Coumarin Derivatives: The Influence of Cycloalkyl Groups at the C-3 Position on Intermolecular Interactions—Synthesis, Structure and Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.1.1. General
2.1.2. X-ray Crystallography
2.2. General Procedure for Synthesis of Coumarins CMR 1–4
3. Results and Discussion
3.1. Synthesis of Coumarins
3.2. NMR and FTIR Spectra of Coumarins
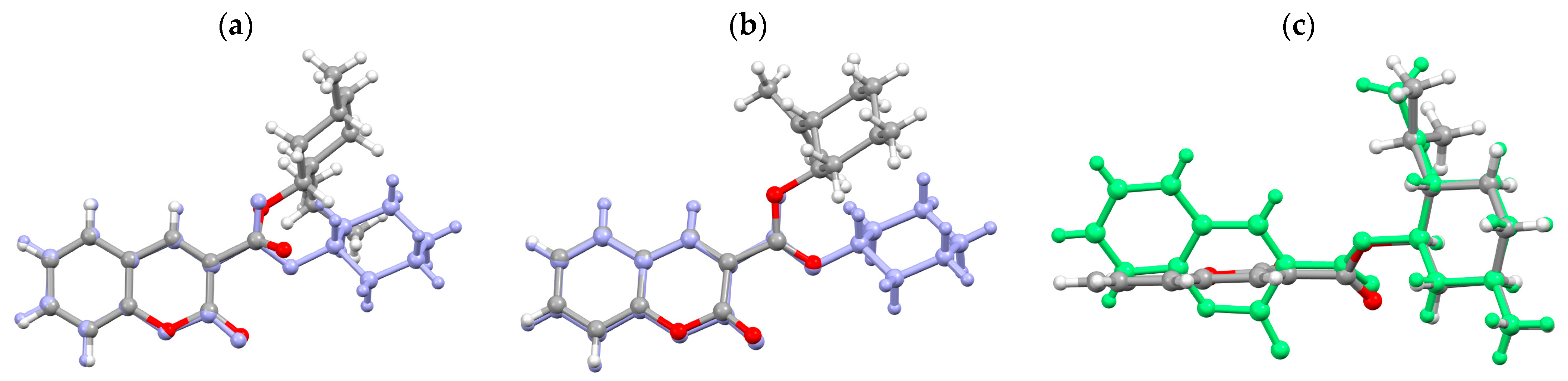
3.3. Molecular Structure
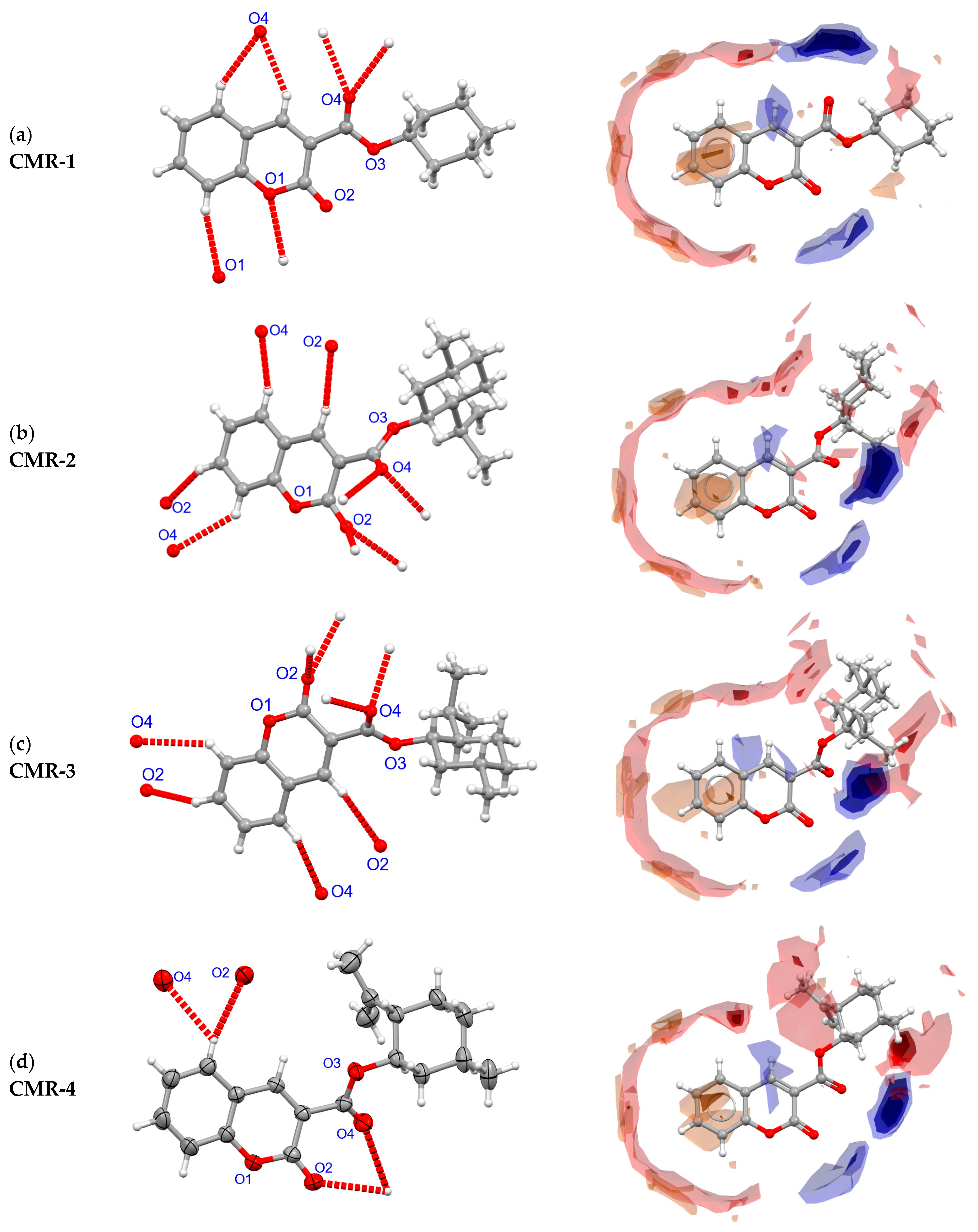
3.4. Crystal Structure and Intermolecular Contacts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Todorov, L.; Saso, L.; Kostova, I. Antioxidant Activity of Coumarins and Their Metal Complexes. Pharmaceuticals 2023, 16, 651. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, P.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F.; et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms, Oxidative Medicine and Cellular Longevity. Oxidative Med. Cell. Longev. 2021, 2021, 6492346. [Google Scholar] [CrossRef]
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules 2023, 28, 2413. [Google Scholar] [CrossRef]
- Patil, S.A.; Kandathil, V.; Sobha, A.; Somappa, S.B.; Feldman, M.R.; Bugarin, A.; Patil, S.A. Comprehensive Review on Medicinal Applications of Coumarin-Derived Imine–Metal Complexes. Molecules 2022, 27, 5220. [Google Scholar] [CrossRef]
- Shaik, B.B.; Katari, N.K.; Seboletswe, P.; Gundla, R.; Kushwaha, N.D.; Kumar, V.; Singh, P.; Karpoormath, R.; Bala, M.D. Recent Literature Review on Coumarin Hybrids as Potential Anticancer Agents. Anticancer Agents Med. Chem. 2023, 23, 142–163. [Google Scholar] [CrossRef]
- Toan, D.N.; Thanh, N.D.; Truong, M.X.; Van, D.T.; Thanh, N.N. Design, synthesis, molecular docking study and molecular dynamics simulation of new coumarin-pyrimidine hybrid compounds having anticancer and antidiabetic activity. Med. Chem. Res. 2023, 32, 1143–1162. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.S.; Kumar, A.; Kaur, K.; Jaitak, V. Recent Developments in Coumarin Derivatives as Neuroprotective Agents. Curr. Med. Chem. 2023; in press. [Google Scholar] [CrossRef]
- Shefali, S.; Pragya, G.; Ameya, K.; Arush, A.; Sharda, P. Exploring Coumarin and Chalcone Analogues as Potential Antimycobacterial Agents. Anti-Infect. Agents 2017, 15, 69–86. [Google Scholar] [CrossRef]
- Sarmah, M.; Chutia, K.; Dutta, D.; Gogoi, P. Overview of coumarin-fused-coumarins: Synthesis, photophysical properties and their applications. Org. Biomol. Chem. 2022, 20, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Mirmahalleh, S.-A.; Golshan, M.; Gheitarani, B.; Hosseini, M.S.; Salami-Kalajahi, M. A review on applications of coumarin and its derivatives in preparation of photo-responsive polymers. Eur. Polym. J. 2023, 198, 112430. [Google Scholar] [CrossRef]
- Szwaczko, K. Fluorescent Coumarin-based Probe for Detection of Biological Thiols. Curr. Org. Chem. 2023, 27, 1329–1335. [Google Scholar] [CrossRef]
- Foroozesh, M.; Sridhar, J.; Goyal, N.; Liu, J. Coumarins and P450s, Studies Reported to-Date. Molecules 2019, 24, 1620. [Google Scholar] [CrossRef]
- Berestetskiy, A. Modern Approaches for the Development of New Herbicides Based on Natural Compounds. Plants 2023, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-X.; Wang, Z.-X.; Peng, J.-F.; Zou, Y.-L.; Hui, Y.-Z.; Chen, Y.-Z.; Gao, S.; Fu, Y.; Ye, F. Design, synthesis, and herbicidal activity of novel phenoxypyridine derivatives containing natural product coumarin. Pest Manag. Sci. 2021, 77, 4785–4798. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L. Progress in the Chemistry of Naturally Occurring Coumarins. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer: Cham, Switzerland, 2017; Volume 106, pp. 241–304. [Google Scholar] [CrossRef]
- Liu, Y.P.; Yan, G.; Xie, Y.T.; Lin, T.-C.; Zhang, W.; Li, J.; Wu, Y.-J.; Zhou, Y.-J.; Fu, Y.-H. Bioactive prenylated coumarins as potential anti-inflammatory and anti-HIV agents from Clausena lenis. Bioorg. Chem. 2020, 97, 103699. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Luo, F.; Manse, Y.; Sugita, H.; Saeki, S.; Chaipech, S.; Pongpiriyadacha, Y.; Muraoka, O.; Ninomiya, K. Geranylated Coumarins From Thai Medicinal Plant Mammea siamensis with Testosterone 5α-Reductase Inhibitory Activity. Front. Chem. 2020, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.A.; Shevchenko, O.G.; Chukicheva, I.Y.; Kutchin, A.V. Synthesis and biological evaluation of novel coumarins with tert-butyl and terpene substituents. Chem. Biodivers. 2019, 16, e1800317. [Google Scholar] [CrossRef]
- Tayarani-Najaran, Z.; Tayarani-Najaran, N.; Eghbali, S. A Review of Auraptene as an Anticancer Agent. Front. Pharmacol. 2021, 12, 698352. [Google Scholar] [CrossRef] [PubMed]
- Bibak, B.; Shakeri, F.; Barreto, G.E.; Keshavarzi, Z.; Sathyapalan, T.; Sahebkar, A. A Review of the Pharmacological and Therapeutic Effects of Auraptene. BioFactors 2019, 45, 867–879. [Google Scholar] [CrossRef]
- Zafar, S.; Sarfraz, I.; Rasul, A.; Shah, M.A.; Hussain, G.; Zahoor, M.K.; Shafiq, N.; Riaz, A.; Selamoglu, Z.; Sarker, S.D. Osthole: A Multifunctional Natural Compound with Potential Anticancer, Antioxidant and Anti-inflammatory Activities. Mini Rev. Med. Chem. 2021, 21, 2747–2763. [Google Scholar] [CrossRef]
- Chen, J.; Liao, X.; Gan, J. Review on the protective activity of osthole against the pathogenesis of osteoporosis. Front. Pharmacol. 2023, 14, 1236893. [Google Scholar] [CrossRef]
- Zili, R.; Min, L.; Hui, X. Osthole: Synthesis, Structural Modifications, and Biological Properties. Mini Rev. Med. Chem. 2022, 22, 2124–2137. [Google Scholar] [CrossRef]
- Kozioł, E.; Skalicka-Woźniak, K. Imperatorin–pharmacological meaning and analytical clues: Profound investigation. Phytochem. Rev. 2016, 15, 627–649. [Google Scholar] [CrossRef]
- Neel, M.; Gouin, J.; Voituriez, A.; Marinetti, A. Phosphine-catalyzed [3+2] cyclizations: Applications to the enantioselective synthesis of cyclopentene-fused chromanones and dihydroquinolinones. Synthesis 2011, 12, 2003–2009. [Google Scholar] [CrossRef]
- Ichikawa, A.; Ono, H.; Harada, N. Stereochemical Studies of Chiral Resolving Agents, M9PP and H9PP Acids. Chirality 2004, 16, 559–567. [Google Scholar] [CrossRef]
- Xu, C.L.; Liu, S.Y.; Chen, G.; Yang, G.Y.; Zhao, M.Q. Menthyl 2-oxo-2H-chromene-3-carboxyl-ate. Acta Cryst. 2009, E65, o2431. [Google Scholar] [CrossRef]
- Škoch, K.; Císařová, I.; Štěpnička, P. Crystal structure of prop-2-en-1-yl 2-oxo-2H-1-benzopyran-3-carboxylate, C13H10O4. Z. Kristallogr. NCS 2016, 231, 609–611. [Google Scholar] [CrossRef]
- Saeed, A.; Ibrar, A.; Arshad, M.; Bolte, M. Methyl 2-oxo-2H-chromene-3-carboxylate. Acta Cryst. 2012, E68, o3024. [Google Scholar] [CrossRef]
- Nowatschin, V.; Nather, C.; Luning, U. Synthesis and crystal structure of allyl 7-(diethyl-amino)-2-oxo-2H-chromene-3-carboxylate. Acta Cryst. 2021, 77, 331–334. [Google Scholar] [CrossRef]
- Yavari, I.; Djahaniani, H.; Nasiri, F. The crystal structure of tert-butyl coumarin-3-carboxylate. Iran. Chem. Soc. 2006, 3, 46–50. [Google Scholar] [CrossRef]
- Gu, J.; Xiao, P.L.; Wang, J.; Zhong, L.; Nie, X.-Y.; Peng, D.-Y. Synthesis, crystal structure, spectroscopic characterization and anti-fungal activity of Ethyl 2-Oxo-2H-chromene-3-carboxylate Derivatives. J. Mol. Struct. 2022, 1257, 132576. [Google Scholar] [CrossRef]
- User Manual Agilent Technologies Inc.: Yarnton, UK, 2014.
- CrysAlisPro 1.171.42.79a; Rigaku Oxford Diffraction: Tokyo, Japan, 2022.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dinparast, L.; Hemmati, S.; Zengin, G.; Alizadeh, A.A.; Bahadori, M.B.; Kafil, H.S.; Dastmalchi, S. Rapid, efficient, and green synthesis of coumarin derivatives via Knoevenagel condensation and investigating their biological effects. Chem. Sel. 2019, 4, 9211–9215. [Google Scholar] [CrossRef]
- He, X.; Shang, Y.; Zhou, Y.; Yu, Z.; Han, G.; Jin, W.; Chen, J. Synthesis of coumarin-3-carboxylic esters via FeCl3-catalyzed multicomponent reaction of salicylaldehydes, Meldrum’s acid and alcohols. Tetrahedron 2015, 71, 863–868. [Google Scholar] [CrossRef]
- Neises, B.; Steglich, W. Simple Method for the Esterification of Carboxylic Acids. Angew. Chem. Int. 1978, 17, 522–524. [Google Scholar] [CrossRef]
- Shinde, S.V.N.; Kumar, A. KPF6-Mediated esterification and amidation of carboxylic acids. J. Org. Chem. 2022, 87, 2651–2661. [Google Scholar] [CrossRef]
- Munawar, S.; Zahoor, A.F.; Hussain, S.M.; Ahmad, S.; Mansha, A.; Parveen, B.; Ali, K.G.; Irfan, A. Steglich esterification: A versatile synthetic approach toward the synthesis of natural products, their analogues/derivatives. Heliyon 2024, 10, e23416. [Google Scholar] [CrossRef]
- Jordan, A.; Whymark, K.D.; Sydenham, J.; Sneddon, H.F. A solvent-reagent selection guide for Steglich-type esterification of carboxylic acids. Green Chem. 2021, 23, 6405–6413. [Google Scholar] [CrossRef]
- Brahmachari, G. Room temperature one-pot green synthesis of coumarin-3-carboxylic acids in water: A practical method for the large-scale synthesis. ACS Sustain. Chem. Eng. 2015, 3, 2350–2358. [Google Scholar] [CrossRef]
- Kuang, Y.; Liu, X.; Chang, L.; Wang, M.; Lin, L.; Feng, X. Catalytic Asymmetric Conjugate Allylation of Coumarins. Org. Lett. 2011, 13, 3814–3817. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwaczko, K.; Kamiński, D.M.; Koziol, A.E. Coumarin Derivatives: The Influence of Cycloalkyl Groups at the C-3 Position on Intermolecular Interactions—Synthesis, Structure and Spectroscopy. Crystals 2024, 14, 196. https://doi.org/10.3390/cryst14020196
Szwaczko K, Kamiński DM, Koziol AE. Coumarin Derivatives: The Influence of Cycloalkyl Groups at the C-3 Position on Intermolecular Interactions—Synthesis, Structure and Spectroscopy. Crystals. 2024; 14(2):196. https://doi.org/10.3390/cryst14020196
Chicago/Turabian StyleSzwaczko, Katarzyna, Daniel M. Kamiński, and Anna E. Koziol. 2024. "Coumarin Derivatives: The Influence of Cycloalkyl Groups at the C-3 Position on Intermolecular Interactions—Synthesis, Structure and Spectroscopy" Crystals 14, no. 2: 196. https://doi.org/10.3390/cryst14020196




