Measurement of Reverse-Light-Induced Excited Spin State Trapping in Spin Crossover Systems: A Study Case with Zn1−xFex(6-mepy)3tren(PF6)2·CH3CN; x = 0.5%
Abstract
:1. Introduction
2. Materials and Methods
3. Results
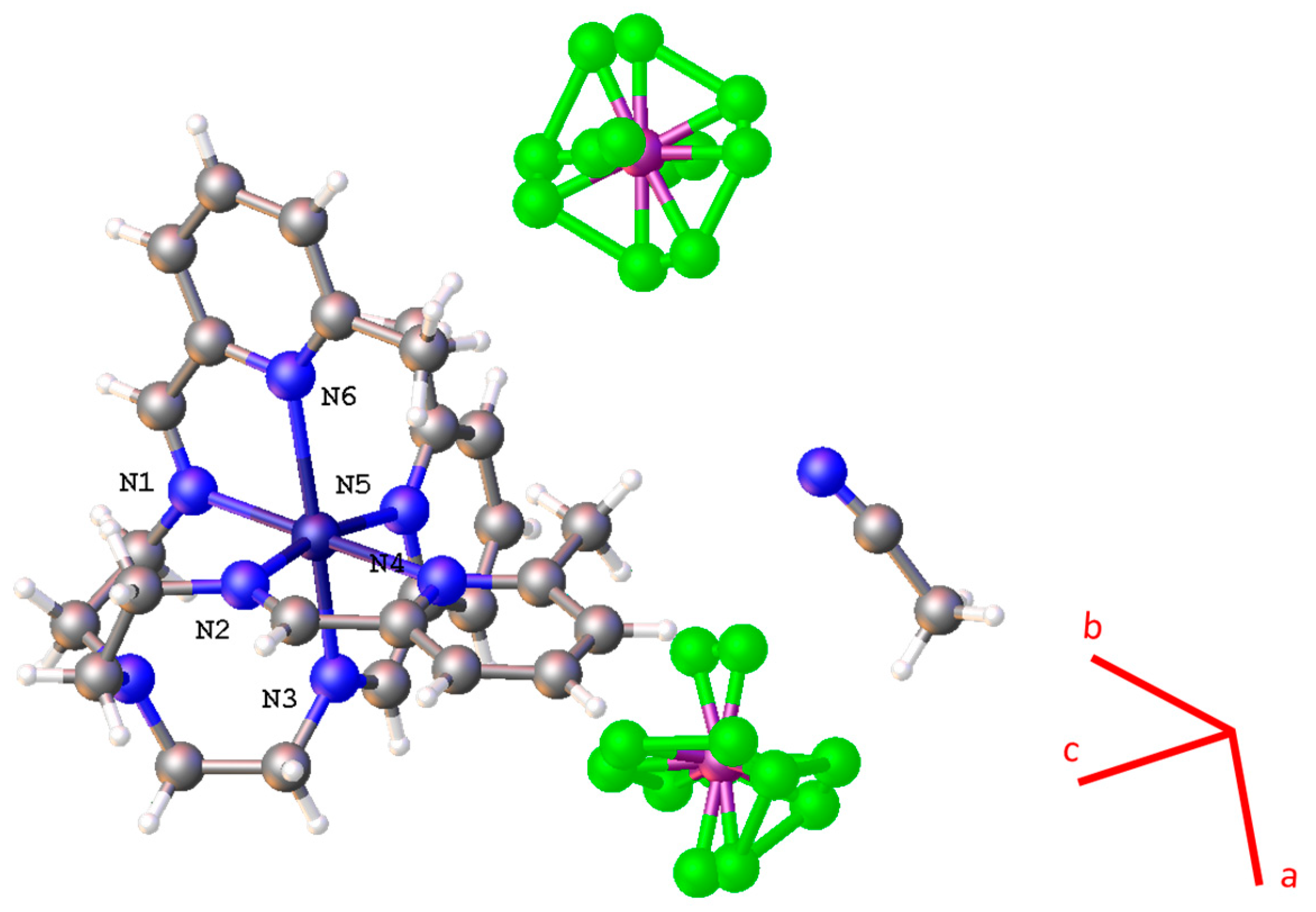
3.1. Crystallography
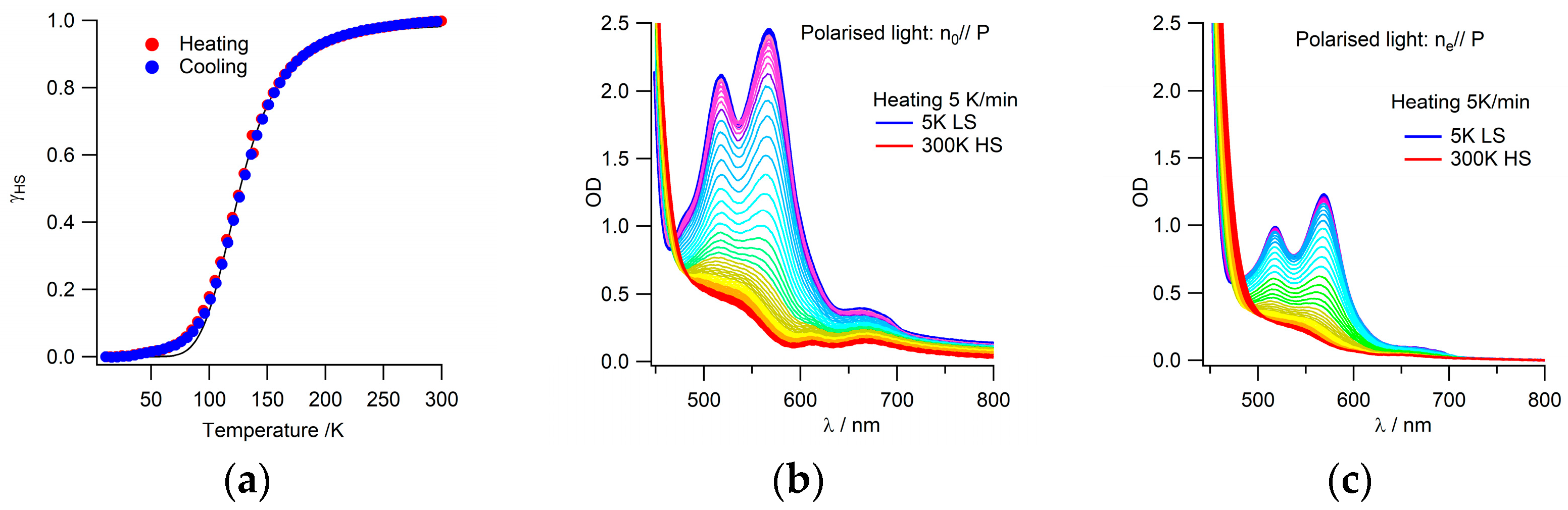
3.2. Thermal Spin Transition
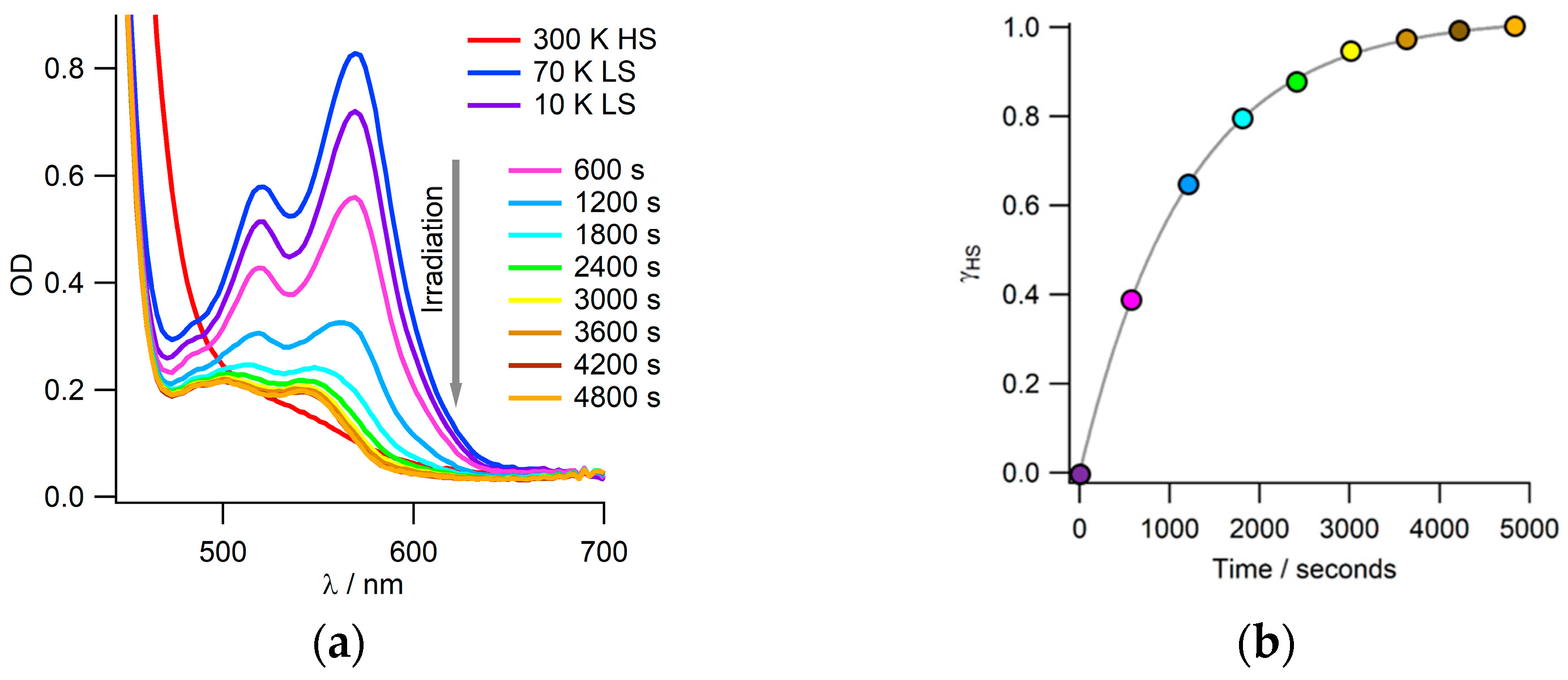
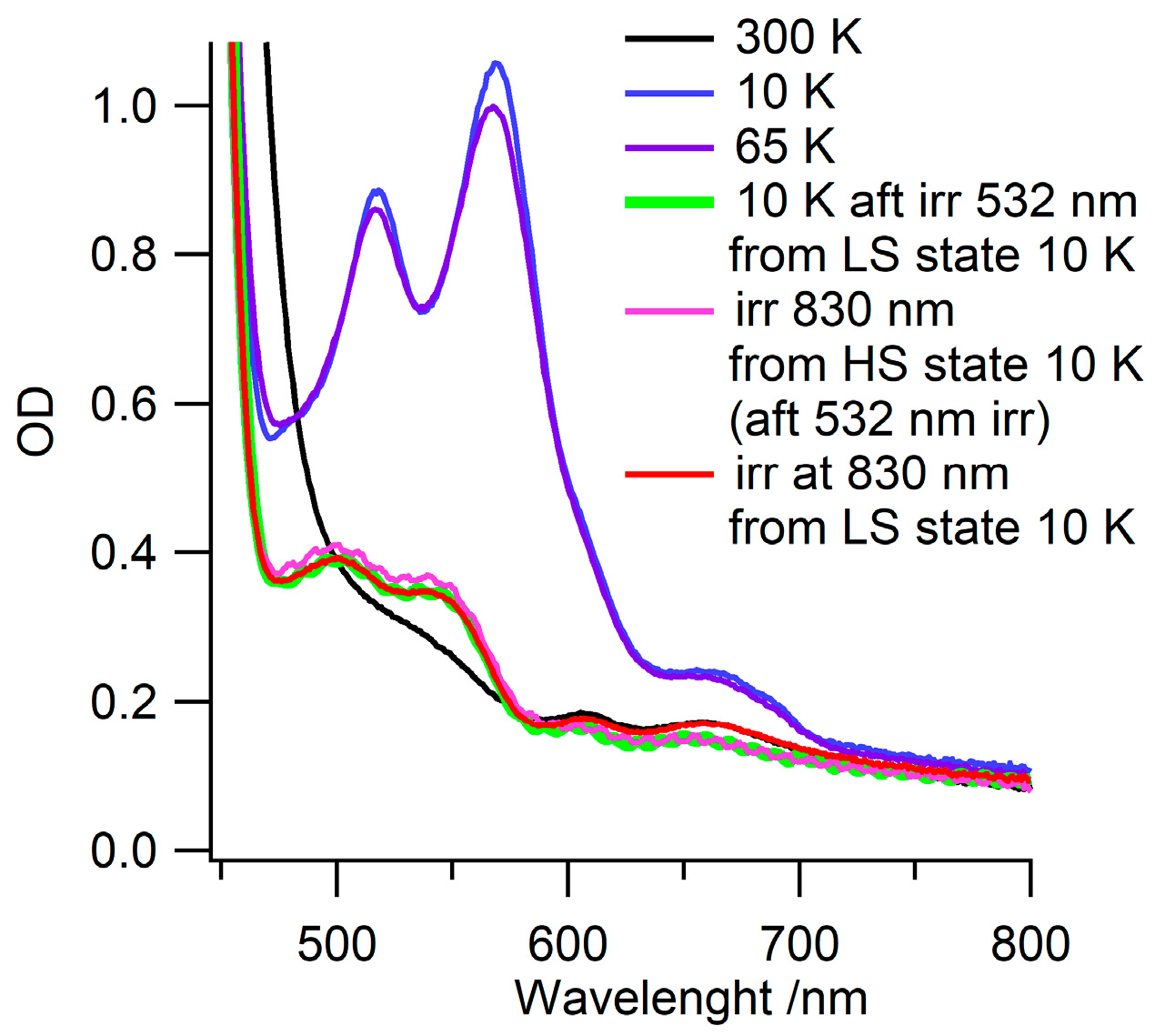
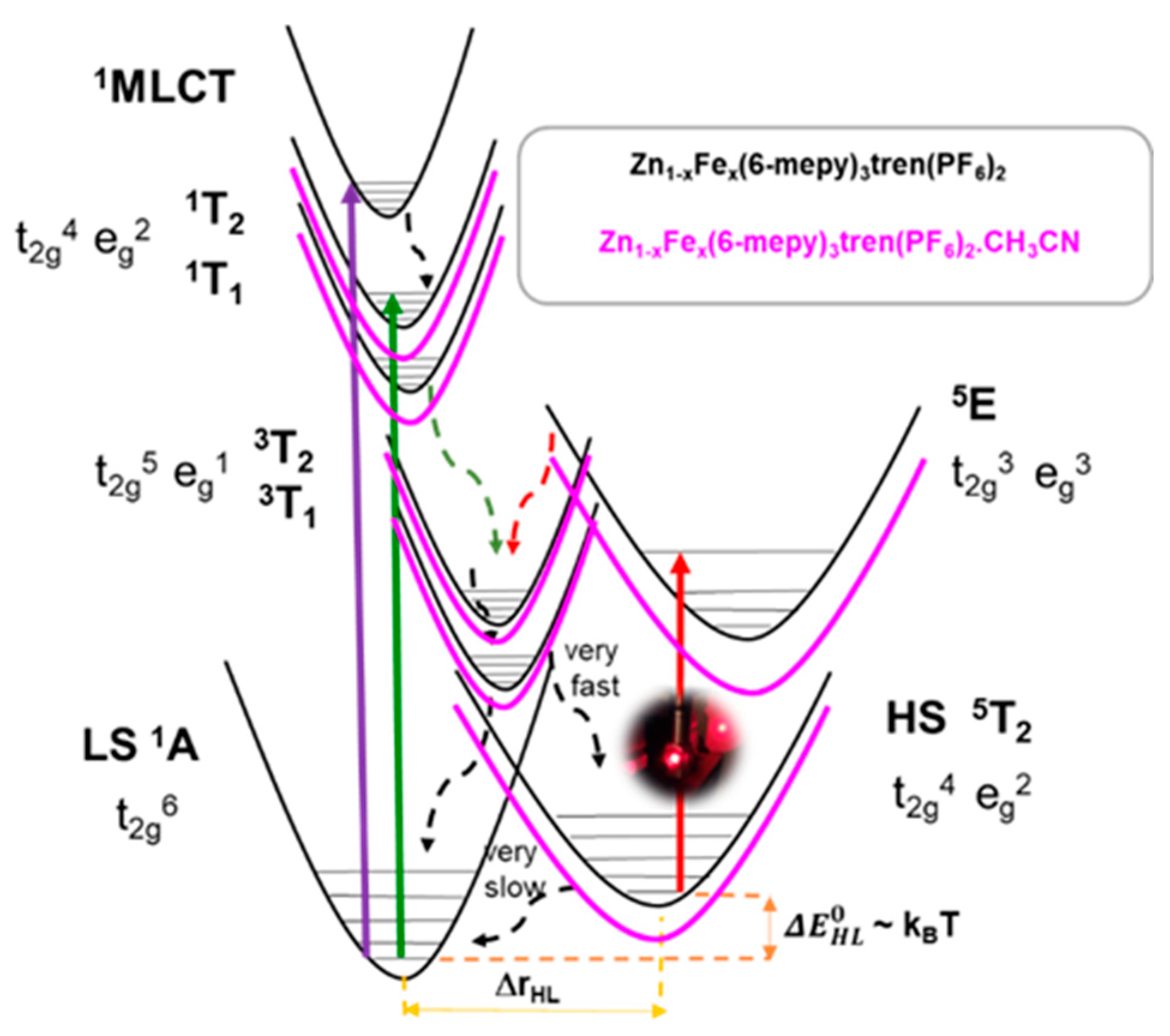
3.3. Photo-Induced LS-to-HS Spin Transition: LIESST Effect
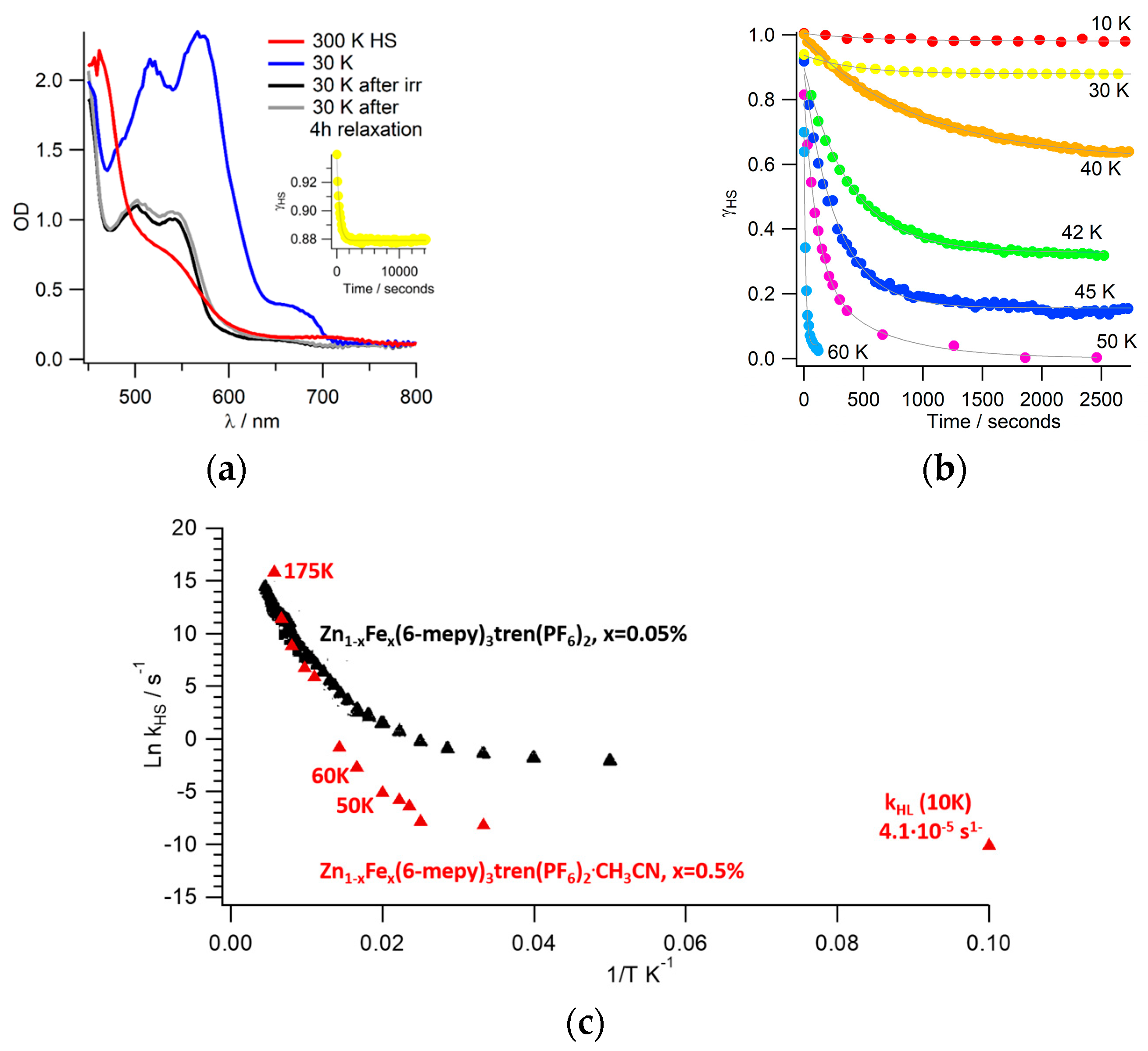
3.4. HS-to-LS Relaxation after LIESST
3.5. Reverse-LIESST
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gütlich, P.; Hauser, A.; Spiering, H. Thermal and Optical Switching of Iron(II) Complexes. Angew. Chem. Int. Ed. Engl. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Guetlich, P.; Ksenofontov, V.; Gaspar, A.B. Pressure effect studies on spin crossover systems. Coord. Chem. Rev. 2005, 249, 1811–1829. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular Spin Crossover Phenomenon: Recent Achievements and Prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef]
- Halcrow, M.A. (Ed.) Spin-Crossover Materials: Properties and Applications; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Delgado, T.; Villard, M. Spin Crossover Nanoparticles. J. Chem. Educ. 2022, 99, 1026–1035. [Google Scholar] [CrossRef]
- Amin, N.A.A.M.; Said, S.M.; Salleh, M.F.M.; Afifi, A.M.; Ibrahim, N.M.J.N.; Hasnan, M.M.I.M.; Tahir, M.; Hashim, N.Z.I. Review of Fe-based spin crossover metal complexes in multiscale device architectures. Inorg. Chim. Acta 2023, 544, 121168. [Google Scholar] [CrossRef]
- Sato, O. Dynamic Molecular Crystals with Switchable Physical Properties. Nat. Chem. 2016, 8, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.-P.; Liu, J.-L.; Hoque, M.N.; Liu, W.; Li, J.-Y.; Chen, Y.-C.; Tong, M.-L. Recent Advances in Guest Effects on Spin-Crossover Behavior in Hofmann-Type Metal-Organic Frameworks. Coord. Chem. Rev. 2017, 335, 28–43. [Google Scholar] [CrossRef]
- Arcís-Castillo, Z.; Muñoz-Lara, F.J.; Muñoz, M.C.; Aravena, D.; Gaspar, A.B.; Sánchez-Royo, J.F.; Ruiz, E.; Ohba, M.; Matsuda, R.; Kitagawa, S.; et al. Reversible Chemisorption of Sulfur Dioxide in a Spin Crossover Porous Coordination Polymer. Inorg. Chem. 2013, 52, 12777–12783. [Google Scholar] [CrossRef]
- Bartual-Murgui, C.; Akou, A.; Thibault, C.; Molnár, G.; Vieu, C.; Salmon, L.; Bousseksou, A. Spin-Crossover Metal–Organic Frameworks: Promising Materials for Designing Gas Sensors. J. Mater. Chem. C 2015, 3, 1277–1285. [Google Scholar] [CrossRef]
- Turo-Cortés, R.; Meneses-Sánchez, M.; Delgado, T.; Bartual-Murgui, C.; Muñoz, M.C.; Real, J.A. Coexistence of luminescence and spin-crossover in 2D iron (II) Hofmann clathrates modulated through guest encapsulation. J. Mater. Chem. C 2022, 10, 10686–10698. [Google Scholar] [CrossRef]
- Yi, C.; Zhang, S.; Zhao, L.; Yao, N.; Zhao, G.; Meng, Y.; Liu, T. Constructing spin crossover-fluorescence bifunctional iron(ii) complexes based on tetraphenyl ethylene-decorated AIEgens. Dalton Trans. 2023, 52, 5169–5175. [Google Scholar] [CrossRef] [PubMed]
- Ohba, M.; Yoneda, K.; Agusti, G.; Munoz, M.C.; Gaspar, A.B.; Real, J.-A.; Yamasaki, M.; Ando, H.; Nakao, Y.; Sakaki, S.; et al. Bidirectional Chemo-Switching of Spin State in a Microporous Framework. Angew. Chem. 2009, 121, 4861. [Google Scholar] [CrossRef]
- Mallah, T.; Cavallini, M. Surfaces, thin films and patterning of spin crossover compounds. C. R. Chim. 2018, 21, 1270–1286. [Google Scholar] [CrossRef]
- Hauser, A. Intersystem Crossing in the [Fe(Ptz)6](BF4)2 Spin Crossover System (Ptz = 1-propyltetrazole). J. Chem. Phys. 1991, 94, 2741–2748. [Google Scholar] [CrossRef]
- Chastanet, G.; Lorenc, M.; Bertoni, R.; Desplanches, C. Light-Induced Spin Crossover—Solution and Solid-State Processes. Spin Crossover Phenom. Phénom. Transit. Spin 2018, 21, 1075–1094. [Google Scholar] [CrossRef]
- Hauser, A. Light-induced spin crossover and the high-spin→low-spin relaxation. Top. Curr. Chem. 2004, 234, 155–198. [Google Scholar]
- Ye, Y.S.; Chen, X.Q.; Cai, Y.D.; Fei, B.; Dechambenoit, P.; Rouzières, M.; Mathonière, C.; Clérac, R.; Bao, X. Slow Dynamics of the Spin-Crossover Process in an Apparent High-Spin Mononuclear Fe II Complex. Angew. Chem. Int. Ed. 2019, 58, 18888–18891. [Google Scholar] [CrossRef] [PubMed]
- Delgado, T.; Tissot, A.; Guénée, L.; Hauser, A.; Valverde-Muñoz, F.J.; Seredyuk, M.; Real, J.A.; Pillet, S.; Bendeif, E.-E.; Besnard, C. Very Long-Lived Photogenerated High-Spin Phase of a Multistable Spin-Crossover Molecular Material. J. Am. Chem. Soc. 2018, 140, 12870–12876. [Google Scholar] [CrossRef]
- Hauser, A.; Enachescu, C.; Daku, M.L.; Vargas, A.; Amstutz, N. Low-Temperature Lifetimes of Metastable High-Spin States in Spin-Crossover and in Low-Spin Iron(II) Compounds: The Rule and Exceptions to the Rule. Coord. Chem. Rev. 2006, 250, 1642–1652. [Google Scholar] [CrossRef]
- Hauser, A.; Jeftić, J.; Romstedt, H.; Hinek, R.; Spiering, H. Cooperative Phenomena and Light-Induced Bistability in Iron(II) Spin-Crossover Compounds. Coord. Chem. Rev. 1999, 190–192, 471–491. [Google Scholar] [CrossRef]
- Delgado, T.; Enachescu, C.; Tissot, A.; Hauser, A.; Guénée, L.; Besnard, C. Evidencing Size-Dependent Cooperative Effects on Spin Crossover Nanoparticles Following Their HS→LS Relaxation. J. Mater. Chem. C 2018, 6, 12698–12706. [Google Scholar] [CrossRef]
- Tchougrkeff, A.L. Lattice Relaxation and Cooperativity in the Low-Spin to High-Spin Transitions in Molecular Crystals. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1995, 274, 17–23. [Google Scholar] [CrossRef]
- Hoselton, M.A.; Wilson, L.J.; Drago, R.S. Substituent Effects on the Spin Equilibrium Observed with Hexadentate Ligands on Iron(II). J. Am. Chem. Soc. 1975, 97, 1722–1729. [Google Scholar] [CrossRef]
- Hauser, A.; Vef, A.; Adler, P. Intersystem Crossing Dynamics in Fe(II) Coordination Compounds. J. Chem. Phys. 1991, 95, 8710–8717. [Google Scholar] [CrossRef]
- Schenker, S.; Hauser, A.; Wang, W.; Chan, I.Y. High-Spin→low-Spin Relaxation in [Zn1−xFex(6-Mepy)3−y(Py)Ytren](PF6)2. J. Chem. Phys. 1998, 109, 9870–9878. [Google Scholar] [CrossRef]
- Chakraborty, P.; Tissot, A.; Peterhans, L.; Guénée, L.; Besnard, C.; Pattison, P.; Hauser, A. Determination of the Molecular Structure of the Short-Lived Light-Induced High-Spin State in the Spin-Crossover Compound [Fe(6-Mepy)3tren](PF6)2. Phys. Rev. B 2013, 87, 214306. [Google Scholar] [CrossRef]
- Tissot, A.; Rivière, E.; Guillot, R.; Toupet, L.; Collet, E.; Boillot, M.-L. Light-Induced Excited Spin State Trapping Effect on [Fe(Mepy)3tren](PF6)2 Solvated Crystals. Dalton Trans. 2014, 43, 1063–1071. [Google Scholar] [CrossRef]
- Guionneau, P. Crystallography and Spin-Crossover. A View of Breathing Materials. Dalton Trans. 2014, 43, 382–393. [Google Scholar] [CrossRef]
- Atkins, P.; Overton, T.; Rourke, J.; Weller, M.; Armstrong, F.; Hagerman, M. Inorganic Chemistry; Oxford University Press: Oxford, UK, 2009. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, T.; Pelé, A.-L. Measurement of Reverse-Light-Induced Excited Spin State Trapping in Spin Crossover Systems: A Study Case with Zn1−xFex(6-mepy)3tren(PF6)2·CH3CN; x = 0.5%. Crystals 2024, 14, 210. https://doi.org/10.3390/cryst14030210
Delgado T, Pelé A-L. Measurement of Reverse-Light-Induced Excited Spin State Trapping in Spin Crossover Systems: A Study Case with Zn1−xFex(6-mepy)3tren(PF6)2·CH3CN; x = 0.5%. Crystals. 2024; 14(3):210. https://doi.org/10.3390/cryst14030210
Chicago/Turabian StyleDelgado, Teresa, and Anne-Laure Pelé. 2024. "Measurement of Reverse-Light-Induced Excited Spin State Trapping in Spin Crossover Systems: A Study Case with Zn1−xFex(6-mepy)3tren(PF6)2·CH3CN; x = 0.5%" Crystals 14, no. 3: 210. https://doi.org/10.3390/cryst14030210





