.T.; Alonso, J.A. New Rhenium-Doped SrCo1-XRexO3-δ Perovskites Performing as Cathodes in Solid Oxide Fuel Cells. Materials 2016, 9, 717. [Google Scholar] [CrossRef]
- Wain-Martin, A.; Campana, R.; Morán-Ruiz, A.; Larrañaga, A.; Arriortua, M.I. Synthesis and Processing of SOFC Components for the Fabrication and Characterization of Anode Supported Cells. Bol. Soc. Esp. Ceram. Vidr. 2022, 61, 264–274. [Google Scholar] [CrossRef]
- Marcucci, A.; Zurlo, F.; Sora, I.N.; Placidi, E.; Casciardi, S.; Licoccia, S.; Di Bartolomeo, E. A Redox Stable Pd-Doped Perovskite for SOFC Applications. J. Mater. Chem. A Mater. 2019, 7, 5344–5352. [Google Scholar] [CrossRef]
- Grunbaum, N.; Mogni, L.; Prado, F.; Caneiro, A. Phase Equilibrium and Electrical Conductivity of SrCo0.8Fe0.2O3-δ. J. Solid. State Chem. 2004, 177, 2350–2357. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, H.; Xu, N.; Shen, Y.; Ding, W.; Lu, X.; Li, F. Electrical Conductivity and Structural Stability of SrCo1-XFexO3-δ. J. Phys. Chem. Solids 2011, 72, 50–55. [Google Scholar] [CrossRef]
- Guo, W.; Cui, L.; Xu, H.; Gong, C. Selective Dissolution of A-Site Cations of La0.6Sr0.4Co0.8Fe0.2O3 Perovskite Catalysts to Enhance the Oxygen Evolution Reaction. Appl. Surf. Sci. 2020, 529, 147165. [Google Scholar] [CrossRef]
- Li, L.; Song, J.; Lu, Q.; Tan, X. Synthesis of Nano-Crystalline Sm0.5Sr0.5Co(Fe)O3-δ Perovskite Oxides by a Microwave-Assisted Sol-Gel Combustion Process. Ceram. Int. 2014, 40, 1189–1194. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G.; Molera, P.; Salán Ballesteros, M.N. Ciencia e Ingeniería de Los Materiales; Callister, W.D., Translator; Reverté, S.A., Ed.; Cengage: Barcelona, Spain, 2016; ISBN 9788429172515. [Google Scholar]
- Skakle, J.M.S.; West, A.R. Superconducting La1.5-xBa1.5+x-yCayCu3Oz Solid Solutions I. Phase Diagram, Cation Stoichiometry and Tc Data. Phys. C 1994, 220, 187–194. [Google Scholar] [CrossRef]
- Barón-González, A.J.; Frontera, C.; García-Muñoz, J.L.; Blasco, J.; Ritter, C.; Valencia, S.; Feyerhermd, R.; Dudzik, E. Exploration of Magnetic Order in Pr0.5Ca0.5CoO3-δ (δ=0) below the Metal-Insulator Transition. Phys. Procedia 2010, 8, 73–77. [Google Scholar] [CrossRef]
- Webber, K.G.; Vögler, M.; Khansur, N.H.; Kaeswurm, B.; Daniels, J.E.; Schader, F.H. Review of the Mechanical and Fracture Behavior of Perovskite Lead-Free Ferroelectrics for Actuator Applications. Smart Mater. Struct. 2017, 26, 063001. [Google Scholar] [CrossRef]
- Misra, R.K.; Cohen, B.A.-E.; Etgar, L. Low-Dimensional Organic-Inorganic Halide Perovskite: Structure, Properties, and Applications. Chem. Sus. Chem. 2017, 10, 3712–3721. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Tomioka, Y. Colossal Magnetoresistive Manganites. J. Magn. Magn. Mater. 1999, 200, 1–23. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; El-Ads, E.H. Perovskite Nanomaterials—Synthesis, Characterization, and Applications. In Perovskite Materials—Synthesis, Characterisation, Properties, and Applications; InTech: London, UK, 2016. [Google Scholar]
- Pavel, C.C.; Lacal-Arántegui, R.; Marmier, A.; Schüler, D.; Tzimas, E.; Buchert, M.; Jenseit, W.; Blagoeva, D. Substitution Strategies for Reducing the Use of Rare Earths in Wind Turbines. Resour. Policy 2017, 52, 349–357. [Google Scholar] [CrossRef]
- Fan, J.H.; Omura, A.; Roca, E. Geopolitics and Rare Earth Metals. Eur. J. Polit. Econ. 2023, 78, 102356. [Google Scholar] [CrossRef]
- Massari, S.; Ruberti, M. Rare Earth Elements as Critical Raw Materials: Focus on International Markets and Future Strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Mogensen, M.; Jensen, K.V.; Jørgensen, J.; Primdahl, S. Progress in Understanding SOFC Electrodes. Solid. State Ion. 2002, 150, 123–129. [Google Scholar] [CrossRef]
- Simner, S.P.; Anderson, M.D.; Pederson, L.R.; Stevenson, J.W. Performance Variability of La(Sr)FeO3 SOFC Cathode with Pt, Ag, and Au Current Collectors. J. Electrochem. Soc. 2005, 152, A1851. [Google Scholar] [CrossRef]
- Mogensen, M.S.S. Kinetic and Geometric Aspects of Solid Oxide Fuel Cell Electrodes. Solid. State Ion. 1996, 86–88, 1151–1160. [Google Scholar] [CrossRef]
- Nielsen, J.; Hjelm, J. Impedance of SOFC Electrodes: A Review and a Comprehensive Case Study on the Impedance of LSM:YSZ Cathodes. Electrochim. Acta 2014, 115, 31–45. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, Q.; Ni, M.; Lyu, R.; Li, P.; Chan, S.H. Activation and Failure Mechanism of La0.6Sr0.4Co0.2Fe0.8O3−δ Air Electrode in Solid Oxide Electrolyzer Cells under High-Current Electrolysis. Int. J. Hydrogen Energy 2018, 43, 5437–5450. [Google Scholar] [CrossRef]
- Wain-Martin, A.; Morán-Ruiz, A.; Laguna-Bercero, M.A.; Campana, R.; Larrañaga, A.; Slater, P.R.; Arriortua, M.I. SOFC Cathodic Layers Using Wet Powder Spraying Technique with Self Synthesized Nanopowders. Int. J. Hydrogen Energy 2019, 44, 7555–7563. [Google Scholar] [CrossRef]
- Jabbari, M.; Bulatova, R.; Tok, A.I.Y.; Bahl, C.R.H.; Mitsoulis, E.; Hattel, J.H. Ceramic Tape Casting: A Review of Current Methods and Trends with Emphasis on Rheological Behaviour and Flow Analysis. Mater. Sci. Eng. B 2016, 212, 39–61. [Google Scholar] [CrossRef]
- Wang, Z.; Qian, J.; Cao, J.; Wang, S.; Wen, T. A Study of Multilayer Tape Casting Method for Anode-Supported Planar Type Solid Oxide Fuel Cells (SOFCs). J. Alloys Compd. 2007, 437, 264–268. [Google Scholar] [CrossRef]
- Song, J.H.; Park, S.I.; Lee, J.H.; Kim, H.S. Fabrication Characteristics of an Anode-Supported Thin-Film Electrolyte Fabricated by the Tape Casting Method for IT-SOFC. J. Mater. Process Technol. 2008, 198, 414–418. [Google Scholar] [CrossRef]
- Carpanese, M.P.; Barbucci, A.; Canu, G.; Viviani, M. BaCe0.85Y0.15O2.925 Dense Layer by Wet Powder Spraying as Electrolyte for SOFC/SOEC Applications. Solid. State Ion. 2015, 269, 80–85. [Google Scholar] [CrossRef]
- Marrero López, D. Síntesis y Caracterización de Nuevos Conductores Iónicos Basados En La2Mo2O9; Servicio de Publicaciones de la Universisdad de La Laguna: San Cristóbal de La Laguna, Spain, 2006; ISBN 84-7756-710-7. [Google Scholar]
- Yang, Z.; Yang, C.; Jin, C.; Han, M.; Chen, F. Ba0.9Co0.7Fe0.2Nb0.1O3−δ as Cathode Material for Intermediate Temperature Solid Oxide Fuel Cells. Electrochem. Commun. 2011, 13, 882–885. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L.; Guo, Y.; Jia, D.; Li, Y.; Wei, W.C.J. La0.6Sr0.4Fe0.8Cu0.2O3−δ Perovskite Oxide as Cathode for IT-SOFC. Int. J. Hydrog. Energy 2012, 37, 11963–11968. [Google Scholar] [CrossRef]
- Zhu, G.; Fang, X.; Xia, C.; Liu, X. Preparation and Electrical Properties of La0.4Sr0.6Ni0.2Fe0.8O3 Using a Glycine Nitrate Process. Ceram. Int. 2005, 31, 115–119. [Google Scholar] [CrossRef]
- Qiu, P.; Li, J.; Jia, L.; Chi, B.; Pu, J.; Li, J.; Chen, F. LaCoO3−δ Coated Ba0.5Sr0.5Co0.8Fe0.2O3-δ Cathode for Intermediate Temperature Solid Oxide Fuel Cells. Electrochim. Acta 2019, 319, 981–989. [Google Scholar] [CrossRef]




| Hkl Positions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| h | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 3 | 2 | 2 |
| k | 0 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 2 | 1 |
| l | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| 2θ | 22.9 | 32.7 | 40.3 | 46.9 | 52.8 | 58.3 | 68.5 | 73.3 | 73.3 | 78.0 |
| Sample | a (Å) | V(Å3) | RBragg | Rp | Rwp | Rexp | Χ2 |
|---|---|---|---|---|---|---|---|
| SrCo0.5Fe0.5O3 | 3.862 | 57.635 | 2.27 | 33.1 | 20.8 | 7.30 | 8.12 |
| La0.01Sr0.99 Co0.5Fe0.5O3 | 3.870 | 57.979 | 1.85 | 82.8 | 39.4 | 27.08 | 2.12 |
| Pr0.01Sr0.99Co0.5Fe0.5O3 | 3.868 | 57.915 | 0.036 | 37.6 | 25.7 | 7.50 | 11.7 |
| Sm0.01Sr0.99Co0.5Fe0.5O3 | 3.865 | 57.765 | 0.0141 | 60.3 | 37.1 | 11.07 | 11.2 |
| Sample | %Sr | %Fe | %Co | %O | %Rare Earth |
|---|---|---|---|---|---|
| SrCo0.5Fe0.5O3 | 35.43 | 30.93 | 9.63 | 24.01 | - |
| La0.01Sr0.99Co0.5Fe0.5O3 | 31.59 | 27.71 | 16.97 | 16.97 | 1.07 |
| Pr0.01Sr0.99Co0.5Fe0.5O3 | 35.63 | 27.35 | 13.57 | 22.48 | 0.97 |
| Sm0.01Sr0.99Co0.5Fe0.5O3 | 21.11 | 17.02 | 38.01 | 22.20 | 1.06 |
| T (°C) | SrCo0.5Fe0.5O3 | La0.01Sr0.99Co0.5Fe0.5O3 | Pr0.01Sr0.99Co0.5Fe0.5O3 | Sm0.01Sr0.99Co0.5Fe0.5O3 | Pt | |
|---|---|---|---|---|---|---|
| 750 | Rohm (Ω∙cm2) | 0.53 | 0.16 | 0.37 | 0.43 | 0.72 |
| 750 | Rpol (Ω∙cm2) | 3.01 | 2.37 | 5.2 | 8.81 | 3.84 |
| 750 | Rtotal (Ω∙cm2) | 3.54 | 2.53 | 5.57 | 9.24 | 4.56 |
| 800 | Rohm (Ω∙cm2) | 0.28 | 0.12 | 0.26 | 0.30 | 0.62 |
| 800 | Rpol (Ω∙cm2) | 1.84 | 1.30 | 3.16 | 5.04 | 3.38 |
| 800 | Rtotal (Ω∙cm2) | 2.12 | 1.42 | 3.42 | 5.34 | 4.01 |
| 850 | Rohm (Ω∙cm2) | 0.26 | 0.10 | 0.21 | 0.24 | 0.51 |
| 850 | Rpol (Ω∙cm2) | 1.32 | 1.20 | 1.82 | 2.80 | 2.63 |
| 850 | Rtotal (Ω∙cm2) | 1.58 | 1.30 | 2.03 | 3.04 | 3.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-González, S.; Campana, R.; Andújar, R.; Pardo, A.; Gil-Hernández, B.; Lozano-Gorrín, A.D. RE0.01Sr0.99Co0.5Fe0.5O3 (RE = La, Pr, and Sm) Cathodes for SOFC. Crystals 2024, 14, 143. https://doi.org/10.3390/cryst14020143
Díaz-González S, Campana R, Andújar R, Pardo A, Gil-Hernández B, Lozano-Gorrín AD. RE0.01Sr0.99Co0.5Fe0.5O3 (RE = La, Pr, and Sm) Cathodes for SOFC. Crystals. 2024; 14(2):143. https://doi.org/10.3390/cryst14020143
Chicago/Turabian StyleDíaz-González, Selene, Roberto Campana, Rocío Andújar, Adrián Pardo, Beatriz Gil-Hernández, and Antonio D. Lozano-Gorrín. 2024. "RE0.01Sr0.99Co0.5Fe0.5O3 (RE = La, Pr, and Sm) Cathodes for SOFC" Crystals 14, no. 2: 143. https://doi.org/10.3390/cryst14020143
APA StyleDíaz-González, S., Campana, R., Andújar, R., Pardo, A., Gil-Hernández, B., & Lozano-Gorrín, A. D. (2024). RE0.01Sr0.99Co0.5Fe0.5O3 (RE = La, Pr, and Sm) Cathodes for SOFC. Crystals, 14(2), 143. https://doi.org/10.3390/cryst14020143







